Annual plants usually show continuous flower generation after flowering has been induced. This works reveals an epigenetic basis for flowering commitment as Polycomb-group mutants display floral reversion that is dependent on the key flowering repressors FLC and SVP. In addition, a daylength-independent function for the florigen FT is identified that maintains flower generation.
Abstract
The switch from vegetative to reproductive growth is extremely stable even if plants are only transiently exposed to environmental stimuli that trigger flowering. In the photoperiodic pathway, a mobile signal, florigen, encoded by FLOWERING LOCUS T (FT) in Arabidopsis thaliana, induces flowering. Because FT activity in leaves is not maintained after transient photoperiodic induction, the molecular basis for stable floral commitment is unclear. Here, we show that Polycomb-group (Pc-G) proteins, which mediate epigenetic gene regulation, maintain the identity of inflorescence and floral meristems after floral induction. Thus, plants with reduced Pc-G activity show a remarkable increase of cauline leaves under noninductive conditions and floral reversion when shifted from inductive to noninductive conditions. These phenotypes are almost completely suppressed by loss of FLOWERING LOCUS C (FLC) and SHORT VEGETATIVE PHASE, which both delay flowering and promote vegetative shoot identity. Upregulation of FLC in Pc-G mutants leads to a strong decrease of FT expression in inflorescences. We find that this activity of FT is needed to prevent floral reversion. Collectively, our results reveal that floral meristem identity is at least partially maintained by a daylength-independent role of FT whose expression is indirectly sustained by Pc-G activity.
INTRODUCTION
The time of flowering in plants is critically important so that flower and fruit production is coordinated with pollinators and seasons. It is regulated by external environmental factors, notably photoperiod and temperature, which provide seasonal cues, and also by internal factors such as age; most plants are not competent to respond to inductive signals unless they have first completed a juvenile phase (reviewed in Kobayashi and Weigel, 2007; Srikanth and Schmid, 2011). Once triggered, the switch from vegetative growth to flowering is usually irreversible. This is likely important in natural environments so that plants do not make sterile flower/shoot intermediates if inductive signals such as temperature fluctuate. Floral commitment is most apparent in annual plants, which usually do not revert to vegetative growth. In perennial plants, which undergo repeated yearly cycles of vegetative and reproductive growth, although not all meristems switch to reproductive growth in any one year, those that do usually show stable floral commitment (Tooke et al., 2005; Wang et al., 2009). However, there are a few examples of plant species and mutants that show distinct types of floral reversion: inflorescence reversion in which the inflorescence reverts to vegetative growth and flower reversion in which the flower itself has features of the inflorescence or even vegetative identity. In these species, floral reversion occurs primarily if inductive signals are not maintained (Tooke et al., 2005). Despite this, the molecular basis for floral commitment is poorly understood, even in plants like Arabidopsis thaliana where the molecular mechanisms triggering flowering are well defined.
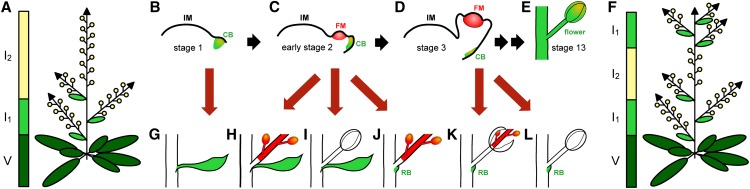
Aerial parts of plants are formed from shoot apical meristems (SAMs), which undergo several changes in identity before producing flowers. These changes in meristem identity cause modifications in the shoot structure through changes within the basic unit of the metamer, which is composed of an internode and a node. The latter consists of a leaf or bract that harbors a secondary meristem in its axil that give rise to a side shoot (Evans and Grover, 1940; Schultz and Haughn, 1991). In Arabidopsis, the SAM sequentially produces three types of metamers: in the vegetative phase, the SAM generates rosette leaves subtending a lateral shoot; after floral induction, the stem elongates (bolting) and cauline leaves with a side shoot, the paraclade, are formed (I1 stage), finally leading to production of flowers without bracts (I2 stage) (Figure 1A; Schultz and Haughn, 1991; Haughn et al., 1995). Detailed analysis of the SAM at the time of floral induction suggests that the I1 stage is a continuation of the vegetative phase and that the SAM directly produces floral primordia on its flanks after induction (Hempel and Feldman, 1994, 1995). Occasionally, e.g., by shifting wild-type plants from short-day (SD) to continuous light, flowers subtended by bracts (cauline leaves) can be observed, indicating that floral bracts can occur in Arabidopsis (Hempel et al., 1998). Furthermore, analysis of SHOOTMERISTEMLESS (STM) and AINTEGUMENTA (ANT) expression, which are markers for meristems and organs respectively, indicate that primordia are initially leaf-like in character as they are marked by absence of STM expression and presence of ANT (flower development stages 0 and 1 in the classification of Smyth et al., 1990; Long and Barton, 2000). In early stage 2, the primordium is partitioned into a meristem, marked by STM expression, and a “cryptic” bract marked by absence of STM (Long et al., 1996; Long and Barton, 2000) and briefly morphologically discernable (Kwiatkowska, 2006). During later stages of flower development, cryptic bract outgrowth is suppressed; hence, it is not visible at maturity. Nonetheless, in mutants with compromised floral meristem identity, such as leafy (lfy) or apetala1 (ap1), the cryptic bract develops more fully and is visible at maturity as a bract subtending flowers or paraclades (Mandel et al., 1992; Weigel et al., 1992). The floral meristem (FM) differs from vegetative or inflorescence SAMs not only in that it makes floral organs, but also in that it is determinate and does not give rise to more meristems or branches.
Figure 1.
Schematic Diagrams of Normal Arabidopsis Inflorescence and Flower Development and Floral Reversion.
(A) The wild-type SAM produces three metameric types sequentially: rosette leaves are formed at the vegetative state (V), after floral induction, the stem elongates and cauline leaves subtending lateral branches, named paraclades, are generated (I1), followed by production of flowers (I2). After floral induction, flower production is stable even if plants are shifted from LD to SD.
(B) to (E) Development of the flower metamers (according to Smyth et al. [1990], Chandler [2012], and Long and Barton [2000]): In stage 1 (B), the floral primordium emerges as cryptic bract (CB) and thus has leaf-like identity. In early stage 2 (C), the FM develops between the IM and the cryptic bract. The formation of the sepal primordia at the flanks of the FM marks stage 3 (D). The CB is morphologically not detectable. Stage 13 flower is in (E).
(F) Pc-G mutants shifted from LD to SD show floral reversion and, thus, an additional I1 phase after I2.
(G) to (L) Likely scenario for origin of distinct types of reversion nodes: empty cauline leaf (G), cauline leaf subtending a paraclade (H), cauline leaf subtending a flower (I), paraclade without a cauline leaf but with a rudimentary bract (RB) (J), late reversion of the FM, an inflorescence emerges inside of sepals (K), and flower with RB (L).
Arabidopsis FLOWERING LOCUS T (FT) is the key gene mediating the vegetative to floral transition and is widely conserved in flowering plants (Kardailsky et al., 1999; Kobayashi et al., 1999; Izawa et al., 2002; Kojima et al., 2002). Thus ft mutants show delayed flowering but produce normal flowers once the transition occurs. The FT product likely corresponds to florigen, the long-sought mobile signal that moves from leaves to the SAM in response to inductive photoperiods and promotes flowering (reviewed in Kobayashi and Weigel, 2007). FT is expressed in leaves in inductive photoperiods (long days in Arabidopsis) and produces a small protein that moves through the phloem to the shoot apex where it forms a complex with a bZIP transcription factor, FD, to activate targets promoting flower primordium identity including AP1 and FRUITFULL (FUL) (Abe et al., 2005; Wigge et al., 2005). A second gene promoting the floral transition is SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), which encodes a MADS box transcription factor (Samach et al., 2000; Searle et al., 2006). SOC1, together with FUL, are likely targets needed for FT action; thus, soc1 ful double mutants largely prevent the early flowering triggered by 35Spro:FT transgenes. In addition, soc1 ful double mutants show reversion from inflorescence to vegetative growth, indicating that floral commitment is impaired (Melzer et al., 2008).
Once floral induction has occurred, LFY and AP1 are expressed. Expression of AP1 is initiated in floral primordia at stage 1 and is thought to mark floral commitment, whereas LFY expression initiates in floral anlagen in incipient primordia (stage 0) and is thus expressed before flowers are discernible (Mandel et al., 1992; Weigel et al., 1992). Studies using transient activation of a steroid-dependent LFY transgene indicate that persistent LFY activity is needed to prevent flower-to-shoot reversion (Wagner and Meyerowitz, 2011), consistent with lfy mutants displaying floral reversion under certain conditions (Okamuro et al., 1996). LFY and AP1 proteins bind each other’s promoters and upregulate one another’s transcription, suggesting that once LFY and AP1 expression are activated in floral primordia they are likely self-perpetuating through positive feedback loops (Wagner et al., 1999; Adrian et al., 2009; Kaufmann et al., 2010).
In addition to flower-promoting factors like LFY and AP1, the switch to floral meristem identity also requires repression of factors that promote vegetative or inflorescence shoot identity in the early floral primordia. Thus, transcriptional profiling indicates that during the early stages of floral primordium development the majority of genes whose expression significantly changes are downregulated (Wellmer et al., 2006). The downregulated genes include SHORT VEGETATIVE PHASE (SVP) and SOC1 which are both direct targets of AP1 and promote shoot identity (Liu et al., 2009; Kaufmann et al., 2010). Paradoxically, activity of these genes is needed during the earliest stages (stages 0 to 2) of flower development and acts together with LFY to activate the floral homeotic genes that specify floral organ identity and determinacy (Liu et al., 2009). Subsequently, SVP/SOC1 need to be switched off for floral meristem identity to be maintained and flower patterning to occur. Thus, expression of SVP/SOC1 transgenes during later stages of flower development causes floral reversion (Liu et al., 2007).
