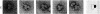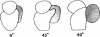Abstract
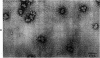
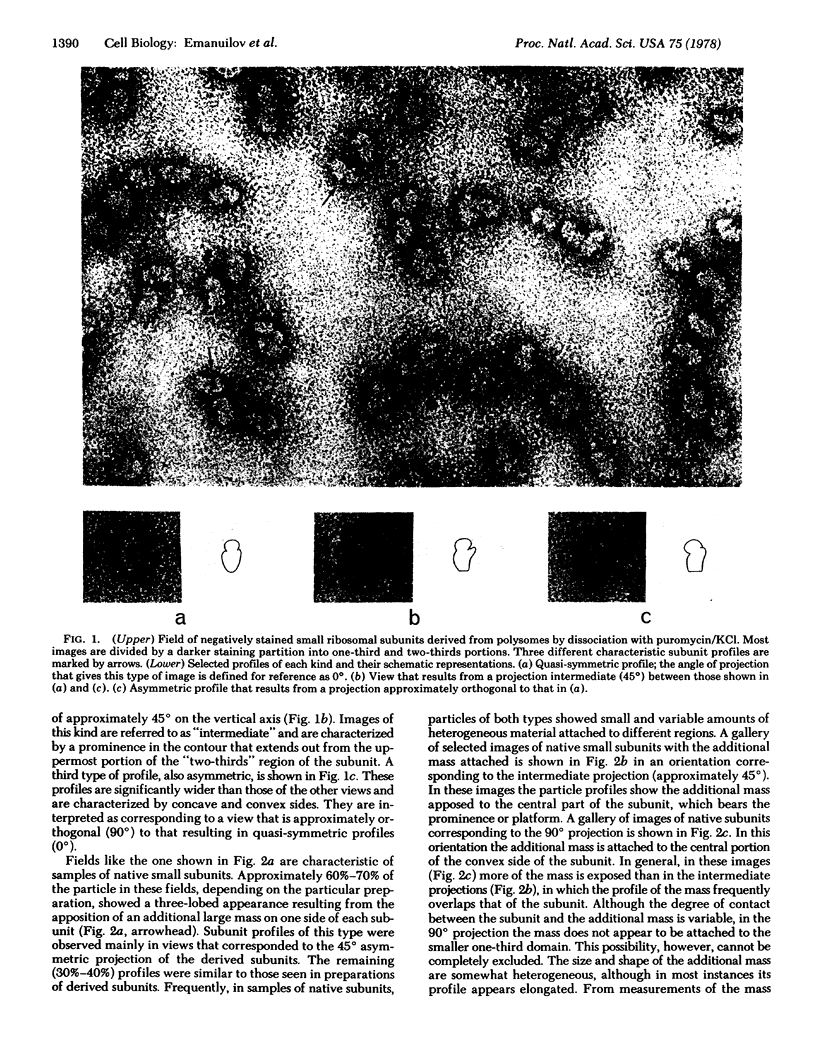
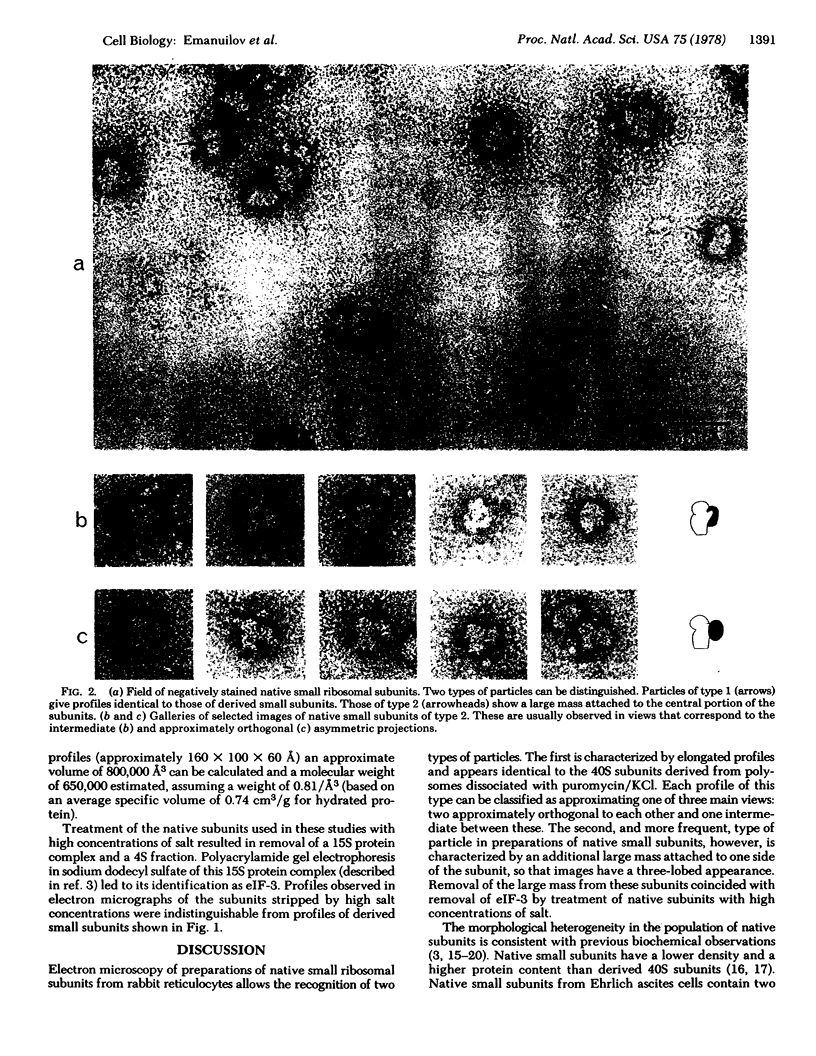
The localization of eukaryotic initiation factor 3(eIF-3) on native small ribosomal subunits has been established by electron microscopy through a comparison of native small ribosomal subunits with derived subunits and with native subunits stripped of eIF-3. Small subunits derived from reticulocyte ribosomes by the puromycin/KCl method are seen in electron micrographs as elongated particles, divided by a heavily stained partition into approximately one-third and two-third domains. Most particles (60-70%) observed in electron micrographs of native small subunit preparations resemble derived small subunits, but have an additional mass attached to one side, thus producing profiles with a three-lobed appearance. The mass measures approximately 160 × 100 × 60 Å, and its particle weight is estimated to be about one-third to one-half that of a 40S subunit. The site of attachment of the additional mass is located on a prominence extending from the central part of the small subunit and is separated by a cleft from the smaller third of the subunit. The remaining particles in preparations of native subunits resemble the profiles seen in electron micrographs of derived subunits. After removal of eIF-3 by treatment with high concentrations of salt, profiles observed in electron micrographs of washed, native subunits were indistinguishable from those of derived subunits. Since removal of eIF-3 coincided with removal of a mass of the correct molecular weight, subunits with the three-lobed appearance are identified as native small subunits carrying eIF-3.
Keywords: protein synthesis, electron microscopy, ribosomes
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayuso-Parilla M., Henshaw E. C., Hirsch C. A. The ribosome cycle in mammalian protein synthesis. 3. Evidence that the nonribosomal proteins bound to the native smaller subunit are initiation factors. J Biol Chem. 1973 Jun 25;248(12):4386–4393. [PubMed] [Google Scholar]
- BORSOOK H., DEASY C. L., HAAGENSMIT A. J., KEIGHLEY G., LOWY P. H. Incorporation in vitro of labeled amino acids into proteins of rabbit reticulocytes. J Biol Chem. 1952 May;196(2):669–694. [PubMed] [Google Scholar]
- Baglioni C. Heterogeneity of the small ribosomal subunit and mechanism of chain initiation in eukaryotes. Biochim Biophys Acta. 1972 Nov 16;287(1):189–193. doi: 10.1016/0005-2787(72)90341-3. [DOI] [PubMed] [Google Scholar]
