Abstract
Contraction of 3D collagen matrices by fibroblasts frequently is used as an in vitro model of wound closure. Different iterations of the model -- all conventionally referred to as “contraction” – involve different morphological patterns. During floating matrix contraction, cells initially are round without stress fibers and subsequently undergo spreading. During stressed matrix contraction, cells initially are spread with stress fibers and subsequently undergo shortening. In the current studies, we used siRNA silencing of myosin IIA (MyoIIA) and myosin IIB (MyoIIB) to test the role of myosin II isoforms in fibroblast interactions with 3D collagen matrices and collagen matrix contraction. We found that MyoIIA but not MyoIIB was required for cellular global inward contractile force, formation of actin stress fibers, and morphogenic cell clustering. Stressed matrix contraction required MyoIIA but not MyoIIB. Either MyoIIA or MyoIIB was sufficient for floating matrix contraction (FMC) stimulated by platelet-derived growth factor. Neither MyoIIA or MyoIIB was necessary for FMC stimulated by serum. Our findings suggest that myosin II-dependent motor mechanisms for collagen translocation during extracellular matrix remodeling differ depending on cell tension and growth factor stimulation.
Keywords: Myosin, 3D Collagen Matrix, Cell Contraction, Morphogenic Cell Clustering, Wound Repair
INTRODUCTION
Two different mechanisms of wound closure have been described. One depends on smooth muscle-like fibroblasts (myofibroblasts) undergoing contraction within newly synthesized wound fibrous connective tissue known as granulation tissue [1, 2]. The other depends on the motile activity of fibroblasts at wound margins and occurs independently of granulation tissue [3]. Myofibroblasts are activated by mechanical tension [4] and have been implicated in pathological wound repair conditions such as wound contracture and hypertrophic scar [5-7]. However, myofibroblasts often appear late relative to the timing of wound closure [8-11] and are not required for fetal wounds to close [12]. Similarly, in a mouse model of hypertrophic scarring, the mechanical tension sensor focal adhesion kinase (FAK) [13-15] was required for the fibrotic response but not for incisional wound closure [16]. The above findings are consistent with the idea that wound closure can occur by different mechanisms depending on mechanical tension within the wound [17].
Collagen matrix contraction by fibroblasts was introduced as an in vitro model of wound closure [18]. Different iterations of this model -- all conventionally referred to as “contraction” --involve different cellular mechanisms. That is, contraction refers to the change that occurs in the collagen matrix but not to the cellular mechanism responsible for collagen translocation leading to matrix remodeling. In the case of floating matrix contraction assays, cells initially are round without stress fibers and become spread during matrix contraction. In the case of stressed matrix contraction, cells initially are spread with stress fibers and subsequently undergo shortening and stress fiber disruption during matrix contraction [17].
Non-muscle myosin II plays central roles in cell adhesion, migration and contraction with different isoforms exhibiting different functional roles [e.g., reviewed in 19, 20, 21]. The myosin II inhibitor blebbistatin inhibited stressed collagen matrix contraction by human fibroblasts but was less effective in blocking floating matrix contraction depending on growth factor conditions [22-24]. This observations raised the possibility that the role of myosin II in collagen matrix contraction was tension and growth factor dependent. In the current studies, we tested the effects of siRNA silencing of MyoIIA and MyoIIB on stressed and floating collagen matrix contraction to determine what roles the different isoforms play in the cellular mechanisms involved. Details are reported herein.
MATERIALS AND METHODS
Materials
Dulbecco's modified Eagle's Medium (DMEM), 0.25% trypsin/EDTA and antibiotic-antimycotic solutions were purchased from GIBCO (Grand Island, NY). PureCol (Bovine Collagen Solution, Type I) was purchased from Advanced BioMatrix (San Diego, CA). Rat tail Type I collagen was obtained from BD Biosciences (Bedford, MA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). Platelet-derived growth factor (PDGF) was obtained from Upstate Biotechnology (Lake Placid, NY). Fatty acid–free bovine serum albumin (BSA), focal adhesion kinase inhibitor PF-228, and monoclonal anti-actin antibody were obtained from Sigma (St. Louis, MO). BSA (fraction V) was obtained from Equitech (Kerrville, TX). Blebbistatin was obtained from TRC (North York, Ontario, Canada). Polyclonal anti- MyoIIA and anti-MyoIIB were purchased from Covance (Alice, TX). Hoechst 33342, Alexa Fluor 488 phalloidin, Alexa Fluor 594 phalloidin, and Alexa 488 Fluor and 568 Fluor conjugated antibodies against mouse and rabbit IgG (H+L) were obtained from Invitrogen-Molecular Probes (Eugene, OR). Polyclonal anti-FN and horseradish peroxidase-conjugated goat anti-mouse IgG were obtained from Abcam (Cambridge,MA). Fluoromount-G was purchased from Southern Biotechnology (Birmingham, AL). Horseradish peroxidase-conjugated goat anti-rabbit IgG was obtained from Thermo Scientific (Pittsburgh, PA). Pierce ECL Western blotting substrate was purchased from Thermo Scientific (Rockford, IL). ON-TARGETplus siRNA for human MYH9 and MYH10 were purchased from Dharmacon (Chicago, IL). Oligofectamine solution was purchased from Invitrogen (Gaithersburg, MD)
Cell culture
Use of human foreskin fibroblasts was approved by the University Institutional Review Board (Exemption #4). BR5 cells (early passage, hTERT immortalized, human foreskin fibroblasts) [25] were cultured in DMEM supplemented with 10% FBS in a 37°C and 5% CO2 humidified incubator. Experimental incubation media consisted of DMEM supplemented with 5 mg/ml BSA (fatty-acid-free) (DMEM/BSA), 5 mg/ml BSA and 50 ng/ml PDGF (DMEM/PDGF), and 10% FBS (DMEM/FBS).