In Arabidopsis, as with most plants, flowering persists after transient photoperiodic induction (Corbesier et al., 1996). However, analysis of plants shifted from inductive long-day (LD) to SD conditions shows that both FT expression in leaves and SOC1 expression in the SAM is rapidly lost after the shift (Corbesier et al., 2007; Torti et al., 2012). Floral commitment has similarity with the vernalization response in that both involve a stable memory of a transient environmental stimulus (cold in the case of vernalization), raising the question of whether they share a common mechanistic basis. The memory of vernalization is mediated by Polycomb-group (Pc-G) genes that confer stable epigenetic silencing of a repressor of flowering, FLOWERING LOCUS C (FLC) (Gendall et al., 2001), which in turn represses expression of FT and SOC1 (Searle et al., 2006). Consistent with their epigenetic role, the Pc-G proteins have biochemical activity toward chromatin; in particular, a complex of four core proteins termed Polycomb Repressive Complex 2 (PRC2) catalyzes the methylation of Lys-27 on histone H3 (H3K27me3), a modification associated with transcriptional repression (reviewed in Margueron and Reinberg, 2011). In Arabidopsis, the MEDEA, CURLY LEAF (CLF), and SWINGER (SWN) genes encode homologs of Drosophila melanogaster Enhancer of zeste, the catalytic subunit of the PRC2, while FERTILIZATION INDEPENDENT SEED2, EMBRYONIC FLOWER2 (EMF2), and VERNALIZATION2 (VRN2) show similarity to a second core PRC2 component, Drosophila Suppressor of zeste 12 (Chanvivattana et al., 2004). In clf swn mutant seedlings, H3K27me3 methylation is completely lost, suggesting that Pc-G activity is eliminated (Lafos et al., 2011). Whereas double mutants for the severe alleles emf2-3 and vrn2-1 resemble clf swn mutants phenotypically (Schubert et al., 2005), combination of the weaker emf2-10 allele with vrn2-1 results in viable, fertile plants, although global H3K27me3 levels are highly reduced (Lafos et al., 2011).
Here, we show that Pc-G proteins are required for floral commitment. Thus, Pc-G mutants show floral reversion such that FMs revert to an earlier inflorescence meristem (IM) identity. Floral reversion in Pc-G mutants requires activity of the two MADS box transcription factors, FLC and SVP. We show that in plants given a transient photoperiodic induction, FT expression does not persist in leaves as previously reported (Corbesier et al., 2007), although it is activated in inflorescences. Furthermore, reduced FT expression in inflorescences, both in Pc-G mutants and as a consequence of FT mutation, causes floral reversion. Thus, additional to its known role in triggering the switch to flowering, FT has a second role in maintaining flower primordium identity in the inflorescence. Thus, Pc-G proteins maintain commitment to flowering at least partially by promoting FT activity in the inflorescence.
RESULTS
Generation of Plant Lines with Strongly Depleted Pc-G Activity
After germination, seedlings of mutants lacking Pc-G activity fail to develop leaves or flowers but instead produce embryogenic callus (Figures 2A and 2B; Chanvivattana et al., 2004). Hence, null Pc-G mutants are uninformative for analyzing Pc-G function during vegetative and reproductive development. To reveal whether Pc-G proteins are also required to initiate and maintain reproductive identity, we circumvented this early developmental arrest by constructing a conditional mutant (iCLF; see Methods; Figure 2; Supplemental Figure 1) in which a steroid-dependent CLF transgene (CLFpro:CLF-GR described in Schubert et al., 2006) was introduced into the clf-28 swn-7 background that lacks all Pc-G activity (Lafos et al., 2011). We rescued the early defects by germinating iCLF seedlings on steroid-containing media, then withdrew steroid by transplanting to soil. When Pc-G activity was progressively depleted in this way, development continued normally except that leaves were more serrated than normal (Figures 2B and 2C) and most flowers were sterile (Supplemental Figures 1A and 1B). Similar leaf serration is observed in the Pc-G double mutant emf2-10 vrn2-1 (Figure 2D), which shows a strong global reduction of H3K27me3 levels (Chanvivattana et al., 2004; Lafos et al., 2011). Therefore, these two plant lines, which are deficient in different components of the PRC2, were used to investigate the role of Pc-G in the vegetative and reproductive phase.
Figure 2.
Floral Reversion and Vegetative Phenotype of Mutants or Transgenic Lines with Reduced Pc-G Activity.
(A) clf-50 swn-3 callus tissue, 4 months after germination.
(B) Wild-type rosette.
(C) iCLF plant 14 d after withdrawal from dexamethasone.
(D) emf2-10 vrn2-1 double mutants. Plants in (B) to (D) were grown in SD, 28 DAG.
(E) emf2-10 vrn2-1 stem grown continuously in SD, 85 DAG.
(F) emf2-10 vrn2-1 ft-13 stem shows a similar phenotype under LD (85 DAG) as (E) in SD.
(G) to (K) Stems of plants 35 DAS from LD to SD. iCLF (G) and emf2-10 vrn2-1 (H). Arrowhead indicates reversion nodes. emf2-10 vrn2-1 flc-5 stem (I) carries fewer reversion nodes than (H). Arrowhead indicates reversion node with cauline leaf. emf2-10 vrn2-1 svp-32 (J) and emf2-10 vrn2-1 flc-5 svp-32 (K) stem with single reversion node. Arrowheads in (J) and (K) indicate paraclades without leaf.
(L) Floral reversion in ft-10 under LD, 4 months after germination. Arrowhead indicates leaf at a reversion node.
Asterisks in (G) to (L) indicate prereversion flowers. Bars = 10 mm (A) to (C) and (E) to (L) and 10 mm in (D). See Tables 1, 2, and 4 and Supplemental Table 2 for detailed organ counts.
Flower Formation Is Delayed in Plants with Reduced Pc-G Activity
To examine potential defects in flowering time regulation, we grew emf2-10 vrn2-1 and iCLF plants under LD and SD conditions and counted their rosette and cauline leaf number. The emf2-10 vrn2-1 double mutants flowered appreciably later than the wild type in SD, whereas in LD, their flowering was only slightly delayed (Table 1). The rosette leaf number of iCLF plants was also significantly increased in both LD and SD (Supplemental Table 1). Both lines displayed additional developmental defects, including homeotic transformations in organ identity, increased floral organ number, and a lack of floral meristem determinacy evident as a meristematically active fifth whorl (Supplemental Figures 2A and 2B), suggesting misregulation of a similar set of target genes. In addition, when emf2-10 vrn2-1 mutants were grown in SD, they produced inflorescences with an abnormally high number of cauline leaves (>60) so that flowers were not produced until very late in development (Figure 2E, Table 1). A similar, but less pronounced, phenotype and overall more variability in plant size was observed for iCLF plants (Supplemental Figure 1C and Supplemental Table 1). Most of the cauline leaves in emf2-10 vrn2-1 and iCLF in SD did not carry paraclades or any other secondary structure in their axils (73.6%, n > 750 for emf2-10 vrn2-1; 54.4%, n > 500 for iCLF). Thus, emf2-10 vrn2-1 and iCLF lines displayed an uncoupling of bolting and flower production under SD conditions, indicating an expanded I1 phase and a requirement for Pc-G proteins to promote the transition from I1 to I2 (Figure 1).
Table 1. Flowering Time of Mutants with Reduced Pc-G Activity Measured by Leaf Number.
|
LD |
SD |
Ratio SD/LD |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | n | RL | CL | TL | N | RL | CL | TL | RL | CL |
| La-0 | 40 | 6.8 ± 0.2 | 2.7 ± 0.1 | 9.5 ± 0.5 | 35 | 20.3 ± 1.5 | 8.6 ± 0.8 | 28.9 ± 2.1 | 3.0 | 3.2 |
| ev | 50 | 12.3 ± 0.3 | 4.5 ± 0.2 | 16.8 ± 0.6 | 50 | 45.7 ± 1.5 | 61.7 ± 4.5 | 107.5 ± 5.2 | 3.7 | 13.7 |
| ev flc | 45 | 7.9 ± 0.2 | 4.0 ± 0.2 | 11.9 ± 0.5 | 40 | 23.4 ± 1.2 | 49.0 ± 2.9 | 72.4 ± 3.5 | 3.0 | 12.4 |
| ev svp | 20 | 8.9 ± 0.4 | 4.8 ± 0.3a | 13.7 ± 0.6 | 20 | 44.4 ± 1.7a | 15.3 ± 0.8 | 59.6 ± 1.8 | 5.0 | 3.2 |
| ev flc svp | 50 | 5.8 ± 0.3 | 4.0 ± 0.3 | 9.8 ± 0.6b | 40 | 15.5 ± 1.0 | 10.6 ± 0.6 | 26.0 ± 1.3b | 2.7 | 2.6 |
| ev ft | 30 | 42.1 ± 1.2 | >360 | >400 | 10 | 43.7 ± 1.3a | >360 | >400 | 1.0 | – |
P > 0.05. Data are ± se. RL, rosette leaves; CL, cauline leaves; TL, total leaves; La-0, Landsberg-0; ev, emf2-10 vrn2-1; ev flc, emf2-10 vrn2-1 flc-5; ev svp, emf2-10 vrn2-1 svp-32; ev flc svp, emf2-10 vrn2-1 flc-5 svp-32; ev ft-13, emf2-10 vrn2-1 ft-13.
Not significantly different from ev.
Not significantly different from La-0.
Notably, we observed single flowers in the axils of the last one to five cauline leaves of emf2-10 vrn2-1 mutants and rudimentary bracts at the base of the pedicels of the flowers in both Pc-G-depleted lines (Figure 1L; Supplemental Figure 2G), indicating a gradual switch from the I1 to I2 phase. Surprisingly, many of the flowers produced in SD in the wild type also showed rudimentary bracts at the base of their pedicels (84.0% for La-0 and 42.5% for Columbia-0 (Col-0), first 20 flowers of 10 plants) and occasionally also stipules at their base (Supplemental Figures 2E and 2F). Thus, even in Arabidopsis wild-type, SD conditions induce leaf-like characteristics on flower metamers.
The leafy shoot phenotype of emf2-10 vrn2-1 in SD strongly resembled that described for ft lfy or fd lfy double mutants (Ruiz-Garcia et al., 1997; Abe et al., 2005; Wigge et al., 2005) but LFY expression was only moderately reduced (Supplemental Figures 3A, 3B, and 4). Consistent with the extensive uncoupling between bolting and flower production, expression of AP1, which is a marker for floral meristem identity, was largely eliminated during the early stages of emf2-10 vrn2-1 inflorescence development in SD but increased as flowers were produced (Supplemental Figures 3C, 3D, and 4). In addition, expression of FT followed a similar trend as AP1, whereas SOC1 and FUL, two other key regulators of flowering, showed wild-type expression levels in all stages analyzed (Supplemental Figure 4). Importantly, the floral repressor FLC but not SVP was upregulated in leafy emf2-10 vrn2-1 inflorescence apices (Supplemental Figure 4). Using a FLCpro:FLC-GUS (β-glucuronidase) reporter, we confirmed the higher promoter activity of FLC in emf2-10 vrn2-1 inflorescences compared with the wild type (Supplemental Figures 5A and 5B). Furthermore, the reporter indicated an upregulation of FLC in emf2-10 vrn2-1 rosette leaves, which may explain the delayed flowering of emf2-10 vrn2-1 in SD (Supplemental Figures 5C and 5D).
Collectively, our analyses of leafy emf2-10 vrn2-1 inflorescences revealed changes in gene expression for FLC, AP1, and FT, but not for LFY, SOC1, FUL, and SVP.
