Abstract
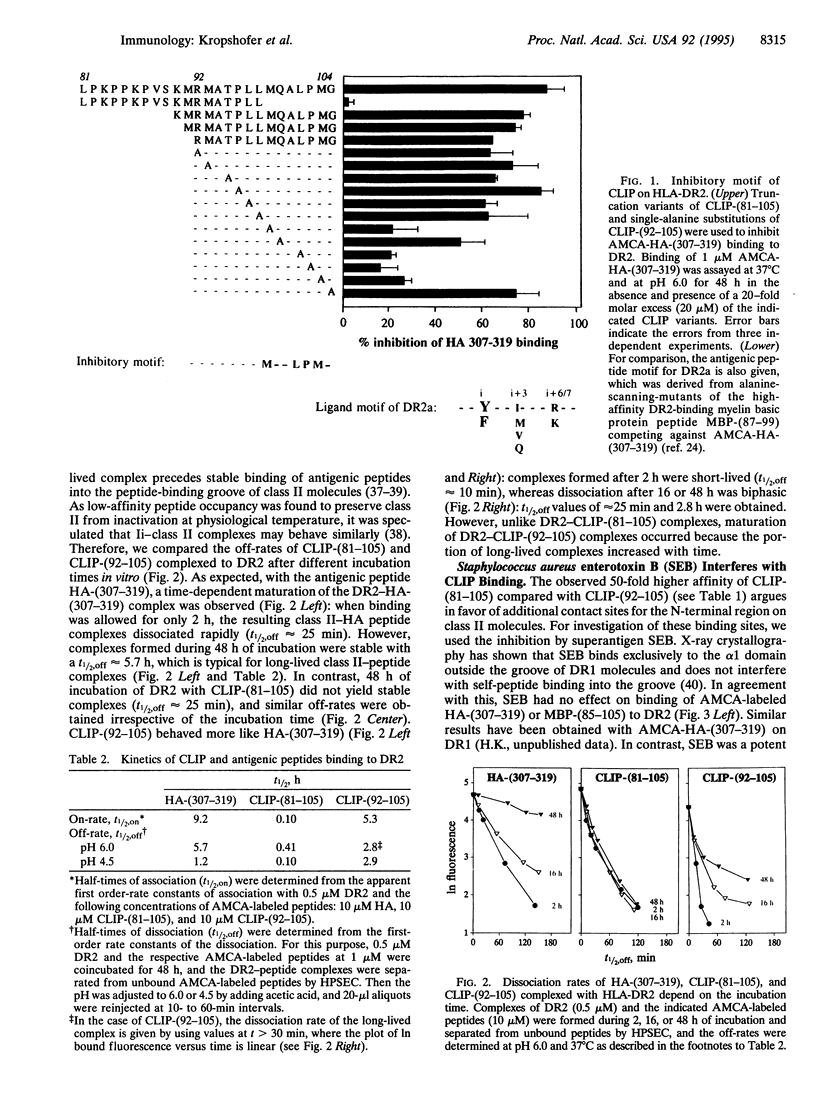
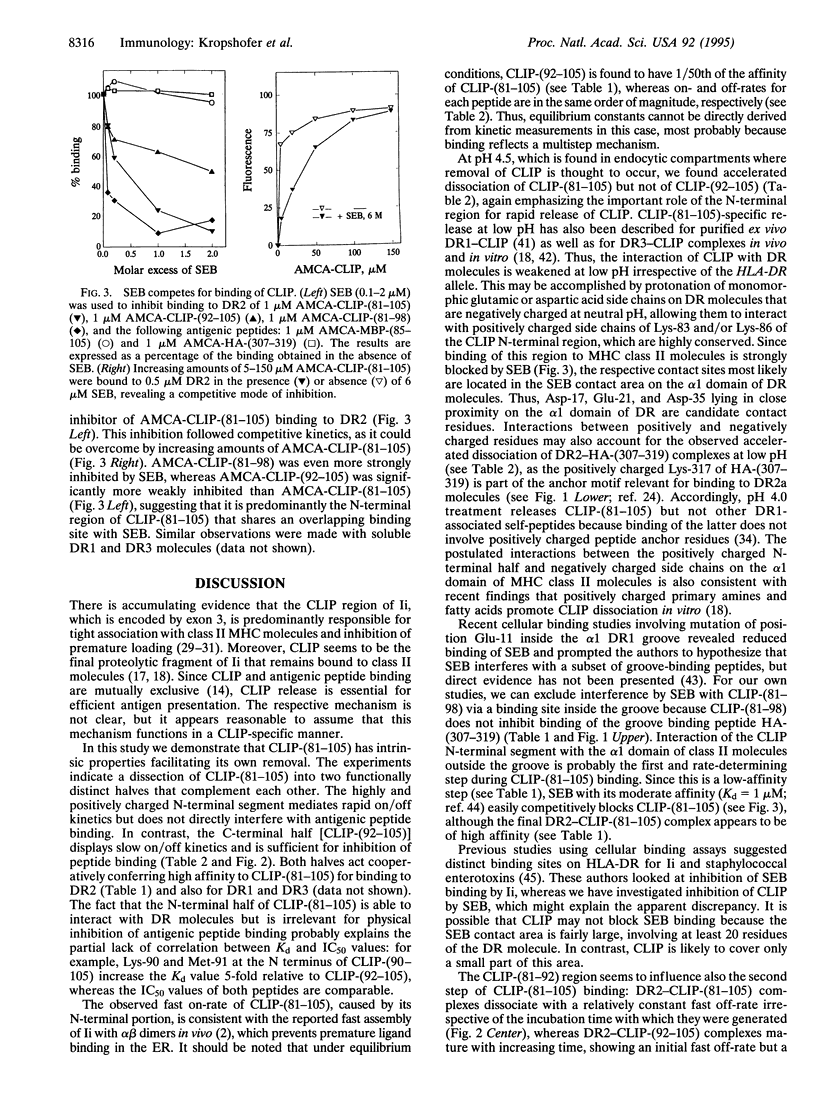
The invariant chain (Ii) prevents binding of ligands to major histocompatibility complex (MHC) class II molecules in the endoplasmic reticulum and during intracellular transport. Stepwise removal of the Ii in a trans-Golgi compartment renders MHC class II molecules accessible for peptide loading, with CLIP (class II-associated Ii peptides) as the final fragment to be released. Here we show that CLIP can be subdivided into distinct functional regions. The C-terminal segment (residues 92-105) of the CLIP-(81-105) fragment mediates inhibition of self- and antigenic peptide binding to HLA-DR2 molecules. In contrast, the N-terminal segment CLIP-(81-98) binds to the Staphylococcus aureus enterotoxin B contact site outside the peptide-binding groove on the alpha 1 domain and does not interfere with peptide binding. Its functional significance appears to lie in the contribution to CLIP removal: the dissociation of CLIP-(81-105) is characterized by a fast off-rate, which is accelerated at endosomal pH, whereas in the absence of the N-terminal CLIP-(81-91), the off-rate of C-terminal CLIP-(92-105) is slow and remains unaltered at low pH. Mechanistically, the N-terminal segment of CLIP seems to prevent tight interactions of CLIP side chains with specificity pockets in the peptide-binding groove that normally occurs during maturation of long-lived class II-peptide complexes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amigorena S., Drake J. R., Webster P., Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994 May 12;369(6476):113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- Avva R. R., Cresswell P. In vivo and in vitro formation and dissociation of HLA-DR complexes with invariant chain-derived peptides. Immunity. 1994 Dec;1(9):763–774. doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- Bakke O., Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990 Nov 16;63(4):707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- Beeson C., McConnell H. M. Kinetic intermediates in the reactions between peptides and proteins of major histocompatibility complex class II. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8842–8845. doi: 10.1073/pnas.91.19.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlmakers M. J., Benaroch P., Ploegh H. L. Assembly of HLA DR1 molecules translated in vitro: binding of peptide in the endoplasmic reticulum precludes association with invariant chain. EMBO J. 1994 Jun 1;13(11):2699–2707. doi: 10.1002/j.1460-2075.1994.tb06560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlmakers M. J., Benaroch P., Ploegh H. L. Mapping functional regions in the lumenal domain of the class II-associated invariant chain. J Exp Med. 1994 Aug 1;180(2):623–629. doi: 10.1084/jem.180.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. S., Cresswell P. Role for intracellular proteases in the processing and transport of class II HLA antigens. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3975–3979. doi: 10.1073/pnas.85.11.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A. G., Campbell P. L., Reynolds P., Gautam A. M., McCluskey J. Antigen presentation and assembly by mouse I-Ak class II molecules in human APC containing deleted or mutated HLA DM genes. J Immunol. 1994 Dec 15;153(12):5382–5392. [PubMed] [Google Scholar]