siRNA transfection
siRNA transfection was accomplished beginning with trypsin rounded cells as described previously [25, 26]. MyoIIA and MyoIIB silencing incubations contained 800 μl of DMEM (serum and antibiotics-free) plus 200 μl of a mixed solution containing 100nM ONTARGETplus siRNAs for human MYH9 and MYH10 respectively, Opti-MEM (Invitrogen, Carlsbad, CA), and 0.2mM oligofectamine. After an initial 24 h incubation, transfected cells were sub-cultured for an additional 72h. To silence both MyoIIA and MyoIIB, the incubations contained 100nM MYH9 and 100nM MYH10. Preliminary experiments were performed using the 4 siRNA open reading frames provided by the manufacturer. Specific knockdown was first confirmed for all 4 oligonucleotides. A mixture of the two most effective siRNA's was used for subsequent experiments. For mock transfection, cells were treated with the transfection reagent with no siRNA.
Floating and stressed collagen matrix contraction
Methods for preparing cell-containing collagen matrices and measuring floating and stressed collagen matrix contraction have been described previously [26, 27]. Briefly, collagen matrices (200 μl, 1.5mg/ml bovine type I collagen, 2×105 cells/matrix) were polymerized within 12 mm circular scores made on the bottom of 24-well plates in a humidified incubator at 37° C. For floating matrix contraction, polymerized matrices were released from the culture surface after 1 hr and incubated 4 h. For stressed matrix contraction, polymerized matrices were cultured overnight DMEM/FBS, rinsed with DMEM and then released from the culture surface and incubated 1 h. Incubation medium contained FBS and PDGF as indicated.
At the end of the incubations, samples were fixed with 3% paraformaldehyde in phosphate buffered saline. Overall appearance of collagen matrices was recorded using an Epson 4870 photo scanner, and matrix diameter measured. Contraction was quantified as starting (12mm) – final matrix diameter.
Cell clustering and collagen matrix flattening
Methods for preparing collagen matrices with surface-seeded cells and measuring cell clustering and matrix flattening have been described previously [25]. Briefly, collagen matrices (200 μl, 1 mg/ml rat tail type I collagen) were polymerized 1 hr on 12 mm circular scores made on the bottom of 24-well plates. 2×104 cells in 1ml of DMEM/FBS were cultured on collagen matrices for 18 h at 37°C with 5% CO2. At the end of the incubations, samples were fixed 10 min at room temperature with 3% paraformaldehyde in phosphate buffered saline and subjected to immunofluorescence analysis as indicated in the figure legends. The extent of cell clustering was determined using Hoechst-stained samples, defining a cell cluster as ≥10 nucleus within a 100 μm square. Matrix flattening was measured microscopically by focusing from top to bottom in the center of the matrix. Flattening was quantified as height of control matrices (no cells, ~1.8 mm) minus height of experimental samples.
Statistical Analyses
Quantitative data are presented as averages ± standard deviations. All experiments were carried out at least three times. Statistical analyses of the results (Student's T-Test, one-tailed distribution, two-sample unequal variance) was performed with Excel, and the results are shown in Supplemental Table 1.
Immunoblotting
Immunoblotting was performed as previously described [25, 26]. Protein concentration was determined using the Bradford protein assay kit (Expedeon Ltd., Harston, Cambridgeshire, UK). Equal protein samples were subjected to SDS-PAGE and electroblotted onto PVDF membrane (Millpore, Bedford, MA). After blocking with 3% BSA (fraction V)/0.1%TTBS (TBS, 20 mM Tris-Base and 150 mM NaCl, pH 7.5, plus Tween 20) for 1 h at room temperature (RT), the upper part of the blot was incubated with primary antibodies to MyoIIA/IIB and the lower part of the blot was incubated with primary antibodies to actin for 2 h at RT. HRP-conjugated goat-anti-mouse (1:5000) or anti-rabbit (1:5000) was used as secondary antibodies. Proteins were detected by Pierce ECL Western blotting substrate (Thermo Scientific, Rockford, IL).
Fluorescence microscopy
Cell preparations were processed for immunofluorescence staining as described previously [25, 26, 28]. Samples were fixed for 10 min at room temperature with 3% paraformaldehyde in PBS, blocked with 2% BSA (fraction V) /1% glycine in PBS for 30 min, and permeabilized for 10 min using 0.5 % Triton X-100 in PBS. Staining for actin was accomplished using Alexa fluor 488 phalloidin (1:500). Staining for nuclei was accomplished using propidium iodide (PI) (1:500) or Hoechst 33342 (2 μg/ml). Staining for extracellular FN was accomplished by incubating samples with anti-FN (1:200) before permeabilization. At the end of the incubations, samples were mounted on glass slides with Fluoromount-G. Microscopic images were collected with a Nikon Elipse 400 fluorescent microscope using 10X/0.45 and 20X/0.75 Plan Apo and 40X/0.75 Plan Fluor infinity corrected objectives, Photometrics SenSys camera and MetaView acquisition software (Universal Imaging Corp.). Image processing was carried out using Photoshop (Adobe).