Loss of FLC, SVP, and FT Modulate the emf2-10 vrn2-1 Leafy Shoot Phenotype
To investigate whether the upregulation of FLC is required for the leafy shoot phenotype of emf2-10 vrn2-1 plants in continuous SD, we created emf2-10 vrn2-1 flc-5 triple mutants. Because FLC can act in a repressor complex with SVP (Li et al., 2008), we also created emf2-10 vrn2-1 svp-32 triple and emf2-10 vrn2-1 flc-5 svp-32 quadruple mutants. Surprisingly, removing FLC or SVP activity had clearly distinct effects (Table 1). In SD, lack of FLC strongly reduced the number of rosette leaves but had little effect on cauline leaf number. By contrast, removing SVP activity did not significantly affect rosette leaf number (P = 0.44 Student’s t test), whereas cauline leaf number was reduced to a quarter of that in emf2-10 vrn2-1. Importantly, the combined loss of both MADS box transcription factors in emf2-10 vrn2-1 flc-5 svp-32 quadruple mutants restored cauline leaf number to near wild-type and rosette leaf number was reduced to slightly fewer than the wild type. These results suggested that the prolonged V phase of emf2-10 vrn2-1 in SD was largely caused by increased FLC, whereas the delay in the I1-to-I2 transition required SVP and to lesser extent FLC activity.
The repressor complex of FLC and SVP delays flowering by binding to the promoter of FT (Li et al., 2008; Deng et al., 2011). To determine the role of FT in delayed flower production in emf2-10 vrn2-1 mutants, we characterized emf2-10 vrn2-1 ft-13 triple mutants (Figure 2F, Table 1). In SD, the rosette leaf number of emf2-10 vrn2-1 double (45.7) and emf2-10 vrn2-1 ft-13 triple (43.7) mutants were not significantly different (P = 0.31, Student’s t test), indicating that similar to the wild type, FT is not active during vegetative development in emf2-10 vrn2-1 in SD. Unexpectedly, the emf2-10 vrn2-1 ft-13 plants never produced flowers, but continuously generated cauline leaves after bolting independently of the daylength (>360 after 4 months; Table 1, Figure 2F). Like emf2-10 vrn2-1 in SD, branching in emf2-10 vrn2-1 ft-13 was strongly suppressed in both SD and LD (Figures 2E and 2F).
Thus, FLC and SVP have overlapping and distinct functions in the promotion of bolting and the transition from I1 to I2 in emf2-10 vrn2-1 mutant plants. In addition, based on genetic analyses of emf2-10 vrn2-1 and emf2-10 vrn2-1 ft-13 mutants, FT is active in promoting the I1-to-I2 transition in emf2-10 vrn2-1 inflorescences in SD.
Photoperiod Shifts Induce Floral Reversion in Plants with Reduced Pc-G Activity
When flowering in continuous LDs, a few (<1% of plants) iCLF inflorescences reverted to an earlier developmental stage, so that after producing a series of flowers as normal they went back to producing a single paraclade, suggesting that floral commitment was impaired (Supplemental Figure 1B). To test this further, we provided a transient photoperiodic flowering induction by shifting iCLF or emf2-10 vrn2-1 seedlings from inductive LD to SD 21 to 25 d after germination (DAG). Several weeks later, iCLF and emf2-10 vrn2-1 plants made some flowers but then reverted to cauline leaves and paraclade generation before eventually resuming making flowers (Figures 1A, 1F, 2G, and 2H; Supplemental Figure 1D). By contrast, wild-type plants began flowering in LD and did not show obvious floral reversion of IM and FM when they were shifted to SD, except the presence of rudimentary bracts (Table 2; Supplemental Table 2 and Supplemental Figure 2). These findings indicate that Pc-G function is essential for maintenance of floral meristem identity. We observed a reduction of reversion nodes in both emf2-10 vrn2-1 and iCLF if we delayed the shift to SD, suggesting that plants became less susceptible to reversion as time in LD increased.
Table 2. Strong Floral Reversion Nodes at Main Shoot of Strong Hypomorphic Pc-G Mutants.
| Genotype | n | PC without CL | CL | CL with PC | CL with F | ∑ |
|---|---|---|---|---|---|---|
| La-0 | 42 | 0 # | 0 # | 0 # | 0 # | 0 # |
| ev | 80 | 2.3 ± 0.3 | 2.7 ± 0.6 | 18.5 ± 1.4 | 3.6 ± 0.3 | 27.2 ± 1.6 |
| ev f | 82 | 2.9 ± 0.2 | 1.6 ± 0.2 | 5.4 ± 0.4 # | 2.6 ± 0.2 # | 12.6 ± 0.5 # |
| ev s | 91 | 10.1 ± 0.6 ‡ | 0.3 ± 0.1 # | 0.1 ± 0.0 # | 0.9 ± 0.1 # | 11.4 ± 0.7 # |
| ev fs | 68 | 1.0 ± 0.2 # | 0 # | 0 # | 0.04 ± 0.03 # | 1.0 ± 0.2 # |
Data are ± se. Significantly fewer reversion nodes than ev (Student's t test): #P < 0.001. Significantly more reversion nodes than ev (Student's t test): ‡P < 0.001. CL, cauline leaves; PC, paraclades; F, flowers; La-0, Landsberg-0; ev, emf2-10 vrn2-1; ev flc, emf2-10 vrn2-1 flc-5; ev svp, emf2-10 vrn2-1 svp-32; ev flc svp, emf2-10 vrn2-1 flc-5 svp-32.
To resolve spatial and temporal origin of inflorescence and flower reversion in emf2-10 vrn2-1 and iCLF, we carefully analyzed the floral reversion nodes. Four main classes were revealed, which were the result of either flower reversion or inflorescence reversion or combined flower and inflorescence reversion and usually occurred progressively on the bolting stem (Table 2; Supplemental Table 2): (1) paraclades without cauline leaves but often subtended by a rudimentary bract (Figure 1J; Supplemental Figure 2I); (2) “empty” cauline leaves, i.e., not subtending any secondary structure in their axils (Figure 1G; Supplemental Figures 2I and 2K); (3) cauline leaves subtending a paraclade (Figure 1H; Supplemental Figure 2J); and (4) cauline leaves subtending a normal flower (Figure 1I; Supplemental Figure 2K). The different categories likely result from a loss of floral identity at different stages in primordium development, as shown in Figures 1B to 1E and 1G to 1K. Interestingly, flower reversion also occurred after sepals were initiated (<5% of strong reversion nodes; Figure 1K; Supplemental Figure 2D) or even from the fifth whorl after carpels had formed so that inflorescences emerged from within the fourth whorl siliques (<1% of strong reversion nodes; Supplemental Figures 2B and 2C). Notably, although the different reversion nodes usually appeared in the order (1) to (4) (see above), we observed fluctuations in the order of successive reversion nodes in emf2-10 vrn2-1 and iCLF (e.g., leaf/flower nodes appeared frequently between nodes with empty cauline leaves; Supplemental Figure 2L), suggesting that meristem identity was unstable and fluctuating around a threshold between the vegetative and reproductive state.
To investigate the morphological changes in the IM and in young primordia in the early stage of floral reversion, we analyzed the distribution of ANT and STM RNA in tissue sections of shifted emf2-10 vrn2-1 apices 6 d after shift (DAS) as these two genes provide indirect markers for the cryptic bracts that subtend early stage floral primordia (Long and Barton, 2000). The cryptic bracts were more prominent in shifted emf2-10 vrn2-1 plants than in the wild type; notably, some primordia at stage 2 did not express STM and therefore likely lacked meristematic activity (Supplemental Figures 6A to 6D), suggesting that reversions occur mostly in very early primordium development.
Thus, our detailed analyses of floral reversion in Pc-G mutants indicate that Pc-G proteins are not only required for a timely transition to flower production after bolting in SD but are also needed to maintain floral formation when plants are shifted from inducing to noninducing photoperiods.
Floral Reversion in Pc-G Mutants Depends on FLC and SVP
We next sought to determine whether the Pc-G target genes FLC and SVP are not only required for a delayed bolting and transition from I1 to I2 in Pc-G mutants, but also responsible for floral reversion. Analysis of FLC expression by RT-PCR and an FLCpro:FLC-GUS reporter in inflorescence apices revealed an increase in emf2-10 vrn2-1 and iCLF plants relative to the wild type, most notably when plants were shifted to SD (Figures 3A and 4A; Supplemental Figures 5E to 5H and 7A). For SVP, RT-PCR revealed a significant upregulation after the shift for emf2-10 vrn2-1 but not for iCLF inflorescence apices (Figure 4A; Supplemental Figure 7B). Analysis of the spatial expression pattern by in situ hybridization showed stronger accumulation of SVP mRNA in inflorescence meristems and in the vasculature beneath the meristem of emf2-10 vrn2-1 plants shifted to SD compared with emf2-10 vrn2-1 plants continuously grown in LD (Figure 3B). However, the overall expression pattern of SVP was not changed in shifted stage 2 emf2-10 vrn2-1 floral primordia and was excluded from the cryptic bract as in the wild type (Supplemental Figures 6E and 6F). Chromatin immunoprecipitation (ChIP) assays using wild-type inflorescence apices revealed H3K27me3 enrichment at FLC and SVP (Figure 4B; Supplemental Figure 7D), confirming that Pc-G proteins directly regulate FLC and SVP in the inflorescence as well as in seedlings. While H3K27me3 at FLC was strongly reduced in emf2-10 vrn2-1 and iCLF in LD and SD, no decrease was detected at SVP (Figure 4B; Supplemental Figure 7D).
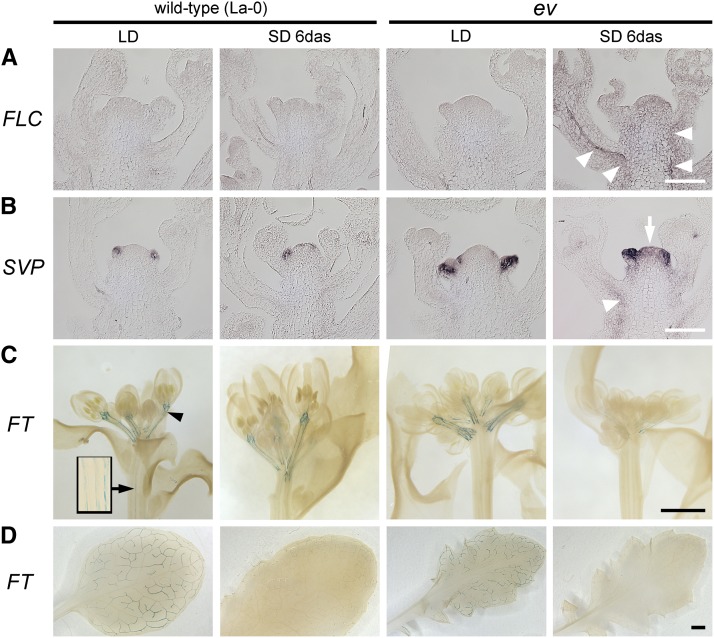
Figure 3.