- Benne R., Hershey J. W. Purification and characterization of initiation factor IF-E3 from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3005–3009. doi: 10.1073/pnas.73.9.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen A., Heimark R. L., Cozzone A., Traut R. R., Hershey J. W. Cross-linking of initiation factor IF-2 to Escherichia coli 30 S ribosomal proteins with dimethylsuberimidate. J Biol Chem. 1975 Jun 10;250(11):4310–4314. [PubMed] [Google Scholar]
- Freienstein C., Blobel G. Nonribosomal proteins associated with eukaryotic native small ribosomal subunits. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3392–3396. doi: 10.1073/pnas.72.9.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freienstein C., Blobel G. Use of eukaryotic native small ribosomal subunits for the translation of globin messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3435–3439. doi: 10.1073/pnas.71.9.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S. M., Kennedy D. S., Bester A. J. Separation of specific initiation factors involved in the translation of myosin and myoglobin messenger RNAs and the isolation of a new RNA involved in translation. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2428–2431. doi: 10.1073/pnas.71.6.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S. M. Specificity of mRNA binding factor in eukaryotes. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1782–1788. doi: 10.1073/pnas.67.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch C. A., Cox M. A., van Venrooij W. J., Henshaw E. C. The ribosome cycle in mammalian protein synthesis. II. Association of the native smaller ribosomal subunit with protein factors. J Biol Chem. 1973 Jun 25;248(12):4377–4385. [PubMed] [Google Scholar]
- Kaempfer R., Kaufman J. Translational control of hemoglobin synthesis by an initiation factor required for recycling of ribosomes and for their binding to messenger RNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3317–3321. doi: 10.1073/pnas.69.11.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A., Kahan L. Ribosomal proteins S5, S11, S13 and S19 localized by electron microscopy of antibody-labeled subunits. J Mol Biol. 1975 Dec 25;99(4):631–644. doi: 10.1016/s0022-2836(75)80177-x. [DOI] [PubMed] [Google Scholar]
- Lake J. A., Pendergast M., Kahan L., Nomura M. Localization of Escherichia coli ribosomal proteins S4 and S14 by electron microscopy of antibody-labeled subunits. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4688–4692. doi: 10.1073/pnas.71.12.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A. Ribosome structure determined by electron microscopy of Escherichia coli small subunits, large subunits and monomeric ribosomes. J Mol Biol. 1976 Jul 25;105(1):131–139. doi: 10.1016/0022-2836(76)90200-x. [DOI] [PubMed] [Google Scholar]