RESULTS
Fibroblast spreading on collagen matrices
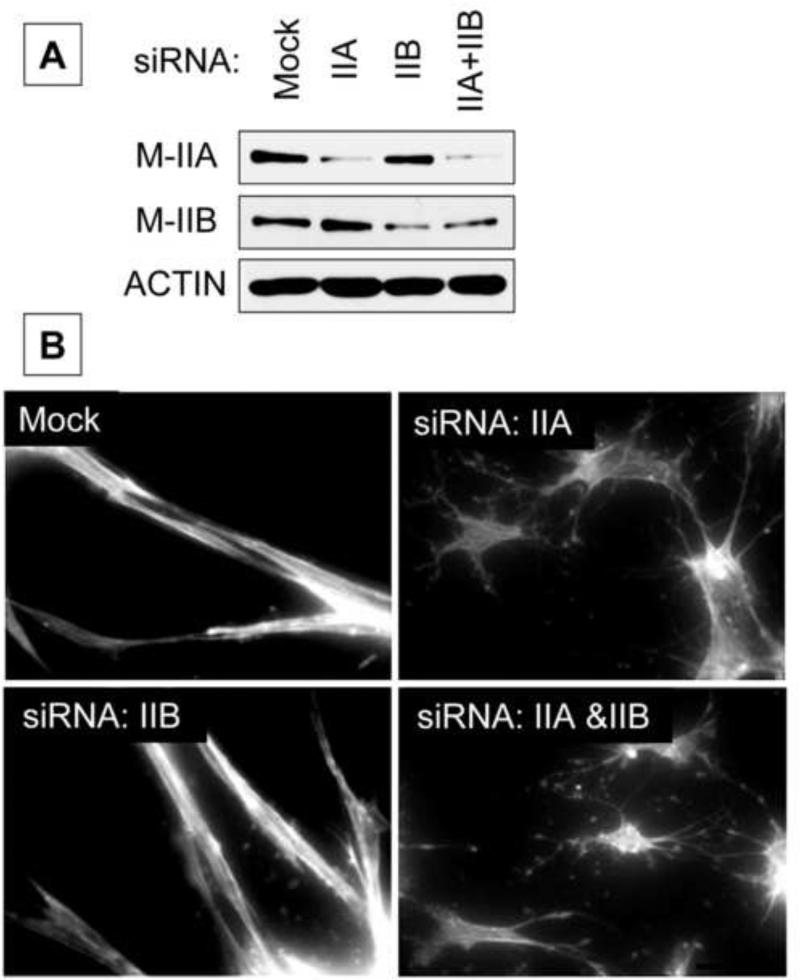
Fibroblasts cultured in or on 3D collagen matrices become well spread with bipolar morphology and prominent actin stress fibers [17]. Figure 1 shows a representative immunoblot confirming substantial knockdown of MyoIIA and MyoIIB by isoform-specific siRNA gene silencing (Figure 1A) and the effects of silencing MyoIIA and MyoIIB on fibroblast spreading on collagen matrices (Fig 1B). Mock-treated cells cultured overnight on the surfaces of collagen matrices developed bipolar shape and actin stress fibers. Cell-cell interactions were observed. MyoIIA silencing by siRNA, although not complete, was sufficient to inhibit cells from undergoing these morphological changes. As a result, fibroblasts exhibited dendritic morphology without stress fibers (Fig 1B). The effect was specific for MyoIIA since cells treated with siRNA to silence MyoIIB showed morphological changes identical to mock-treated cells.
Figure 1. Silencing MyoIIA prevents fibroblasts from spreading in bipolar shape with actin stress fibers during overnight culture.
A) Representative immunoblotting results showing MyoIIA and MyoIIB expression after treatment of cells by isoform-specific siRNA gene silencing. B) Mock and myosin II isoform-silenced cells as indicated were cultured 18 h on collagen matrices in DMEM/FBS. At the end of the incubations, samples were fixed and stained for actin. Silencing MyoIIA but not MyoIIB inhibited cells from developing actin stress fibers. The effect was specific for MyoIIA since cells treated with siRNA to silence MyoIIB showed morphological changes identical to mock-treated cells. Bar = 50 μm.