- Chicz R. M., Lane W. S., Robinson R. A., Trucco M., Strominger J. L., Gorga J. C. Self-peptides bound to the type I diabetes associated class II MHC molecules HLA-DQ1 and HLA-DQ8. Int Immunol. 1994 Nov;6(11):1639–1649. doi: 10.1093/intimm/6.11.1639. [DOI] [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Gorga J. C., Vignali D. A., Lane W. S., Strominger J. L. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993 Jul 1;178(1):27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Lane W. S., Gorga J. C., Stern L. J., Vignali D. A., Strominger J. L. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992 Aug 27;358(6389):764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- Denzin L. K., Robbins N. F., Carboy-Newcomb C., Cresswell P. Assembly and intracellular transport of HLA-DM and correction of the class II antigen-processing defect in T2 cells. Immunity. 1994 Oct;1(7):595–606. doi: 10.1016/1074-7613(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Pool sequencing of natural HLA-DR, DQ, and DP ligands reveals detailed peptide motifs, constraints of processing, and general rules. Immunogenetics. 1994;39(4):230–242. doi: 10.1007/BF00188785. [DOI] [PubMed] [Google Scholar]
- Freisewinkel I. M., Schenck K., Koch N. The segment of invariant chain that is critical for association with major histocompatibility complex class II molecules contains the sequence of a peptide eluted from class II polypeptides. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9703–9706. doi: 10.1073/pnas.90.20.9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga J. C., Horejsí V., Johnson D. R., Raghupathy R., Strominger J. L. Purification and characterization of class II histocompatibility antigens from a homozygous human B cell line. J Biol Chem. 1987 Nov 25;262(33):16087–16094. [PubMed] [Google Scholar]
- Hedley M. L., Urban R. G., Strominger J. L. Assembly and peptide binding of major histocompatibility complex class II heterodimers in an in vitro translation system. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10479–10483. doi: 10.1073/pnas.91.22.10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Brown J. H., Gorga J. C., Stern L. J., Urban R. G., Chi Y. I., Stauffacher C., Strominger J. L., Wiley D. C. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994 Apr 21;368(6473):711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- Karp D. R., Jenkins R. N., Long E. O. Distinct binding sites on HLA-DR for invariant chain and staphylococcal enterotoxins. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9657–9661. doi: 10.1073/pnas.89.20.9657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropshofer H., Bohlinger I., Max H., Kalbacher H. Self and foreign peptides interact with intact and disassembled MHC class II antigen HLA-DR via tryptophan pockets. Biochemistry. 1991 Sep 24;30(38):9177–9187. doi: 10.1021/bi00102a008. [DOI] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Lotteau V., Teyton L., Peleraux A., Nilsson T., Karlsson L., Schmid S. L., Quaranta V., Peterson P. A. