Abstract
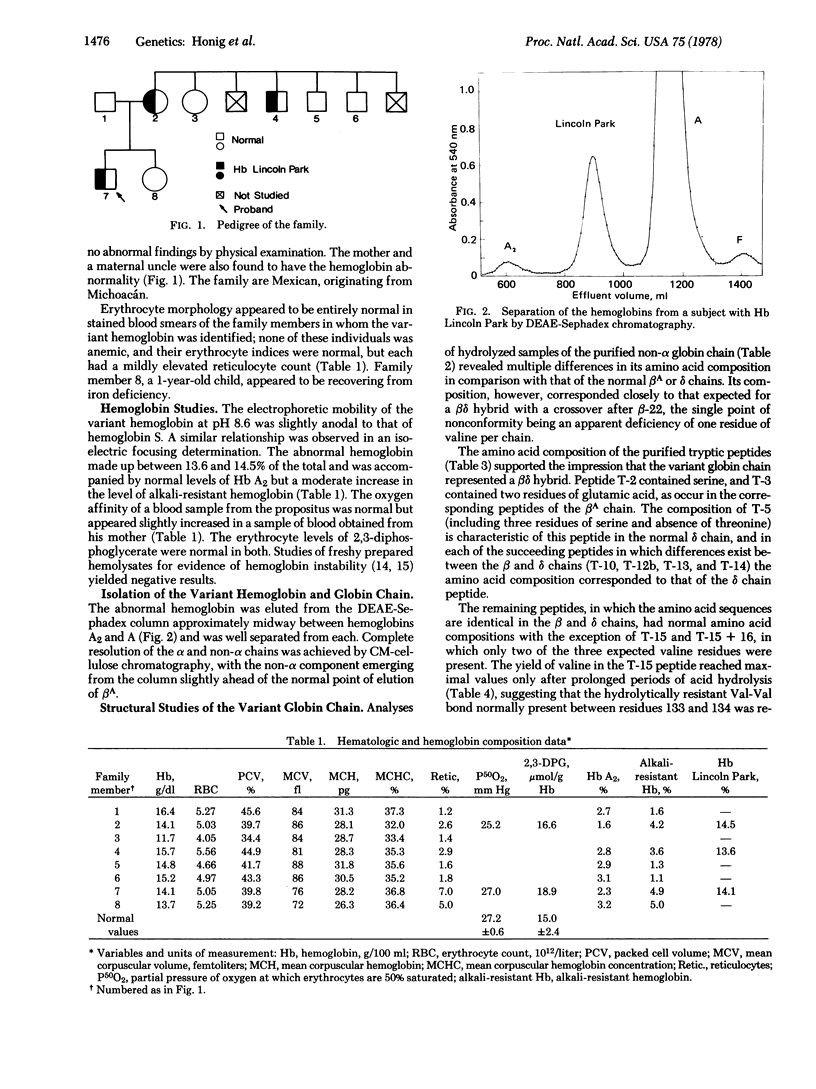
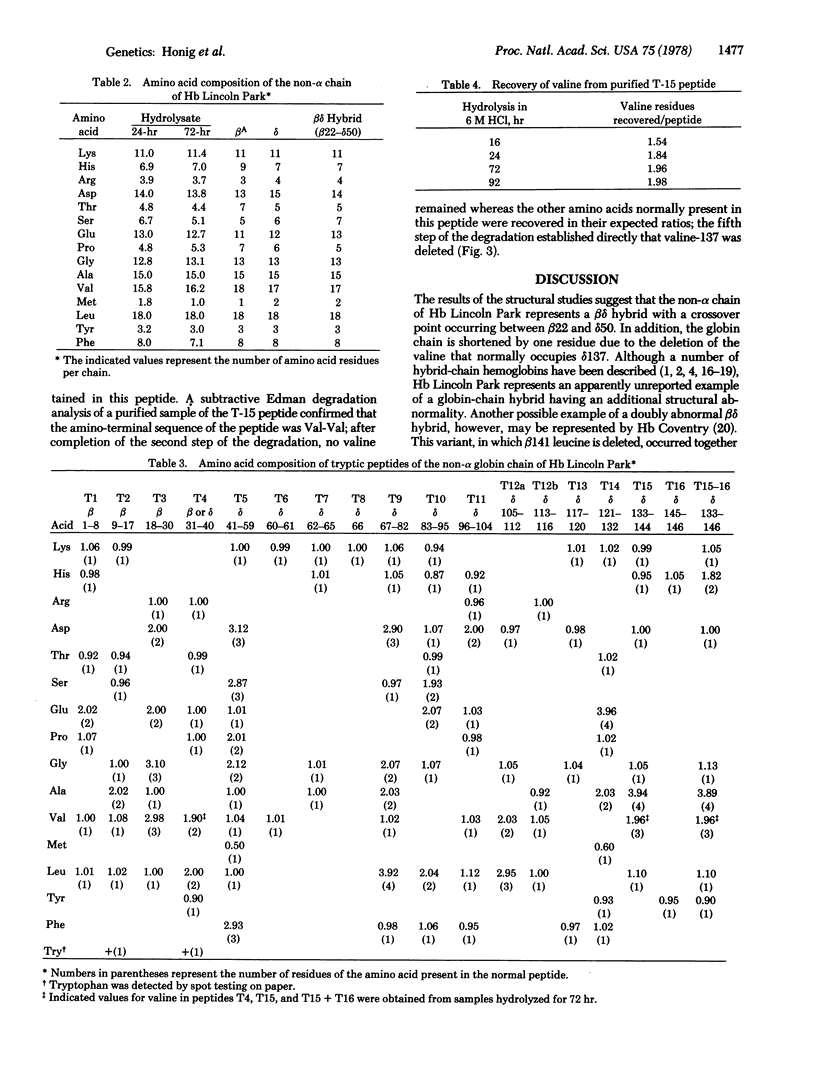
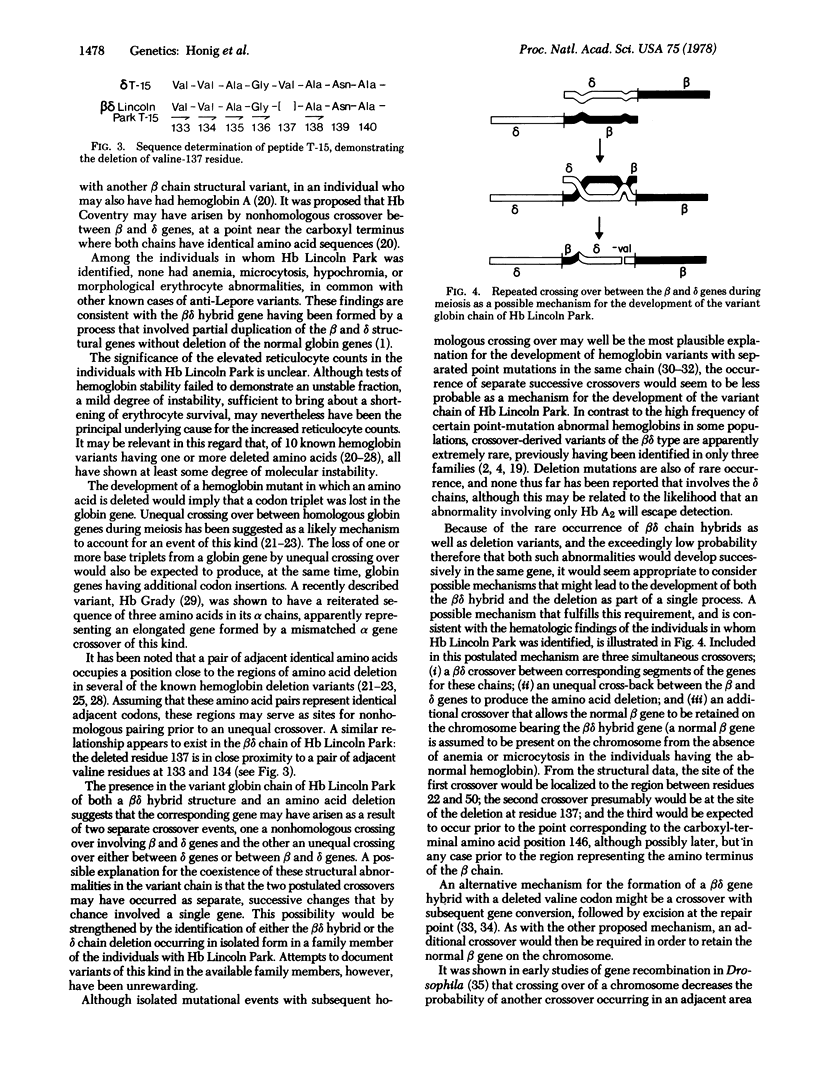
An electrophoretically slow-moving hemoglobin variant was identified in three members of a family originating from Southern Mexico. The variant, Hb Lincoln Park, made up approximately 14% of the total hemoglobin and appeared to have normal stability and functional properties. None of the individuals in whom the abnormal hemoglobin was present was anemic, but each had a mildly elevated reticulocyte count. Structural data suggest that the non-alpha chain of Hb Lincoln Park represents a betadelta gene-fusion product, with normal beta chain structure of the amino-terminal portion of the chain and delta sequences subsequently, the crossover point occurring between animo acid residues 22 and 50. An additional abnormality is the deletion of valine-137, a component of the delta gene-derived segment of the betadelta chain. To account for the development of this abnormal globin chain, a series of intergenic crossovers is proposed; the first, a nonhomologous crossover between the beta and delta genes, presumably gave rise to the betadelta fusion gene; two additional crossovers, one of them unequal, may then have occurred between the same beta and delta genes to produce the amino acid deletion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BETKE K., MARTI H. R., SCHLICHT I. Estimation of small percentages of foetal haemoglobin. Nature. 1959 Dec 12;184(Suppl 24):1877–1878. doi: 10.1038/1841877a0. [DOI] [PubMed] [Google Scholar]
- Badr F. M., Lorkin P. A., Lehmann H. Haemoglobin P-Nilotic containing a - chain. Nat New Biol. 1973 Mar 28;242(117):107–110. doi: 10.1038/newbio242107a0. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L., Ranney H. M., Jacobs A. S. Hemoglobin C Harlem: a sickling variant containing amino acid substitutions in two residues of the beta-polypeptide chain. Biochem Biophys Res Commun. 1966 Apr 19;23(2):122–127. doi: 10.1016/0006-291x(66)90515-8. [DOI] [PubMed] [Google Scholar]