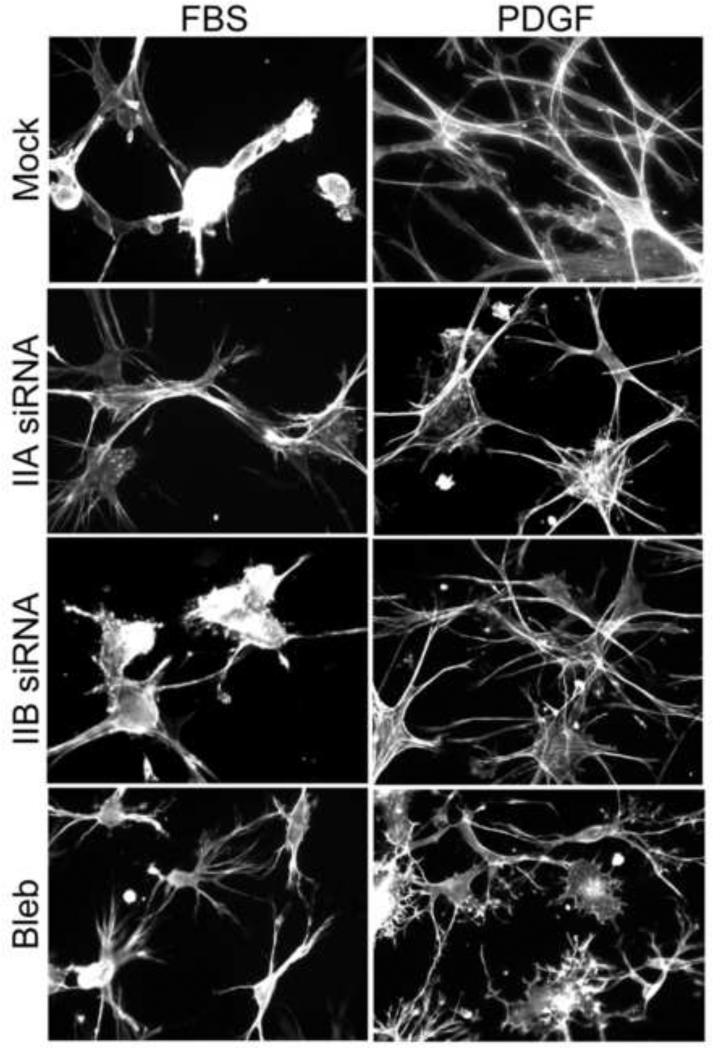
When fibroblasts interact with 3D collagen matrices or with soft 2D collagen-coated polyacrylamide substrates, the trajectory of initial cell spreading depends on the starting growth factor environment. Cells cultured in medium with serum exhibit Rho kinase-dependent global inward contractile force that attenuates cell extensions and causes a delay in cell spreading compared to Rac-activating conditions such as platelet-derived growth factor (PDGF) [27-30]. Figure 2 shows the difference in morphology of control cells (Mock) after spreading 4 hr on 3D collagen matrices. Fibroblasts in fetal bovine serum (FBS)-containing medium tended to be rounded with short extensions; fibroblasts in PDGF-containing medium were well spread with much longer, dendritic extensions. Silencing MyoIIA but not MyoIIB blocked the FBS effect and permitted cells to spread with dendritic morphology as rapidly as fibroblasts in PDGF-containing medium. Blebbistatin treatment had a similar effect as silencing MyoIIA.
Figure 2. Silencing MyoIIA blocks serum-stimulated global inward cell contraction that attenuates initial fibroblast spreading.
Mock and myosin II isoform-silenced cells as indicated and untreated cells plus 20 μM blebbistatin were cultured on polymerized collagen matrices 4 h in DMEM/FBS and DMEM/PDGF as shown. At the end of the incubations, samples were fixed and stained for actin. Blebbistatin treatment or silencing MyoIIA but not MyoIIB blocked FBS-stimulated round cell morphology and permitted cells to spread with dendritic morphology as rapidly as in PDGF-containing medium. Bar = 50 μm.
Fibroblast contraction of collagen matrices
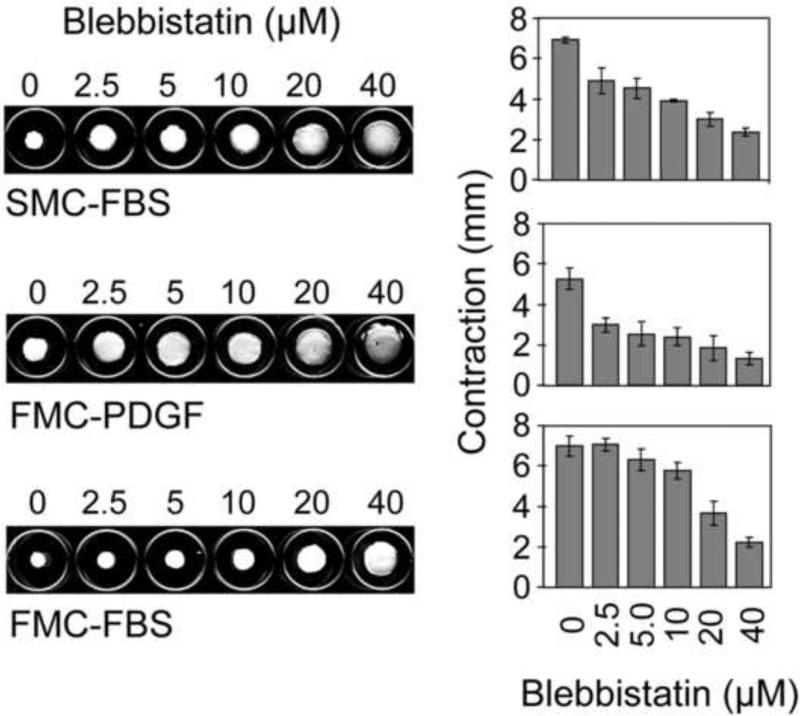
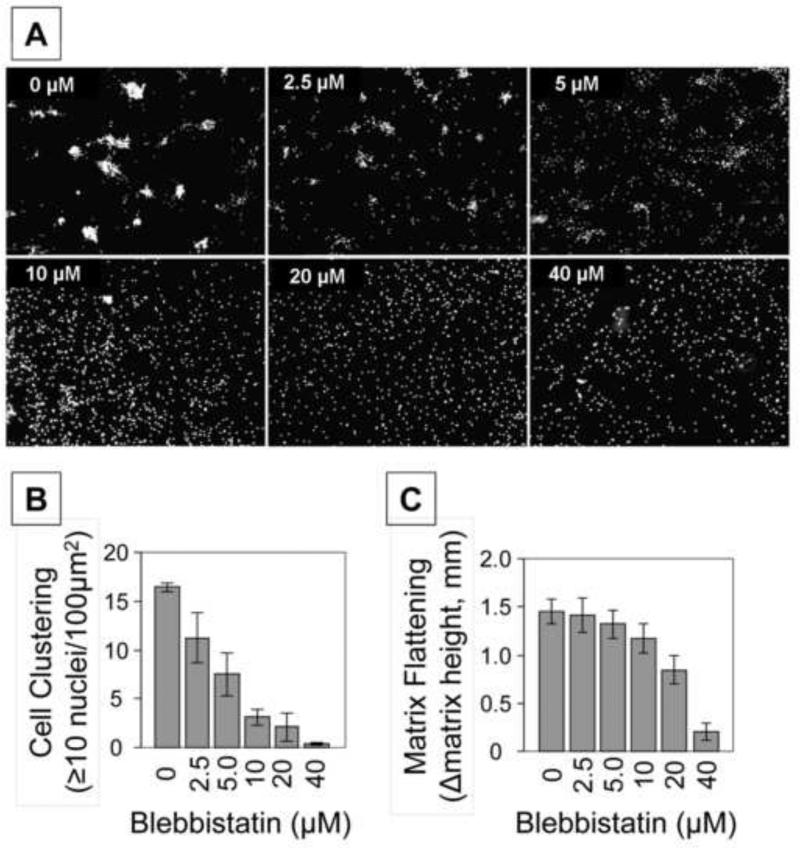