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990 Dec 13;348(6302):600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- Marks M. S., Blum J. S., Cresswell P. Invariant chain trimers are sequestered in the rough endoplasmic reticulum in the absence of association with HLA class II antigens. J Cell Biol. 1990 Sep;111(3):839–855. doi: 10.1083/jcb.111.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason K., McConnell H. M. Short-lived complexes between myelin basic protein peptides and IAk. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12463–12466. doi: 10.1073/pnas.91.26.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max H., Halder T., Kropshofer H., Kalbus M., Müller C. A., Kalbacher H. Characterization of peptides bound to extracellular and intracellular HLA-DR1 molecules. Hum Immunol. 1993 Nov;38(3):193–200. doi: 10.1016/0198-8859(93)90540-h. [DOI] [PubMed] [Google Scholar]
- Monji T., McCormack A. L., Yates J. R., 3rd, Pious D. Invariant-cognate peptide exchange restores class II dimer stability in HLA-DM mutants. J Immunol. 1994 Nov 15;153(10):4468–4477. [PubMed] [Google Scholar]
- Newcomb J. R., Cresswell P. Characterization of endogenous peptides bound to purified HLA-DR molecules and their absence from invariant chain-associated alpha beta dimers. J Immunol. 1993 Jan 15;150(2):499–507. [PubMed] [Google Scholar]
- Peters P. J., Neefjes J. J., Oorschot V., Ploegh H. L., Geuze H. J. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991 Feb 21;349(6311):669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- Riberdy J. M., Avva R. R., Geuze H. J., Cresswell P. Transport and intracellular distribution of MHC class II molecules and associated invariant chain in normal and antigen-processing mutant cell lines. J Cell Biol. 1994 Jun;125(6):1225–1237. doi: 10.1083/jcb.125.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riberdy J. M., Newcomb J. R., Surman M. J., Barbosa J. A., Cresswell P. HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature. 1992 Dec 3;360(6403):474–477. doi: 10.1038/360474a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990 Jun 14;345(6276):615–618. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. Proteolysis of the class II-associated invariant chain generates a peptide binding site in intracellular HLA-DR molecules. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3150–3154. doi: 10.1073/pnas.88.8.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche P. A., Marks M. S., Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991 Dec 5;354(6352):392–394. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- Romagnoli P., Germain R. N. The CLIP region of invariant chain plays a critical role in regulating major histocompatibility complex class II folding, transport, and peptide occupancy. J Exp Med. 1994 Sep 1;180(3):1107–1113. doi: 10.1084/jem.180.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., McConnell H. M. A kinetic intermediate in the reaction of an antigenic peptide and I-Ek. Nature. 1989 Jan 19;337(6204):274–276. doi: 10.1038/337274a0. [DOI] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., Stern L. J., Wiley D. C., Germain R. N. MHC class II function preserved by low-affinity peptide interactions preceding stable binding. Nature. 1994 Aug 25;370(6491):647–650. doi: 10.1038/370647a0. [DOI] [PubMed] [Google Scholar]