Misexpression of FLC and SVP and Downregulation of Their Target Gene FT in Pc-G Mutants.
(A) and (B) In situ RNA hybridization: a shift from LD to SD leads to FLC (A) and SVP (B) upregulation in emf2-10 vrn2-1 (ev), but not in wild-type inflorescence apices (27 DAG). Expression is detected in the vasculature of the stem (arrowheads) and in the inflorescence meristem (arrow). A control experiment using STM RNA probes verified that these differences did not reflect variation in sample preservation (Supplemental Figure 6). Bars = 100 μm.
(C) and (D) FTpro:GUS expression in inflorescence apices ([C]; bars = 400 μm) and rosette leaves ([D]; bars = 1 mm). Arrowhead in (C) indicates strong staining in the vasculature of pedicels; arrow/inset in (C) show weak staining in the vasculature of stem. Plants are 27 d old and were continuously grown in LD or 21 d in LD, shifted to SD, and grown for a further 6 d (SD 6 DAS).
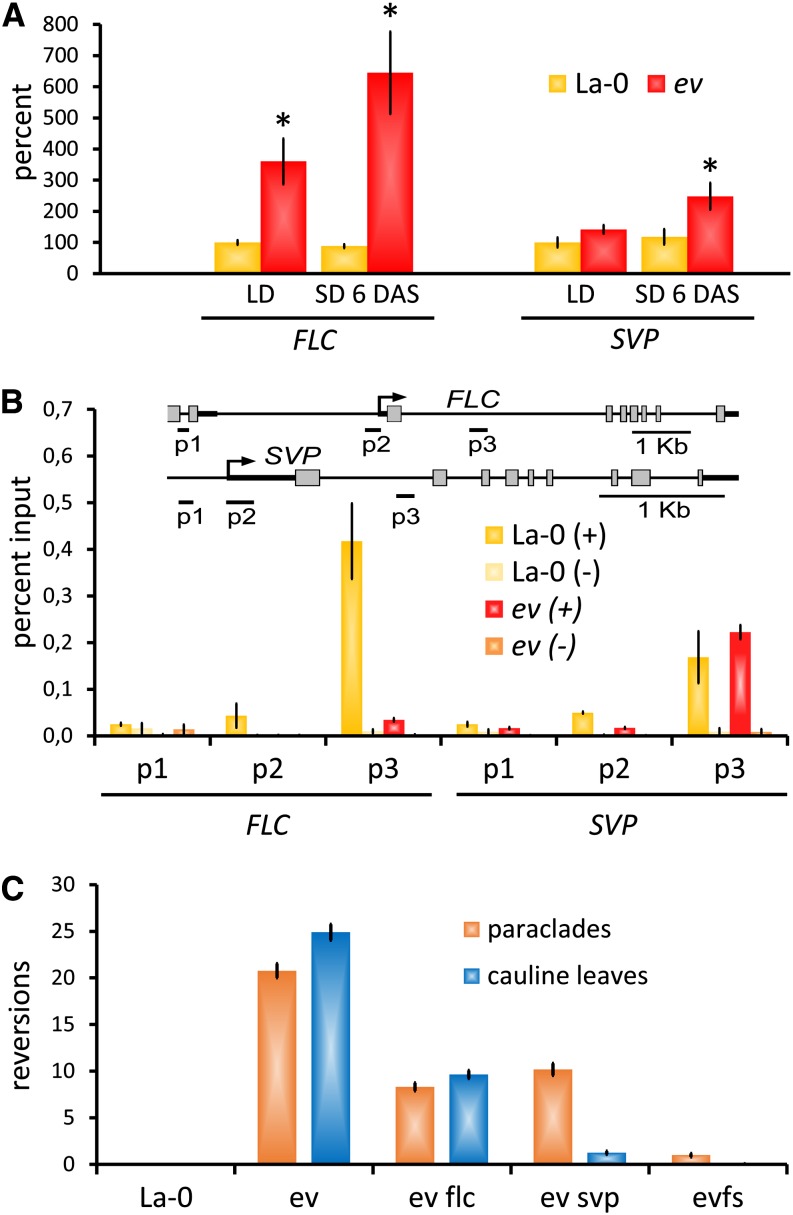
Figure 4.
FLC and SVP Are Required for Floral Reversion in emf2-10 vrn2-1 Mutants.
(A) Quantitative RT-PCR analyses of FLC and SVP mRNA expression in emf2-10 vrn2-1 (ev) inflorescence apices (harvested 8 h after lights on) normalized to elF4, relative to expression in La-0 (LD). Each bar represents the mean of five biological replicates ± se. Asterisks indicate significant change of expression (Student’s t test: P ≤ 0.05) compared with the equally treated wild-type control (La-0).
(B) ChIP assay: FLC and SVP chromatin are enriched for H3K27me3 in inflorescence apices of the wild type (La-0). H3K27me3 is strongly reduced at the FLC locus in emf2-10 vrn2-1 but not at SVP. (+) H3K27me3 antibody; (−) no antibody control. Data represent mean of two biological replicates ± se.
(C) Loss of both FLC and SVP suppress floral reversion in emf2-10 vrn2-1 plants; reversion nodes per plant, reversions of IM to tSAM (indicated by cauline leaves), and of FM to IM (indicated by paraclades) are distinguished ± se (n ≥ 40). See Table 2 for detailed counts of the different reversion node types. evfs, emf2-10 vrn2-1 flc-5 svp-32.
[See online article for color version of this figure.]
Collectively, these results indicate that FLC and SVP are direct targets of Pc-G repression during early floral meristem development, but only alterations in FLC H3K27me3 and expression levels are correlated with floral reversion in emf2-10 vrn2-1 and iCLF. To test for a genetic requirement for FLC and SVP, we analyzed floral reversion in emf2-10 vrn2-1 flc-5 triple, emf2-10 vrn2-1 svp-32 triple, and emf2-10 vrn2-1 flc-5 svp-32 quadruple mutants. Floral reversion was strongly reduced in emf2-10 vrn2-1 flc-5 and emf2-10 vrn2-1 svp-32 triple mutants and almost completely abolished in emf2-10 vrn2-1 flc-5 svp-32 quadruple mutants (Figures 2I to 2K and 4C, Table 2). However, the effects of flc and svp mutation on the different classes of floral reversion were distinct. In emf2-10 vrn2-1 flc-5, reversion nodes consisting of cauline leaves with or without paraclades were similarly reduced (∼40% compared with emf2-10 vrn2-1), whereas in emf2-10 vrn2-1 svp-32, reversion nodes lost their cauline leaves (reduced to 5% compared with emf2-10 vrn2-1) and therefore showed an increase in paraclades lacking cauline leaves. However, also in emf2-10 vrn2-1 svp-32, the total number of floral reversion nodes was reduced (Table 2).
We concluded that floral reversion in Pc-G mutants depends on additive and synergistic function of FLC and SVP and that Pc-G proteins are needed to keep FLC repressed and H3K27me3 decorated in wild-type inflorescences when plants are shifted from LD to SD. As the analyzed Pc-G mutants represent only partial loss-of-Pc-G-function, SVP H3K27me3 may be maintained by the residual Pc-G activity present in the mutants.
Reduced FT Expression in Pc-G Mutant Inflorescences Contributes to Floral Reversion
Next, we aimed to reveal the downstream targets of FLC and SVP that are required for stable floral commitment. FLC and SVP, as part of a repressor complex, delay flowering by binding to the promoters of SOC1 and FT in leaves (Hepworth et al., 2002; Helliwell et al., 2006; Searle et al., 2006; Fujiwara et al., 2008; Li et al., 2008; Jang et al., 2009; Deng et al., 2011). Both genes were expressed in both LD, SD, and after LD to SD shift in wild-type inflorescence apices (Figures 3C and 5A; Supplemental Figures 4 and 8A). Therefore, regulation of FT expression is apparently different in leaves and inflorescences as its expression in leaves was strongly reduced when plants were shifted to SD as previously reported (Corbesier et al., 2007; Hiraoka et al., 2013) (Figures 3D and 5B). We confirmed that FT is active in wild-type inflorescences after the shift to SD using FTpro:LUCIFERASE and FTpro:GUS reporters, which revealed strongest expression in the vasculature of the stem and of pedicels (Figures 3 and 5), consistent with recent studies (Adrian et al., 2010; Hiraoka et al., 2013). Plants grown in continuous SDs showed weaker but detectable FT expression in the inflorescence (Figure 5E), suggesting that high FT expression in the inflorescence under noninductive conditions may require a transient photoperiodic induction. We then analyzed flowering-promoting genes in Pc-G mutant backgrounds. Neither the expression of the FLC/SVP target gene SOC1 nor of FUL, which is needed with SOC1 to prevent floral reversion (Melzer et al., 2008), was significantly decreased in shifted emf2-10 vrn2-1 mutants compared with shifted wild-type plants (Supplemental Figure 8A). Similarly, the expression of AP1 was also not altered, whereas LFY expression was increased in emf2-10 vrn2-1 inflorescences compared with wild-type plants both in LD and SD (Supplemental Figure 8B). By contrast, FT expression in emf2-10 vrn2-1 and iCLF inflorescences was strongly reduced in LD relative to the wild type and further reduced when plants were shifted to SD (Figures 3C and 5A; Supplemental Figure 7C). It was restored to near wild-type levels in emf2-10 vrn2-1 flc-5 and emf2-10 vrn2-1 flc-5 svp-32 inflorescences but not in emf2-10 vrn2-1 svp-32, indicating that FLC but not SVP activity caused the FT downregulation (Figure 5A). Thus, only the activity of the flowering-promoting gene FT was reduced in emf2-10 vrn2-1 after transient photoperiodic induction, whereas the key meristem identity genes SOC1, FUL, and LFY were unchanged or even elevated.