The results in Figures 1 and 2 suggested that for fibroblasts interacting with collagen matrices, serum-stimulated global inward contractile force and formation of actin stress fibers required MyoIIA but not MyoIIB. We then carried out experiments to determine the role of myosin II in the different iterations of the collagen matrix contraction model: stressed matrix contraction (SMC) stimulated by FBS and floating matrix contraction stimulated by PDGF and FBS. Figure 3 shows the appearance of collagen matrices after contraction and quantification of contraction as a function of blebbistatin concentration. Blebbistatin inhibited SMC-FBS and FMC-PDGF at 2.5 μM, but 10-20 μM blebbistatin was required to inhibit FBS-FMC [c.f. 22].
Figure 3. Differential effects of blebbistatin on fibroblast contraction of stressed and floating collagen matrices.
Stressed collagen matrix contraction was carried out in DMEM/FBS. Floating collagen matrix contraction was carried out in DMEM/PDGF and DMEM/FBS. Blebbistatin as indicated was added to the incubations at the times that the matrices were released from the underlying culture dishes to initiate contraction -- 18 hr for SMC and 1 hr for FMC. Data shown are the appearance of collagen matrices from a representative experiment and the averages ± SD of quantitative contraction measurements (starting – final matrix diameter) for triplicate samples. Blebbistatin inhibited SMC-FBS and FMC-PDGF at 2.5 μM, but 10-20 μM blebbistatin was required to inhibit FBS-FMC.
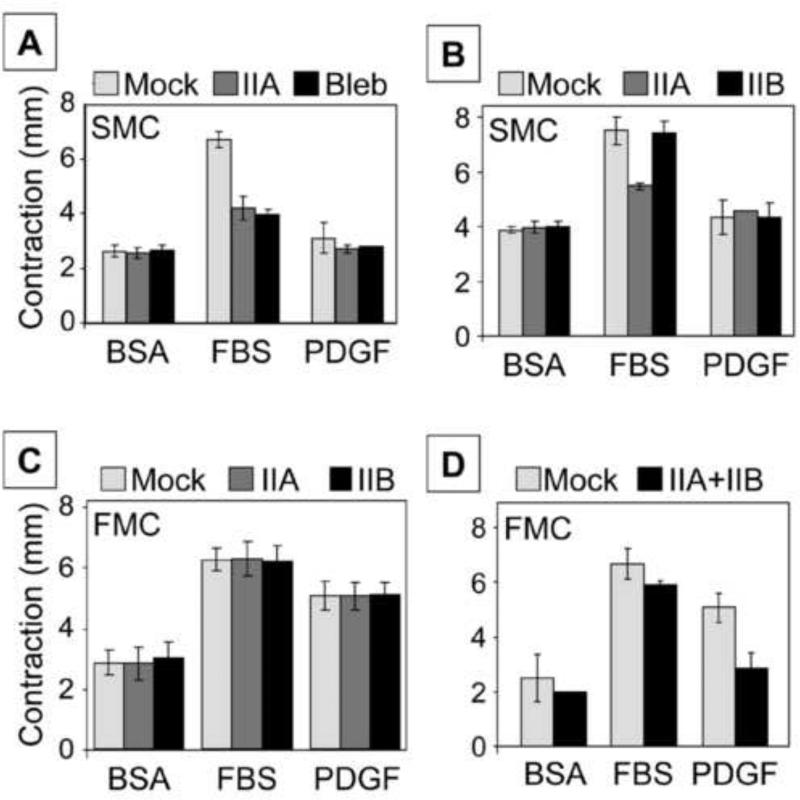
Figures 4A and 4B show experiments analyzing the role of myosin isoforms in stressed matrix contraction. As expected, serum but not PDGF stimulated FMC above basal conditions (BSA, absence of added growth factors) [27]. Silencing MyoIIA inhibited SMC as well as addition of 20 μM blebbistatin (Figure 4A). Parallel experiments demonstrated that silencing MyoIIB did not have a detectable effect on SMC (Figure 4B). Figures 4C and 4D show experiments to analyze the role of myosin isoforms in floating matrix contraction. With FMC, both serum and PDGF stimulated contraction above basal levels. Silencing either MyoIIA or MyoIIB separately had no effect on FMC (Figure 4C). However, silencing both MyoIIA and MyoIIB together inhibited PDGF-FMC but not FBS-FMC (Figure 4D).