- Bradley T. B., Jr, Wohl R. C., Rieder R. F. Hemoglobin Gun Hill: deletion of five amino acid residues and impaired heme-globin binding. Science. 1967 Sep 29;157(3796):1581–1583. doi: 10.1126/science.157.3796.1581. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Kay R. A simple method for the detection of unstable haemoglobins. Br J Haematol. 1972 Nov;23(5):615–619. doi: 10.1111/j.1365-2141.1972.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Chase M, Doermann A H. High Negative Interference over Short Segments of the Genetic Structure of Bacteriophage T4. Genetics. 1958 May;43(3):332–353. doi: 10.1093/genetics/43.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal M., Blouquit Y., Garel M. C., Thillet J., Gaillard L., Creyssel R., Gibaud A., Rosa J. Haemoglobin Lyon (beta17-18 (A 14-15) Lys-Val leads to O). Determination by sequenator analysis. Biochim Biophys Acta. 1974 Jun 7;351(2):306–316. [PubMed] [Google Scholar]
- DACIE J. V., GRIMES A. J., MEISLER A., STEINGOLD L., HEMSTED E. H., BEAVEN G. H., WHITE J. C. HEREDITARY HEINZ-BODY ANAEMIA. A REPORT OF STUDIES ON FIVE PATIENTS WITH MILD ANAEMIA. Br J Haematol. 1964 Jul;10:388–402. doi: 10.1111/j.1365-2141.1964.tb00715.x. [DOI] [PubMed] [Google Scholar]
- DE SERRES F. J. Recombination and interference in the ad-3 region of Neurospora crassa. Cold Spring Harb Symp Quant Biol. 1958;23:111–118. doi: 10.1101/sqb.1958.023.01.015. [DOI] [PubMed] [Google Scholar]
- De Jong W. W., Went L. N., Bernini L. F. Haemoglobin Leiden: deletion of beta-6 or 7 glutamic acid. Nature. 1968 Nov 23;220(5169):788–790. doi: 10.1038/220788a0. [DOI] [PubMed] [Google Scholar]
- Dozy A. M., Kleihauer E. F., Huisman T. H. Studies on the heterogeneity of hemoglobin. 13. Chromatography of various human and animal hemoglobin types on DEAE-Sephadex. J Chromatogr. 1968 Feb 20;32(4):723–727. doi: 10.1016/s0021-9673(01)80551-3. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Righetti P., Bunn H. F. The separation of human and animal hemoglobins by isoelectric focusing in polyacrylamide gel. Biochim Biophys Acta. 1971 Jan 19;229(1):42–50. doi: 10.1016/0005-2795(71)90315-1. [DOI] [PubMed] [Google Scholar]