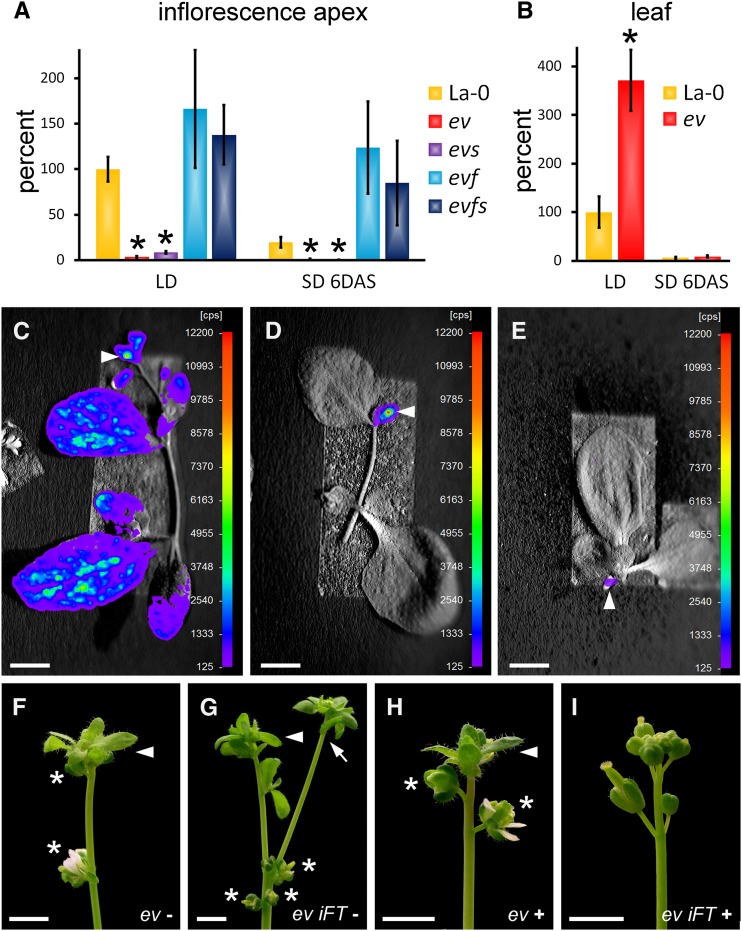
Figure 5.
Downregulation of FT Causes Floral Reversion in PcG Mutants.
(A) and (B) Quantitative RT-PCR analyses of FT expression in inflorescence apices (A) and rosette leaves (B) normalized by elF4, relative to expression in La-0 (LD). Asterisks indicate significant change of expression (Student’s t test: P ≤ 0.05) compared with the equally treated La-0. FT expression is reduced when plants are shifted from LD to SD ([A] and [B]), but less in inflorescence apices compared with rosette leaves. Lack of FLC but not SVP in emf2-10 vrn2-1 (ev) rescues FT expression in apices. Average of three (B) to six (A) (except for evs, where n = 3) biological replicates per bar is shown ± se. ev, emf2-10 vrn2-1; evs, emf2-10 vrn2-1 svp-32; evf, emf2-10 vrn2-1 flc-5; evfs, emf2-10 vrn2-1 flc-5 svp-32; plants were 27 DAG and inflorescence apices were harvested 8 h after lights on.
(C) to (E) FTpro:LUCIFERASE activity in wild-type shoots 50 DAG in LD (C), 11 DAS from LD to SD (D), and in continuous SD (E). Note that after LD to SD shift, no expression in leaves is seen, whereas expression is readily detected in inflorescence apices (arrowhead). Color coding represents relative counts per second (cps).
(F) to (I) Suppression of floral reversion by high FT expression. emf2-10 vrn2-1 ([F] and [H]) and emf2-10 vrn2-1 iFT ([G] and [I]) plants at 62 DAG, shifted from LD to SD at 21 DAG, without estradiol ([F] and [G]) or with estradiol ([H] and [I]). A solution of 10 μM estradiol was supplied for 7 DAS by spraying plants once each day. Bars = 10 mm.
The correlation between FT level and floral reversion together with our genetic analysis showing that the leafy inflorescence phenotype of emf2-10 vrn2-1 plants grown in continuous SD is largely due to reduced FT activity (Figures 2E and 2F; Supplemental Figure 4) suggested that reduced FT expression might be the basis for floral reversion in shifted emf2-10 vrn2-1 plants. To further test this, we used an estradiol-inducible FT construct in transgenic emf2-10 vrn2-1 plants (iFT emf2-10 vrn2-1) to activate FT in specific tissues and at distinct time points. When FT was induced by treating whole plants or apices with estradiol after shifting to SD, floral reversion was partially suppressed (Figures 5F to 5I, Table 3; Supplemental Table 3). Together, these results suggest that FT activity in emf2-10 vrn2-1 inflorescences is required to prevent floral reversion in short days, when FT activity is lacking in leaves.
Table 3. Suppression of Floral Reversion in ev by High FT.
| Genotype/Treatment | PC without CL | CL | CL with PC | CL with F | ∑ |
|---|---|---|---|---|---|
| ev − estradiol | 1.3 ± 0.3 | 0.6 ± 0.3 | 8.8 ± 1.5 | 2.3 ± 0.5 | 13.1 ± 1.5 |
| ev + estradiol | 1.5 ± 0.4 | 0.3 ± 0.1 | 8.3 ± 1.6 | 2.2 ± 0.3 | 12.2 ± 1.8 |
| ev iFT − estradiol | 1.6 ± 0.5 | 0.6 ± 0.2 | 8.8 ± 1.4 | 1.9 ± 0.3 | 12.8 ± 1.6 |
| ev iFT + estradiol | 0.1 ± 0.1* | 0 | 0** | 0* | 0.1 ± 0.1** |
Data are ± se. n = 12 plants. CL, cauline leaves; PC, paraclades; F, flowers; ev, emf2-10 vrn2-1; ev iFT, emf2-10 vrn2-1 iFT. Significantly fewer reversion nodes than ev − estradiol (Student's t test): *P ≤0.01 and **P < 0.001.
FT Maintains Inflorescence and Floral Meristem Identity
Our results raised the question of whether FT activity also has a role in maintaining floral commitment in wild-type inflorescences. FT acts redundantly with LFY to activate AP1 in floral meristems (Ruiz-García et al., 1997; Abe et al., 2005; Wigge et al., 2005), so that ft lfy double mutants bolt and generate cauline leaves but never produce flowers, suggesting a role for FT in specifying floral meristem identity. To reveal a role for ft single mutants in maintaining floral commitment, we performed a careful analysis of metamers in the ft-10 (Col) null allele. In LD, these ft mutant plants resemble SD-grown wild-type plants in lacking FT activity in leaves, but differ in that they also lack FT activity in inflorescences. As previously reported, ft-10 mutants flowered late in LD and SD and showed an increase in cauline leaf number, particularly in SD where ft-10 resembled iCLF (Supplemental Table 1). In addition, we observed inflorescence and flower reversion in ft-10 in both LD and SD, but never in the corresponding wild type (Figure 2L, Table 4). About 70% of the pedicels of ft-10 mutant flowers carried rudimentary bracts under LD (Supplemental Figure 2H; first 10 flowers of 18 plants) phenocopying the rudimentary bracts in the wild type in SD (Supplemental Figures 2E and 2F). Importantly, the ft-10 mutants initiated flower production, but then showed an oscillation of leaf and flower emergence in the following nodes suggesting that FT activity in inflorescences is required for the maintenance of IM and FM identity.
Table 4. Strong Floral Reversion Nodes in ft-10 Mutants.
|
LD |
SD |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | PC | CL | CL + PC | CL + F | ∑ | N | PC | CL | CL + PC | CL + F | ∑ | |
| Col-0 | 12 | 0 | 0 | 0 | 0 | 0 | 15 | 0 | 0 | 0 | 0 | 0 |
| ft-10 | 18 | 0.3 ± 0.1* | 0 | 2.3 ± 0.5* | 0.4 ± 0.2 | 3.0 ± 0.6* | 18 | 0.1 ± 0.1 | 0.1 ± 0.1 | 3.2 ± 0.5* | 0.2 ± 0.1 | 3.6 ± 0.4* |
Data are ± se. Significantly more reversion nodes than Col-0: *P ≤ 0.01 (Student's t test). CL, cauline leaves; PC, paraclades; F, flowers.
DISCUSSION
Pc-G Proteins Are Needed for Floral Commitment
Pc-G proteins have been shown to regulate numerous traits in plants, and a common underlying theme is that they maintain changes in developmental phase by repressing genes that promote an earlier phase. For example, after seed germination, the Pc-G permit transition from embryonic to seedling identity by repressing regulatory genes, such as FUSCA3, that are normally expressed earlier during embryonic development and specify embryonic identity (Chanvivattana et al., 2004; Makarevich et al., 2006). Here, by genetically manipulating Pc-G activity, we reveal roles for Pc-G in maintaining phase transitions as they promote commitment to flowering after transient photoperiodic induction and induce the switch from cauline leaf to flower production in noninductive conditions. These roles are consistent with a function of Pc-G in sustaining developmental transitions as they involve repression of genes such as FLC that are normally expressed during the earlier vegetative phase and repress the floral transition (Michaels and Amasino, 1999; De Lucia et al., 2008; Pien et al., 2008). Our genetic analyses indicate that activities of both FLC and SVP are critical for reversion and the switch to flower production in emf2-10 vrn2-1 mutants (Figure 6). Both genes are direct targets of Pc-G, as they are enriched for H3K27me3 in inflorescence tissue. This is necessary for FLC regulation: FLC loses H3K27me3 and is upregulated in iCLF and emf2-10 vrn2-1 mutants. For SVP, the role of Pc-G is less clear; we did not see consistent decreases in H3K27me3 enrichment at SVP in Pc-G mutants and observed only minor differences in expression in inflorescences. It is therefore possible that the role of SVP in floral reversion is indirect (at least for the Pc-G mutants analyzed in this study), i.e., it is required as a cofactor for another, unknown factor (“X”) that is misexpressed in Pc-G mutants or that chromatin of SVP target genes promoting reversion are more accessible in Pc-G mutants and activated (Figure 6). As the Pc-G mutants analyzed here have residual EMF2 (in emf2-10 vrn2-1 mutants) or CLF activity (in iCLF), SVP H3K27me3 may be conferred by the EMF-PRC2, whereas FLC H3K27me3 and repression may be mediated by both EMF-PRC2 and VRN-PRC2 (Chanvivattana et al., 2004; Figure 6). Nonetheless, it seems likely that the role of Pc-G, FLC, and SVP in floral commitment is conserved in flowering plants. The Pc-G proteins are present in all plants, and recent research suggests that FLC is much more widely conserved in angiosperms than previously thought, having been identified in monocots (Reeves et al., 2007; Ruelens et al., 2013). FLC plays a role in floral reversion in natural environments as the ecotype Skye-0 shows FLC-dependent floral reversion (Poduska et al., 2003). In addition, SVP is widely conserved, and in barley (Hordeum vulgare), a monocot that is distantly related to Arabidopsis, SVP orthologs are also expressed during vegetative development and repressed during FM development (Trevaskis et al., 2007). Furthermore, experiments using transgenics show that misexpression of SVP orthologs in flowers causes floral reversion, particularly when plants were grown in noninductive short days (Trevaskis et al., 2007). It is therefore plausible that the Pc-G/FLC/SVP module provides a general mechanism for floral commitment.