Figure 4. Differential effects of silencing MyoIIA and IIB on floating and stressed collagen matrix contraction.
A,B) Stressed matrix contraction was carried out with mock and myosin II isoform-silenced cells as indicated and untreated cells plus 20 μM blebbistatin (bleb) in DMEM/BSA, DMEM/FBS, and DMEM/PDGF as shown. C,D). Floating collagen matrix contraction was carried out with mock and myosin II isoform-silenced cells as indicated in DMEM/BSA, DMEM/FBS, and DMEM/PDGF as shown. Data presented are the averages ± SD of quantitative contraction measurements (starting – final matrix diameter) for triplicate samples. Complete set of p values are shown in supplemental Table 1. Silencing MyoIIA inhibited SMC as well as addition of 20 μM blebbistatin, whereas silencing MyoIIB did not have a detectable effect. Silencing either MyoIIA or MyoIIB separately had no effect on FMC; silencing both together inhibited PDGF-FMC but not FBS-FMC.
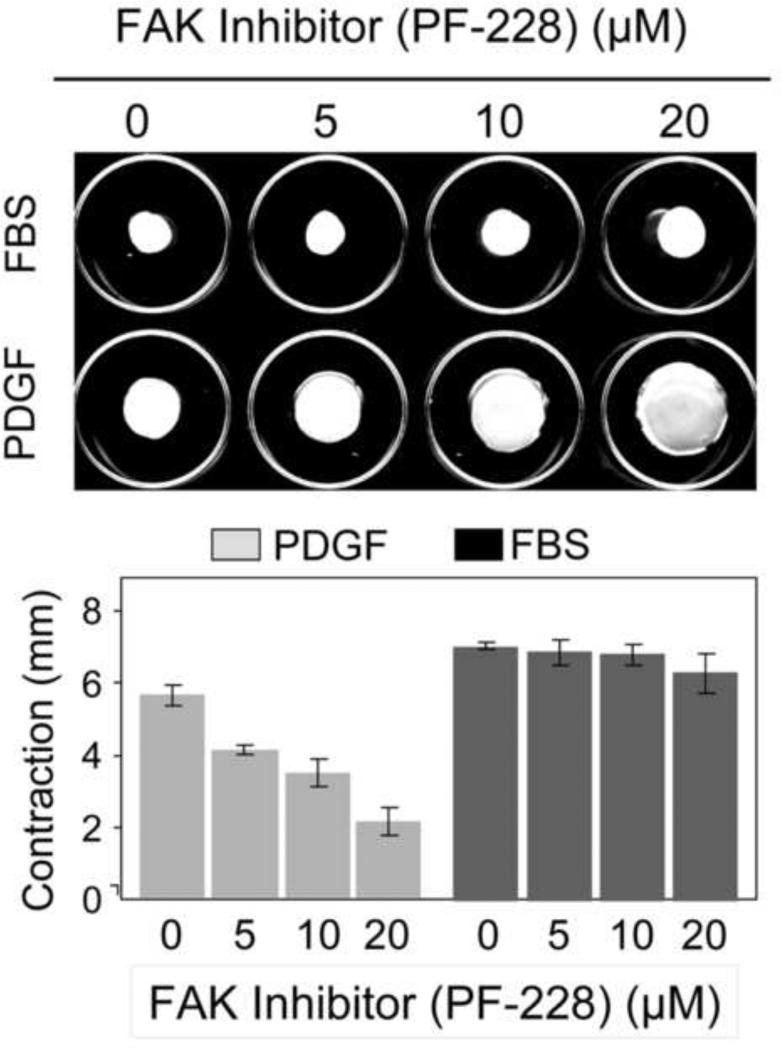
Given the close relationship between myosin II and mechanical tension, the observation that either MyoIIA or MyoIIB was sufficient for PDGF-FMC but that neither was required for FBS-FMC led us to test if there was a growth factor-dependent difference in the role of mechanosensing in FMC. Consistent with this possibility, Figure 5 shows that the focal adhesion kinase inhibitor PF-228 blocked PDGF-FMC but had no effect on FBS-FMC.