- Nonomura Y., Blobel G., Sabatini D. Structure of liver ribosomes studied by negative staining. J Mol Biol. 1971 Sep 14;60(2):303–323. doi: 10.1016/0022-2836(71)90296-8. [DOI] [PubMed] [Google Scholar]
- Prichard P. M., Gilbert J. M., Shafritz D. A., Anderson W. F. Factors for the initiation of haemoglobin synthesis by rabbit reticulocyte ribosomes. Nature. 1970 May 9;226(5245):511–514. doi: 10.1038/226511a0. [DOI] [PubMed] [Google Scholar]
- Safer B., Adams S. L., Kemper W. M., Berry K. W., Lloyd M., Merrick W. C. Purification and characterization of two initiation factors required for maximal activity of a highly fractionated globin mRNA translation system. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2584–2588. doi: 10.1073/pnas.73.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameshima M., Izawa M. Properties and synthesis of multiple components in native small ribosomal subunits of mouse ascites tumor cells. Biochim Biophys Acta. 1975 Feb 10;378(3):405–414. doi: 10.1016/0005-2787(75)90185-9. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of eukaryotic protein synthesis: (Met-tRNA f -40S ribosome) initiation complex catalysed by purified initiation factors in the absence of mRNA. Nat New Biol. 1973 Mar 14;242(115):35–38. doi: 10.1038/newbio242035a0. [DOI] [PubMed] [Google Scholar]
- Strycharz W. A., Ranki M., Dahl H. H. A high-molecular-weight protein component required for natural messenger translation in ascites tumor cells. Eur J Biochem. 1974 Oct 1;48(1):303–310. doi: 10.1111/j.1432-1033.1974.tb03769.x. [DOI] [PubMed] [Google Scholar]
- Sundkvist I. C., McKeehan W. L., Schreier M. H., Staehelin T. Initiation factor activity associated with free 40 S subunits from rat liver and rabbit reticulocytes. J Biol Chem. 1974 Oct 25;249(20):6512–6516. [PubMed] [Google Scholar]
- Sundkvist I. C., Staehelin T. Structure and function of free 40 S ribosome subunits: Characterization of initiation factors. J Mol Biol. 1975 Dec 15;99(3):401–418. doi: 10.1016/s0022-2836(75)80135-5. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Vasiliev V. D. Morphology of the ribosomal 30S subparticle according to electron microscopic data. Acta Biol Med Ger. 1974;33(5-6):779–793. [PubMed] [Google Scholar]
- van Venrooij W. J., Janssen A. P., Hoeymakers J. H., de Man B. M. On the heterogeneity of native ribosomal subunits in Ehrlich-ascites-tumor cells cultured in vitro. Eur J Biochem. 1976 May 1;64(2):429–435. doi: 10.1111/j.1432-1033.1976.tb10319.x. [DOI] [PubMed] [Google Scholar]