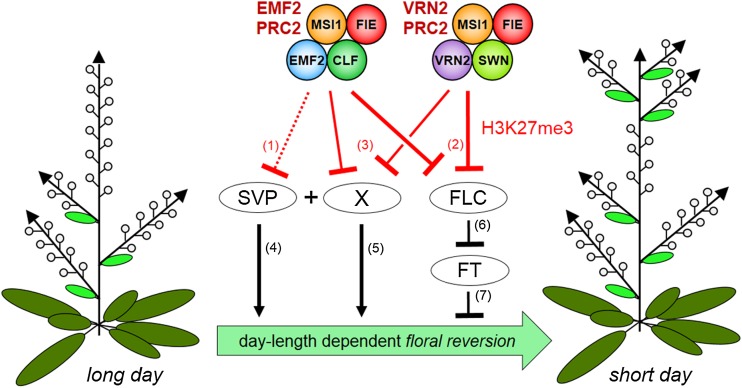
Figure 6.
Model: Prevention of Daylength-Dependent Floral Reversion by Pc-G Proteins and FT.
At least two differently composed PRC2 complexes, the EMF2-PRC2 and the VRN2-PRC2, prevent daylength-dependent floral reversion. (1) SVP is a target of H3K27me3 in inflorescences that is likely mediated by the EMF2-PRC2 as loss of VRN2 (in emf2-10 vrn2-1) or SWN (in iCLF) do not reduce H3K27me3 levels at SVP. (2) In both lines with reduced Pc-G activity, H3K27me3 is strongly reduced at the FLC locus and FLC mRNA accumulates. (3) At least one other H3K27me3 target (X) promotes daylength-dependent floral reversion (5), since lack of SVP (4) and FLC (6) only incompletely suppress daylength-dependent floral reversion in emf2-10 vrn2-1 mutants. Factor X is likely misexpressed and loses H3K27me3 in partially depleted Pc-G mutants and may be a cofactor for SVP. The upregulation of FLC (6) but not SVP (4) represses FT expression in emf2-10 vrn2-1 mutants. (7) ft mutants show photoperiod-independent floral reversion, whereas induced FT suppresses daylength-dependent floral reversion in emf2-10 vrn2-1 mutants. In addition, FT is also regulated by pathways acting in parallel to ev (not indicated; see Discussion).
Differing Roles of FLC and SVP in Reversion
Unexpectedly, our genetic analysis revealed that FLC and SVP have distinct roles in floral reversion. First, the emf2-10 vrn2-1 flc-5 svp-32 quadruple mutant shows less floral reversion than either the emf2-10 vrn2-1 flc-5 or the emf2-10 vrn2-1 svp-32 triple mutant, suggesting that FLC and SVP act in parallel. Second, removing FLC activity in the emf2-10 vrn2-1 background increases FT expression, whereas removing SVP activity does not, suggesting that the role of SVP in promoting reversion is independent of FT (Figure 6). Third, the phenotypes of the two triple mutants differ, with SVP activity being more specifically required for the production of cauline leaves. Arabidopsis is unusual in that its flowers are not subtended by a leaf-like organ termed a bract. However, detailed analysis has suggested that after the floral transition, the youngest primordia to arise on the flanks of the inflorescence meristem are leaf-like organs, which usually do not grow out during later flower stages and hence are termed cryptic bracts (Figure 1) (Long and Barton, 2000; Kwiatkowska, 2006; Chandler, 2012). The different types of reversion nodes observed in the Pc-G mutants likely reflect the time at which floral primordium identity is compromised and reversion occurs. For example, cauline leaves with “empty“ axils may arise if floral primordium identity is inhibited extremely early, likely before or around the time the FM is specified on the adaxial side of cryptic bract primordia. Inhibition at later stages may result in cauline leaves subtending paraclades, paraclades only, or in weaker cases flowers subtended by cauline leaves or rudimentary bracts (Figure 1). SVP therefore seems to inhibit early stages in floral primordium development, whereas FLC has more continuous effects, perhaps consistent with the more general misexpression of FLC in emf2-10 vrn2-1 inflorescences.
FT Is Needed for Floral Commitment
The FT gene was originally identified based on its requirement to accelerate time to flowering in LD in Arabidopsis. Subsequently, FT or its orthologs have been found to act more generally and to control other traits, including bud dormancy, potato tuberization, leaf dissection, and lateral bud outgrowth (Böhlenius et al., 2006; Shalit et al., 2009; Navarro et al., 2011; Hiraoka et al., 2013). Our analyses reveal a daylength-independent role for FT in the maintenance of IM and FM identity.
We and others have detected FTpro:GUS reporter expression in the vasculature of the pedicels of older flowers in inflorescences in LD and SD (Figures 3C and 5C) (Adrian et al., 2010; Hiraoka et al., 2013). We believe this reflects native FT expression as RT-PCR detects FT mRNA in inflorescence tips of shifted wild-type plants but much less so in shifted emf2-10 vrn2-1 plants. Additionally, the reporters that contain 8.1 kb of sequences upstream of FT reliably reflect the expression of FT in vasculature in leaves in LD but not SD and the same promoter fragment driving FT cDNA complements null ft-10 mutations (Adrian et al., 2010). Importantly, our genetic analysis shows that the FT expression we detect in the inflorescence is functionally relevant. In particular, we show that ft mutants grown in LD or SD conditions show floral reversion after bolting (Table 4). Thus, although FT plays a negligible role in triggering flowering in SD, as it is not expressed in leaves (Kobayashi and Weigel, 2007), once bolting and floral induction has occurred it is transcriptionally activated in the inflorescence and here has a role in maintaining floral commitment. Our results further suggest that the decreased FT expression in emf2-10 vrn2-1 inflorescences is partly responsible for their loss of floral commitment. First, in emf2-10 vrn2-1 flc svp quadruple mutants, which show drastically reduced floral reversion relative to emf2-10 vrn2-1 double mutants, FT expression in inflorescences is restored to near wild-type levels. Second, when a FT transgene is induced in LD to SD shifted emf2-10 vrn2-1 plants, floral reversion is reduced. Since removing SVP activity in the emf2-10 vrn2-1 background reduces floral reversion without restoring FT activity, it is likely that additional genes besides FT are involved. It is also notable that emf2-10 vrn2-1 ft-13 triple mutants show a more extreme phenotype than do emf2-10 vrn2-1 double mutants in SD. This reflects the fact that emf2-10 vrn2-1 reduces but does not eliminate FT activity and implies that there are ev-independent pathways that act in parallel to regulate FT.
Persistent FT Activity Is Needed for Floral Commitment
How does FT activity in the inflorescence maintain floral commitment in plants shifted to noninductive conditions? In transiently induced plants, it is likely that the LFY/AP1 autoregulatory loop stabilizes FM identity once LFY/AP1 expression has been initiated in young floral primordia, but this does not explain how flower primordium identity is repeatedly established on the flanks of the SAM. Here, movement of mobile FT protein into the SAM from flower primordia may be important for continued production of flowers. Although the generality of this mechanism is unknown, characterization of FT orthologs from legumes has revealed a correlation between floral commitment and FT expression in inflorescences (Hecht et al., 2011; Sun et al., 2011), and genetic studies in tomato (Solanum lycopersicum) show that FT is needed for floral commitment as well as for floral induction (Molinero-Rosales et al., 2004). In addition, floral commitment in Impatiens balsamina requires a leaf-derived, mobile signal, which is consistent with florigen being the signal (Tooke and Battey, 2000). Thus, there are other examples in diverse plant species that stable floral commitment does not only rely on signaling events in the meristem but requires continuous signaling from lateral organs producing a mobile signal.
In conclusion, FT activity in inflorescences is required for floral commitment, and the Pc-G genes are needed to permit this. However, an important question remains as to how FT transcription is activated or maintained in inflorescences in LD-to-SD-shifted plants. FT itself is unlikely to provide the “memory” of transient inductive signals in shifted plants, but rather is needed for maintaining commitment. Thus, shifting experiments show that plants that are only at a very early stage in inflorescence development lacking stage 3 or older flower primordia at the time of shifting (Torti et al., 2012) are fully committed, yet we do not detect FT transcription in the IM or very early flower primordia. Genes such as FUL, whose expression persists in the IM and which are needed for floral commitment, are strong candidates to provide the initial memory (Melzer et al., 2008; Torti et al., 2012). We detected FT transcription in the vasculature of young flowers (Figure 3) and speculate that FT may be activated downstream of floral organ identity or other genes active during flower development. Regardless of how this occurs, the activation of FT in young flowers would provide a mobile signal (FT protein) that reinforces flower primordium identity on the flanks of the IM. In this way, a promotive signal from older flowers back to the IM would make flowering self-perpetuating once initiated.
METHODS
Plant Materials and Growth Conditions
Plants were grown at 22°C under LD (16 h light/8 h dark) or SD (8 h light/16 h dark) conditions. emf2-10 vrn2-1 was described previously (Lafos et al., 2011). ft-10 (GABI_290E08) is in the Col-0 background and was isolated from a T-DNA library generated by GABI-Kat (Yoo et al., 2005; Kleinboelting et al., 2012). C. Dean kindly provided seeds of FLCpro:FLC-GUS and flc-5 (Greb et al., 2007). svp-32 (Lee et al., 2007) (SALK_072930) was obtained from the Nottingham Arabidopsis Stock Centre. Seeds of FTpro:LUC (Adrian et al., 2010) and FTpro:GUS (Kotake et al., 2003) were kindly provided by F. Turck and K. Goto, respectively.
Construction of iCLF
We generated the iCLF (clf-28 swn-7 CLFpro:CLF-GR) line by introducing the previously described CLFpro:CLF-GR (Schubert et al. 2006) steroid-dependent transgene into the clf-28 swn-7 background by floral dip transformation of swn-7 clf-28/+ plants. In the presence of dexamethasone steroid, iCLF plants are near wild-type (Supplemental Figure 1) as CLF activity complements both swn-7 and clf-28 mutations due to the functional redundancy between the SWN and CLF genes (Chanvivattana et al., 2004). In the absence of steroid, iCLF plants have a weaker phenotype than clf-28 swn-7 mutants (Supplemental Figure 1), suggesting that the CLFpro:CLF-GR transgene is slightly leaky. In depletion experiments, iCLF plants were grown on tissue culture plates containing 10 μM dexamethasone for 10 or 14 d and then transferred to soil, resulting in a progressive decrease in Pc-G activity from day 10 to 14 onwards. iCLF FLCpro:GUS plants were generated by crossing FLCpro:GUS into the clf-50 swn-3 CLFpro:CLF-GR background.
Construction of iFT
iFT was constructed by introducing the FT cDNA into the plasmid pMDC7 (Curtis and Grossniklaus, 2003), which is a derivative of pER8 (Zuo et al., 2000). FT cDNA was amplified with primers FT-ATG (5′-ATGTCTATAAATATAAGAGACCCTCTT-3′) and FT-TAG (5′-CTAAAGTCTTCTTCCTCCGC-3′), cloned into pCR8-GW/Topo (Invitrogen), and introduced into pMDC7 via an LR reaction (Gateway; Invitrogen). Transgenic iFT Arabidopsis plants were generated using Agrobacterium tumefaciens–mediated transformation in emf2-10 vrn2-1.