Figure 5. Differential effects of FAK inhibitor on fibroblast contraction of floating collagen matrices in PDGF and FBS-containing medium.
Floating collagen matrix contraction was carried out in DMEM/PDGF and DMEM/FBS. FAK inhibitor PF-22 as indicated was added to the incubations at the time the matrices were released from the underlying culture dish to initiate contraction. Data shown are the appearance of collagen matrices from a representative experiment and the averages ± SD of quantitative contraction measurements (starting – final matrix diameter) for triplicate samples. FAK inhibitor PF-228 blocked PDGF-FMC but had no effect on FBS-FMC.
Collagen matrices with surface-seeded fibroblasts -- cell clustering and matrix flattening
The observation that FBS-FMC required neither MyoIIA or MyoIIB or both together was an unusual finding. We studied this observation further in a second, independent experimental model -- morphogenic clustering of cells seeded on the surfaces on collagen matrices [25, 31]. Clustering requires contraction-dependent fibronectin matrix assembly and can be blocked by Rho kinase inhibitor and blebbistatin. In addition to but independent of cell clustering, collagen matrices with surface-seeded fibroblasts undergo matrix flattening as the cells spread, a process that resembles collagen matrix contraction [32, 33]. Therefore, we also tested the effects of silencing MyoIIA and MyoIIB on FBS-stimulated matrix flattening.
Figures 6A and 6B show the appearance and quantification of FBS-stimulated cell clustering detected by distribution of cell nuclei and the effect of increasing concentrations of blebbistatin on clustering. Figure 6C shows matrix flattening in the same incubations, which was measured as decrease in matrix height. The results demonstrated that 2.5 μM blebbistatin inhibited cell clustering, whereas 10 μM blebbistatin was required to block matrix flattening. This difference in sensitivity to inhibition by blebbistatin was similar to the findings for FBS-FMC compared to FBS-SMC (Figure 3).
Figure 6. Differential effects of blebbistatin on fibroblast clustering and contraction of attached collagen matrices.
A,B) Fibroblast clustering on collagen matrices was carried out in DMEM/FBS with blebbistatin added to the incubations as indicated. At the end of the incubations, samples were fixed and stained for cell nuclei (Hoechst). Data shown are the appearance of cell clusters from a representative experiment (Bar = 200 μm) and the averages ± SD of quantitative clustering measurements (cell cluster = ≥10 nucleus in 100 μm2 ) for triplicate samples. C) Using the same samples as in A,B, contraction of attached collagen matrices was determined as the difference in height between control matrices (no cells) and experimental matrices. Data shown are the averages ± SD for triplicate samples. Blebbistatin inhibited cell clustering at 2.5-5 μM, whereas 10-20 μM blebbistatin was required to block matrix flattening.
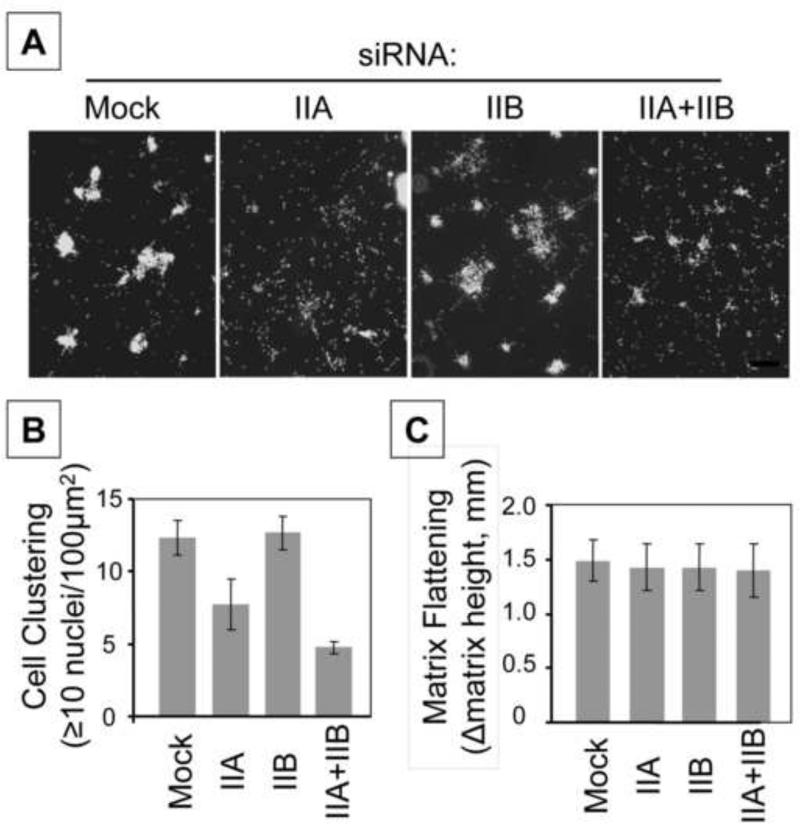