- Goossens M., Garel M. C., Auvinet J., Basset O., Ferreira Gomes P., Rosa J., Arous N. Hemoglobin C Ziguinchor alphaA2 beta62 (A3) Glu leads to Val beta58 (E2) Pro leads to Arg: the second sickling variant with amino acid substitutions in 2 residues of the beta polypeptide chain. FEBS Lett. 1975 Oct 15;58(1):149–154. doi: 10.1016/0014-5793(75)80246-8. [DOI] [PubMed] [Google Scholar]
- Honig G. R., Gunay U., Mason R. G., Vida L. N., Ferenc C. Sickle cell syndromes. I. Hemoglobin SC-alpha-thalassemia. Pediatr Res. 1976 Jun;10(6):613–620. doi: 10.1203/00006450-197606000-00010. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Wilson J. B., Gravely M., Hubbard M. Hemoglobin Grady: the first example of a variant with elongated chains due to an insertion of residues. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3270–3273. doi: 10.1073/pnas.71.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Wrightstone R. N., Wilson J. B., Schroeder W. A., Kendall A. G. Hemoglobin Kenya, the product of fusion of amd polypeptide chains. Arch Biochem Biophys. 1972 Dec;153(2):850–853. doi: 10.1016/0003-9861(72)90408-0. [DOI] [PubMed] [Google Scholar]
- JONES R. T. STRUCTURAL STUDIES OF AMINOETHYLATED HEMOGLOBINS BY AUTOMATIC PEPTIDE CHROMATOGRAPHY. Cold Spring Harb Symp Quant Biol. 1964;29:297–308. doi: 10.1101/sqb.1964.029.01.032. [DOI] [PubMed] [Google Scholar]
- Jones R. T., Brimhall B., Huisman T. H., Kleihauer E., Betke K. Hemoglobin Freiburg: abnormal hemoglobin due to deletion of a single amino acid residue. Science. 1966 Nov 25;154(3752):1024–1027. doi: 10.1126/science.154.3752.1024. [DOI] [PubMed] [Google Scholar]
- Keitt A. S. Reduced nicotinamide adenine dinucleotide-linked analysis of 2,3-diphosphoglyceric acid: spectrophotometric and fluorometric procedures. J Lab Clin Med. 1971 Mar;77(3):470–475. [PubMed] [Google Scholar]