- Seth A., Stern L. J., Ottenhoff T. H., Engel I., Owen M. J., Lamb J. R., Klausner R. D., Wiley D. C. Binary and ternary complexes between T-cell receptor, class II MHC and superantigen in vitro. Nature. 1994 May 26;369(6478):324–327. doi: 10.1038/369324a0. [DOI] [PubMed] [Google Scholar]
- Sette A., Ceman S., Kubo R. T., Sakaguchi K., Appella E., Hunt D. F., Davis T. A., Michel H., Shabanowitz J., Rudersdorf R. Invariant chain peptides in most HLA-DR molecules of an antigen-processing mutant. Science. 1992 Dec 11;258(5089):1801–1804. doi: 10.1126/science.1465617. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Brown J. H., Jardetzky T. S., Gorga J. C., Urban R. G., Strominger J. L., Wiley D. C. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994 Mar 17;368(6468):215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Wiley D. C. The human class II MHC protein HLA-DR1 assembles as empty alpha beta heterodimers in the absence of antigenic peptide. Cell. 1992 Feb 7;68(3):465–477. doi: 10.1016/0092-8674(92)90184-e. [DOI] [PubMed] [Google Scholar]
- Strubin M., Berte C., Mach B. Alternative splicing and alternative initiation of translation explain the four forms of the Ia antigen-associated invariant chain. EMBO J. 1986 Dec 20;5(13):3483–3488. doi: 10.1002/j.1460-2075.1986.tb04673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyton L., O'Sullivan D., Dickson P. W., Lotteau V., Sette A., Fink P., Peterson P. A. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature. 1990 Nov 1;348(6296):39–44. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- Thibodeau J., Cloutier I., Lavoie P. M., Labrecque N., Mourad W., Jardetzky T., Sékaly R. P. Subsets of HLA-DR1 molecules defined by SEB and TSST-1 binding. Science. 1994 Dec 16;266(5192):1874–1878. doi: 10.1126/science.7997881. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Urban R. G., Chicz R. M., Strominger J. L. Selective release of some invariant chain-derived peptides from HLA-DR1 molecules at endosomal pH. J Exp Med. 1994 Aug 1;180(2):751–755. doi: 10.1084/jem.180.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt A. B., Kropshofer H., Kalbacher H., Kalbus M., Rammensee H. G., Coligan J. E., Martin R. Ligand motifs of HLA-DRB5*0101 and DRB1*1501 molecules delineated from self-peptides. J Immunol. 1994 Aug 15;153(4):1665–1673. [PubMed] [Google Scholar]
- West M. A., Lucocq J. M., Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature. 1994 May 12;369(6476):147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Sette A., Southwood S., Oseroff C., Matsui M., Strominger J. L., Hafler D. A. Structural requirements for binding of an immunodominant myelin basic protein peptide to DR2 isotypes and for its recognition by human T cell clones. J Exp Med. 1994 Jan 1;179(1):279–290. doi: 10.1084/jem.179.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Capraro G. A., Daibata M., Reyes V. E., Humphreys R. E. Cathepsin B cleavage and release of invariant chain from MHC class II molecules follow a staged pattern. Mol Immunol. 1994 Jul;31(10):723–731. doi: 10.1016/0161-5890(94)90146-5. [DOI] [PubMed] [Google Scholar]