Shift Experiments
Seeds were sown on soil, grown for 21 d (emf2-10 vrn2-1 and La-0) or 29 d (iCLF and Col-0) in LD, then shifted to SD and retained there for at least 4 weeks before examination of the phenotype. Rosette leaves and inflorescence apices, excluding flowers older than stage 13 (Smyth et al., 1990), were harvested 27 DAG (emf2-10 vrn2-1 and La-0) or 34 DAG (iCLF and Col-0) for gene expression analyses. Additionally, the length of the initial period in LD was varied from 7 d to 5 weeks to determine whether sensitivity to reversion varied with developmental age. Because emf2-10 vrn2-1 flc-5 and emf2-10 vrn2-1 flc-5 svp-32 flowered slightly earlier than emf2-10 vrn2-1 in LD (Table 1), differences in reversion might reflect that they were at a more advanced developmental stage at the time of shift. The time in LD was therefore varied, between 7 and 28 d, before shifting to SD so that each genotype was shifted at a range of different stages.
RNA Extraction and Real-Time RT-PCR Analysis
Total RNA of two to six biological replicates of leaves and inflorescence tips, excluding open flowers (see Shift Experiments above), was extracted with TRIZOL (Invitrogen) and cDNA was synthesized using M-MLV reverse transcriptase (Promega). The primers used are listed in Supplemental Table 4.
In Situ RNA Hybridization
In situ RNA hybridization was performed using digoxigenin-labeled mRNA probes as described previously (Chanvivattana et al., 2004). STM, ANT, and LFY antisense probes were generated using plasmids generously provided by R. Simon. FLC (Pien et al. 2008) and SVP plasmids for antisense probes (Hartmann et al., 2000) were kindly provided by C. Dean and P. Huijser, respectively. AP1 antisense probe was generously provided by Coral Vincent and George Coupland (Torti et al., 2012).
ChIP Assay
ChIP assays were performed as described previously (Schubert et al. 2006). The primers used are listed in Supplemental Table 5.
Luciferase Imaging
Initially, plants were sprayed with a solution of 20 mM luciferine (Synchem) to degrade accumulated LUCIFERASE and kept for 1 h. Plants were then sprayed again and imaged in a NightOWL II LB 983 (Berthold Technologies). Exposition time was 10 min and resolution 1 × 1 pixels.
GUS Staining
Detection of GUS activity in tissue preparations were performed as described with minor modifications (Jefferson et al., 1987).
Imaging
Photoshop adjustment involved only image exposure using adjustment levels and sharpening using unsharp mask.
Accession Numbers
Sequence data from this article can be found in GenBank/EMBL data libraries under accession numbers At4g02020 (SWN), At2g23380 (CLF), At5g51230 (EMF2), At4g16485 (VRN2), At1g65480 (FT), At2g22450 (SVP), At5g10140 (FLC), At2g45660 (SOC1), At5g61850 (LFY), At5g60910 (FUL), and At1g69120 (AP1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Phenotypes of iCLF (clf-28 swn-7 CLFpro:CLF-GR) Plants.
Supplemental Figure 2. Floral Phenotype, Rudimentary Bracts, and Classes of Reversion Nodes.
Supplemental Figure 3. LFY and AP1 Expression in Wild-Type Inflorescences and Leafy emf2-10 vrn2-1 Shoots in SD.
Supplemental Figure 4. Gene Expression of Flowering Time and Meristem Identity Genes in Wild-Type (La-0) and emf2-10 vrn2-1 (ev) Inflorescences in SD.
Supplemental Figure 5. FLCpro:GUS Expression in Wild Type, emf2-10 vrn2-1 Mutants, and iCLF.
Supplemental Figure 6. Gene Expression in Inflorescences and Cryptic Bracts in Wild-Type and emf2-10 vrn2-1 Mutant Floral Primordia.
Supplemental Figure 7. Expression Analyses of FLC, SVP, and FT and H3K27me3 ChIP for FLC and SVP in iCLF versus the Wild Type.
Supplemental Figure 8. qRT-PCR Analyses of LFY, SOC1, FUL, and AP1 in emf2-10 vrn2-1 (ev) and Wild-Type Inflorescence Apices.
Supplemental Table 1. Flowering Time of iCFL and ft-10 Mutants Measured by Leaf Number.
Supplemental Table 2. Strong Reversion Nodes at the Main Shoot of iCLF.
Supplemental Table 3. Suppression of Floral Reversion in ev by High FT.
Supplemental Table 4. RT-PCR Primers.
Supplemental Table 5. ChIP PCR Primers.
Supplementary Material
Acknowledgments
We thank Maurice Wernado, Yu Fu, Erica de Leau, and Nora Lorberg for technical assistance, Andrew Hudson, Franziska Turck, Sara Farrona, and Rüdiger Simon for critical reading of the article, and Gregor Klein for supplying the iFT construct. This work was supported by a BBRSC grant (BB/F007442/1) to R.M.-X. and J.G. as well by grants of the Deutsche Forschungsgemeinschaft (SFB590/6-2 and SCHU1954/1-1) and the Boehringer Ingelheim Foundation to D.S. The plasmids for LFY, ANT, and STM antisense probes were kindly provided by R. Simon, and the AP1 antisense probe was generously provided by Coral Vincent and George Coupland. FTpro:LUC seeds were generously provided by F. Turck, the plasmid for SVP antisense probe by P. Huijser, and seeds of FTpro:GUS by K. Goto. Seeds of FLCpro:GUS and flc-5 and FLC antisense probe were kindly provided by C. Dean.
AUTHOR CONTRIBUTIONS
R.M.-X., J.G., and D.S. designed and performed the research and wrote the article. O.C. and L.P. performed research.
Glossary
- SAM
shoot apical meristem
- SD
short-day
- LD
long-day
- Col-0
Columbia-0
- DAG
days after germination
- IM
inflorescence meristem
- FM
floral meristem
- DAS
days after shift
- ChIP
chromatin immunoprecipitation
Footnotes
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
Articles can be viewed online without a subscription.
References
- Abe M., Kobayashi Y., Yamamoto S., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T. (2005). FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056. [DOI] [PubMed] [Google Scholar]
- Adrian J., Torti S., Turck F. (2009). From decision to commitment: the molecular memory of flowering. Mol. Plant 2: 628–642. [DOI] [PubMed] [Google Scholar]
- Adrian J., Farrona S., Reimer J.J., Albani M.C., Coupland G., Turck F. (2010). cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell 22: 1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlenius H., Huang T., Charbonnel-Campaa L., Brunner A.M., Jansson S., Strauss S.H., Nilsson O. (2006). CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043. [DOI] [PubMed] [Google Scholar]
- Chandler J.W. (2012). Floral meristem initiation and emergence in plants. Cell. Mol. Life Sci. 69: 3807–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanvivattana Y., Bishopp A., Schubert D., Stock C., Moon Y.H., Sung Z.R., Goodrich J. (2004). Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development 131: 5263–5276. [DOI] [PubMed] [Google Scholar]
- Corbesier L., Gadisseur I., Silvestre G., Jacqmard A., Bernier G. (1996). Design in Arabidopsis thaliana of a synchronous system of floral induction by one long day. Plant J. 9: 947–952. [DOI] [PubMed] [Google Scholar]
- Corbesier L., Vincent C., Jang S., Fornara F., Fan Q., Searle I., Giakountis A., Farrona S., Gissot L., Turnbull C., Coupland G. (2007). FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033. [DOI] [PubMed] [Google Scholar]
- Curtis M.D., Grossniklaus U. (2003). A Gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia F., Crevillen P., Jones A.M., Greb T., Dean C. (2008). A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc. Natl. Acad. Sci. USA 105: 16831–16836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Ying H., Helliwell C.A., Taylor J.M., Peacock W.J., Dennis E.S. (2011). FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 6680–6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.W., Grover F.O. (1940). Developmental morphology of the growing point of the shoot and the inflorescence in grasses. J. Agric. Res. 61: 481–520. [Google Scholar]
- Fujiwara S., Oda A., Yoshida R., Niinuma K., Miyata K., Tomozoe Y., Tajima T., Nakagawa M., Hayashi K., Coupland G., Mizoguchi T. (2008). Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering in Arabidopsis. Plant Cell 20: 2960–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendall A.R., Levy Y.Y., Wilson A., Dean C. (2001). The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell 107: 525–535. [DOI] [PubMed] [Google Scholar]
- Greb T., Mylne J.S., Crevillen P., Geraldo N., An H., Gendall A.R., Dean C. (2007). The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr. Biol. 17: 73–78. [DOI] [PubMed] [Google Scholar]
- Hartmann U., Hohmann S., Nettesheim K., Wisman E., Saedler H., Huijser P. (2000). Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. Plant J. 21: 351–360. [DOI] [PubMed] [Google Scholar]
- Haughn G.W., Schultz E.A., Martinez-Zapater J.M. (1995). The regulation of flowering in Arabidopsis thaliana: meristems, morphogenesis, and mutants. Can. J. Bot. 73: 959–981. [Google Scholar]
- Hecht V., Laurie R.E., Vander Schoor J.K., Ridge S., Knowles C.L., Liew L.C., Sussmilch F.C., Murfet I.C., Macknight R.C., Weller J.L. (2011). The pea GIGAS gene is a FLOWERING LOCUS T homolog necessary for graft-transmissible specification of flowering but not for responsiveness to photoperiod. Plant Cell 23: 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell C.A., Wood C.C., Robertson M., James Peacock W., Dennis E.S. (2006). The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 46: 183–192. [DOI] [PubMed] [Google Scholar]
- Hempel F.D., Feldman L.J. (1994). Bi-directional inflorescence development in Arabidopsis thaliana: Acropetal initiation of flowers and basipetal initiation of paraclades. Planta 192: 276–286. [Google Scholar]
- Hempel F.D., Feldman L.J. (1995). Specification of chimeric flowering shoots in wild-type Arabidopsis. Plant J. 8: 725–731. [DOI] [PubMed] [Google Scholar]
- Hempel F.D., Zambryski P.C., Feldman L.J. (1998). Photoinduction of flower identity in vegetatively biased primordia. Plant Cell 10: 1663–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth S.R., Valverde F., Ravenscroft D., Mouradov A., Coupland G. (2002). Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 21: 4327–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka K., Yamaguchi A., Abe M., Araki T. (2013). The florigen genes FT and TSF modulate lateral shoot outgrowth in Arabidopsis thaliana. Plant Cell Physiol. 54: 352–368. [DOI] [PubMed] [Google Scholar]
- Izawa T., Oikawa T., Sugiyama N., Tanisaka T., Yano M., Shimamoto K. (2002). Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev. 16: 2006–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Torti S., Coupland G. (2009). Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J. 60: 614–625. [DOI] [PubMed] [Google Scholar]