- Lutcher C. L., Wilson J. B., Gravely M. E., Stevens P. D., Chen C. J., Lindeman J. G., Wong S. C., Miller A., Gottleib M., Huisman T. H. Hb Leslie, an unstable hemoglobin due to deletion of glutaminyl residue beta 131 (H9) occurring in association with beta0-thalassemia, HbC, and HbS. Blood. 1976 Jan;47(1):99–112. [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Yamaoka K., Sumida I., Yanase T. Haemoglobin Miyada, a beta-delta fusion peptide (anti-Lepore) type discovered in a Japanese family. Nat New Biol. 1971 Sep 15;234(50):218–220. doi: 10.1038/newbio234218a0. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Smith E. W. Hemoglobin-Lepore-Baltimore, a third type of a delta, beta crossover (delta 50, beta 86). Eur J Biochem. 1969 Sep;10(2):371–376. doi: 10.1111/j.1432-1033.1969.tb00700.x. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. CHROMOSOMAL REARRANGEMENTS AND PROTEIN STRUCTURE. Cold Spring Harb Symp Quant Biol. 1964;29:309–319. doi: 10.1101/sqb.1964.029.01.033. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Huisman T. H., Hyman C., Shelton J. R., Apell G. An individual with "Miyada"-like hemoglobin indistinguishable from hemoglobin A2. Biochem Genet. 1973 Oct;10(2):135–147. doi: 10.1007/BF00485761. [DOI] [PubMed] [Google Scholar]
- Stadler D. R. The mechanism of intragenic recombination. Annu Rev Genet. 1973;7:113–127. doi: 10.1146/annurev.ge.07.120173.000553. [DOI] [PubMed] [Google Scholar]
- Wajcman H., Labie D., Schapira G. Two new hemoglobin variants with deletion. Hemoglobin tours: Thr 8 7 (F 3 ) deleted and hemoglobin St Antoine: Gly-Leu 74-75 (E 18-19 deleted. Consequences for oxygen affinity and protein stability. Biochim Biophys Acta. 1973 Feb 21;295(2):495–504. [PubMed] [Google Scholar]
- White J. M., Lang A., Lorkin P. A., Lehmann H., Reeve J. Synthesis of haemoglobin Lepore. Nat New Biol. 1972 Feb 16;235(59):208–210. doi: 10.1038/newbio235208a0. [DOI] [PubMed] [Google Scholar]