- Jefferson R.A., Kavanagh T.A., Bevan M.W. (1987). GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I., Shukla V.K., Ahn J.H., Dagenais N., Christensen S.K., Nguyen J.T., Chory J., Harrison M.J., Weigel D. (1999). Activation tagging of the floral inducer FT. Science 286: 1962–1965. [DOI] [PubMed] [Google Scholar]
- Kaufmann K., Wellmer F., Muiño J.M., Ferrier T., Wuest S.E., Kumar V., Serrano-Mislata A., Madueño F., Krajewski P., Meyerowitz E.M., Angenent G.C., Riechmann J.L. (2010). Orchestration of floral initiation by APETALA1. Science 328: 85–89. [DOI] [PubMed] [Google Scholar]
- Kleinboelting N., Huep G., Kloetgen A., Viehoever P., Weisshaar B. (2012). GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res. 40: D1211–D1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Weigel D. (2007). Move on up, it’s time for change—mobile signals controlling photoperiod-dependent flowering. Genes Dev. 21: 2371–2384. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kaya H., Goto K., Iwabuchi M., Araki T. (1999). A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962. [DOI] [PubMed] [Google Scholar]
- Kojima S., Takahashi Y., Kobayashi Y., Monna L., Sasaki T., Araki T., Yano M. (2002). Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 43: 1096–1105. [DOI] [PubMed] [Google Scholar]
- Kotake T., Takada S., Nakahigashi K., Ohto M., Goto K. (2003). Arabidopsis TERMINAL FLOWER 2 gene encodes a heterochromatin protein 1 homolog and represses both FLOWERING LOCUS T to regulate flowering time and several floral homeotic genes. Plant Cell Physiol. 44: 555–564. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska D. (2006). Flower primordium formation at the Arabidopsis shoot apex: quantitative analysis of surface geometry and growth. J. Exp. Bot. 57: 571–580. [DOI] [PubMed] [Google Scholar]
- Lafos M., Kroll P., Hohenstatt M.L., Thorpe F.L., Clarenz O., Schubert D. (2011). Dynamic regulation of H3K27 trimethylation during Arabidopsis differentiation. PLoS Genet. 7: e1002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Yoo S.J., Park S.H., Hwang I., Lee J.S., Ahn J.H. (2007). Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 21: 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Liu C., Shen L., Wu Y., Chen H., Robertson M., Helliwell C.A., Ito T., Meyerowitz E., Yu H. (2008). A repressor complex governs the integration of flowering signals in Arabidopsis. Dev. Cell 15: 110–120. [DOI] [PubMed] [Google Scholar]
- Liu C., Xi W., Shen L., Tan C., Yu H. (2009). Regulation of floral patterning by flowering time genes. Dev. Cell 16: 711–722. [DOI] [PubMed] [Google Scholar]
- Liu C., Zhou J., Bracha-Drori K., Yalovsky S., Ito T., Yu H. (2007). Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development 134: 1901–1910. [DOI] [PubMed] [Google Scholar]
- Long J., Barton M.K. (2000). Initiation of axillary and floral meristems in Arabidopsis. Dev. Biol. 218: 341–353. [DOI] [PubMed] [Google Scholar]
- Long J.A., Moan E.I., Medford J.I., Barton M.K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379: 66–69. [DOI] [PubMed] [Google Scholar]
- Makarevich G., Leroy O., Akinci U., Schubert D., Clarenz O., Goodrich J., Grossniklaus U., Kohler C. (2006). Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep. 7: 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M.A., Gustafson-Brown C., Savidge B., Yanofsky M.F. (1992). Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360: 273–277. [DOI] [PubMed] [Google Scholar]
- Margueron R., Reinberg D. (2011). The Polycomb complex PRC2 and its mark in life. Nature 469: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer S., Lens F., Gennen J., Vanneste S., Rohde A., Beeckman T. (2008). Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat. Genet. 40: 1489–1492. [DOI] [PubMed] [Google Scholar]
- Michaels S.D., Amasino R.M. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinero-Rosales N., Latorre A., Jamilena M., Lozano R. (2004). SINGLE FLOWER TRUSS regulates the transition and maintenance of flowering in tomato. Planta 218: 427–434. [DOI] [PubMed] [Google Scholar]
- Navarro C., Abelenda J.A., Cruz-Oró E., Cuéllar C.A., Tamaki S., Silva J., Shimamoto K., Prat S. (2011). Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 478: 119–122. [DOI] [PubMed] [Google Scholar]
- Okamuro J.K., den Boer B.G., Lotys-Prass C., Szeto W., Jofuku K.D. (1996). Flowers into shoots: photo and hormonal control of a meristem identity switch in Arabidopsis. Proc. Natl. Acad. Sci. USA 93: 13831–13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pien S., Fleury D., Mylne J.S., Crevillen P., Inzé D., Avramova Z., Dean C., Grossniklaus U. (2008). ARABIDOPSIS TRITHORAX1 dynamically regulates FLOWERING LOCUS C activation via histone 3 lysine 4 trimethylation. Plant Cell 20: 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduska B., Humphrey T., Redweik A., Grbić V. (2003). The synergistic activation of FLOWERING LOCUS C by FRIGIDA and a new flowering gene AERIAL ROSETTE 1 underlies a novel morphology in Arabidopsis. Genetics 163: 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P.A., He Y., Schmitz R.J., Amasino R.M., Panella L.W., Richards C.M. (2007). Evolutionary conservation of the FLOWERING LOCUS C-mediated vernalization response: evidence from the sugar beet (Beta vulgaris). Genetics 176: 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruelens P., de Maagd R.A., Proost S., Theißen G., Geuten K., Kaufmann K. (2013). FLOWERING LOCUS C in monocots and the tandem origin of angiosperm-specific MADS-box genes. Nat. Commun. 4: 2280. [DOI] [PubMed] [Google Scholar]
- Ruiz-García L., Madueño F., Wilkinson M., Haughn G., Salinas J., Martínez-Zapater J.M. (1997). Different roles of flowering-time genes in the activation of floral initiation genes in Arabidopsis. Plant Cell 9: 1921–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach A., Onouchi H., Gold S.E., Ditta G.S., Schwarz-Sommer Z., Yanofsky M.F., Coupland G. (2000). Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288: 1613–1616. [DOI] [PubMed] [Google Scholar]
- Schubert D., Clarenz O., Goodrich J. (2005). Epigenetic control of plant development by Polycomb-group proteins. Curr. Opin. Plant Biol. 8: 553–561. [DOI] [PubMed] [Google Scholar]
- Schubert D., Primavesi L., Bishopp A., Roberts G., Doonan J., Jenuwein T., Goodrich J. (2006). Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J. 25: 4638–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E.A., Haughn G.W. (1991). LEAFY, a Homeotic Gene That Regulates Inflorescence Development in Arabidopsis. Plant Cell 3: 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle I., He Y., Turck F., Vincent C., Fornara F., Kröber S., Amasino R.A., Coupland G. (2006). The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev. 20: 898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit A., Rozman A., Goldshmidt A., Alvarez J.P., Bowman J.L., Eshed Y., Lifschitz E. (2009). The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc. Natl. Acad. Sci. USA 106: 8392–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D.R., Bowman J.L., Meyerowitz E.M. (1990). Early flower development in Arabidopsis. Plant Cell 2: 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth A., Schmid M. (2011). Regulation of flowering time: all roads lead to Rome. Cell. Mol. Life Sci. 68: 2013–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Jia Z., Cao D., Jiang B., Wu C., Hou W., Liu Y., Fei Z., Zhao D., Han T. (2011). GmFT2a, a soybean homolog of FLOWERING LOCUS T, is involved in flowering transition and maintenance. PLoS One 6: e29238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooke F., Battey N.H. (2000). A leaf-derived signal is a quantitative determinant of floral form in Impatiens. Plant Cell 12: 1837–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooke F., Ordidge M., Chiurugwi T., Battey N. (2005). Mechanisms and function of flower and inflorescence reversion. J. Exp. Bot. 56: 2587–2599. [DOI] [PubMed] [Google Scholar]
- Torti S., Fornara F., Vincent C., Andrés F., Nordström K., Göbel U., Knoll D., Schoof H., Coupland G. (2012). Analysis of the Arabidopsis shoot meristem transcriptome during floral transition identifies distinct regulatory patterns and a leucine-rich repeat protein that promotes flowering. Plant Cell 24: 444–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B., Tadege M., Hemming M.N., Peacock W.J., Dennis E.S., Sheldon C. (2007). Short vegetative phase-like MADS-box genes inhibit floral meristem identity in barley. Plant Physiol. 143: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D., Meyerowitz E.M. (2011). Switching on Flowers: Transient LEAFY Induction Reveals Novel Aspects of the Regulation of Reproductive Development in Arabidopsis. Front. Plant Sci. 2: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D., Sablowski R.W., Meyerowitz E.M. (1999). Transcriptional activation of APETALA1 by LEAFY. Science 285: 582–584. [DOI] [PubMed] [Google Scholar]
- Wang R., Farrona S., Vincent C., Joecker A., Schoof H., Turck F., Alonso-Blanco C., Coupland G., Albani M.C. (2009). PEP1 regulates perennial flowering in Arabis alpina. Nature 459: 423–427. [DOI] [PubMed] [Google Scholar]
- Weigel D., Alvarez J., Smyth D.R., Yanofsky M.F., Meyerowitz E.M. (1992). LEAFY controls floral meristem identity in Arabidopsis. Cell 69: 843–859. [DOI] [PubMed] [Google Scholar]
- Wellmer F., Alves-Ferreira M., Dubois A., Riechmann J.L., Meyerowitz E.M. (2006). Genome-wide analysis of gene expression during early Arabidopsis flower development. PLoS Genet. 2: e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P.A., Kim M.C., Jaeger K.E., Busch W., Schmid M., Lohmann J.U., Weigel D. (2005). Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059. [DOI] [PubMed] [Google Scholar]
- Yoo S.K., Chung K.S., Kim J., Lee J.H., Hong S.M., Yoo S.J., Yoo S.Y., Lee J.S., Ahn J.H. (2005). CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol. 139: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J., Niu Q.W., Chua N.H. (2000). Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24: 265–273. [DOI] [PubMed] [Google Scholar]
NOTE ADDED IN PROOF
Liu et al. (2014) also revealed a photoperiod-independent function of FT in maintaining floral commitment.
- Liu L., Farrona S., Klemme S., Turck F.K. (2014). Postfertilization expression of FLOWERING LOCUS T suppresses reproductive reversion. Front. Plant Sci. 5: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.