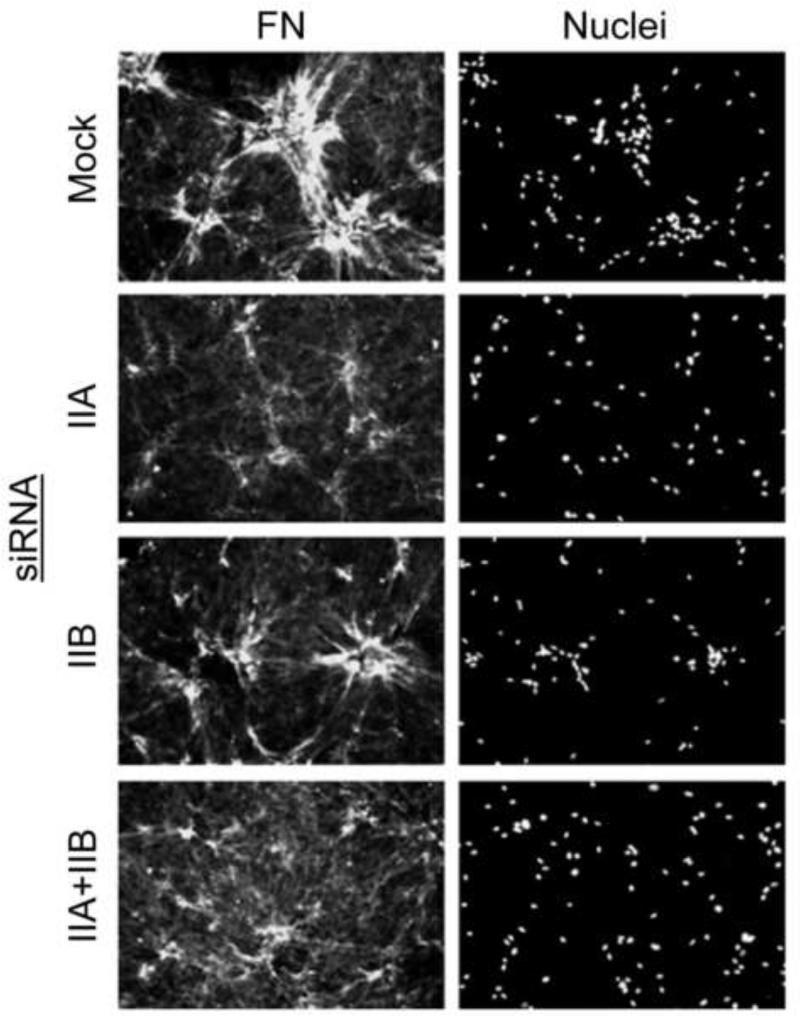
Figures 7 and 8 show parallel experiments testing the effects of siRNA silencing MyoIIA and MyoIIB on cell clustering, FN matrix assembly, and matrix flattening. Silencing MyoIIA but not MyoIIB inhibited cell clustering. However, silencing MyoIIA or MyoIIB or both together had no effect on matrix flattening (Figure 7). Silencing MyoIIA but not MyoIIB also caused a marked change in FN matrix assembly (Figure 8). Therefore, under conditions in which cell contraction-dependent FN matrix assembly was modified and cell clustering was inhibited by silencing MyoIIA, flattening of collagen matrices was unaffected by silencing MyoIIA or MyoIIB or both together.
Figure 7. Silencing MyoIIA blocks cell clustering on collagen matrices not attached gel contraction.
A,B) Cell clustering was carried out with mock and myosin II isoform-silenced cells as indicated. At the end of the incubations, samples were fixed and stained for cell nuclei (Hoechst). Data shown are the appearance of cell clusters from a representative experiment (Bar = 200 mμ) and the averages ± SD of quantitative clustering measurements (cell cluster = ≥10 nucleus in 100 μm2 ) for triplicate samples. C) Using the same samples as in A,B, contraction of attached collagen matrices was determined as the difference in height between control matrices (no cells) and experimental matrices. Data shown are the averages ± SD for triplicate samples. Silencing MyoIIA inhibited cell clustering, whereas silencing MyoIIA or MyoIIB or both together had no effect on matrix flattening.
Figure 8. Silencing MyoIIA modifies fibronectin fibrillar matrix organization.
Samples are the same as in Figure 7 except stained for extracellular fibronectin (FN) and cell nuclei (Hoechst). Silencing MyoIIA but not MyoIIB caused a marked change in FN matrix assembly. Bar = 100 μm.
DISCUSSION
The myosin II inhibitor blebbistatin was reported to inhibit fibroblast contraction of 3D collagen matrices depending on mechanical tension and growth factor environment [22-24]. In the current studies, we tested the effects of siRNA silencing of myosin IIA and myosin IIB to understand better the motor functions of myosin II isoforms in matrix contraction. As summarized in Table I and discussed below, the results suggested three different motor mechanisms: MyoIIA but not MyoIIB was required for stressed matrix contraction; either MyoIIA or MyoIIB was sufficient for PDGF-stimulated floating matrix contraction; neither MyoIIA or MyoIIB appeared to play a role in FBS-stimulated floating matrix contraction.
Table 1.
Summary of myosin II isoform functions in collagen matrix contraction1
| 3D Collagen Matrix Model | Myosin II Isoform | Inhibition by Blebbistatin | Rho Effector |
|---|---|---|---|
| FBS -- Stressed Matrix Contraction | IIA | 2.5 – 5.0 μM | Rho Kinase [22,37] |
| PDGF -- Floating Matrix Contraction | IIA or IIB | 2.5 – 5.0 μM | Rho Kinase [22,26] |
| FBS -- Floating Matrix Contraction | Neither IIA or IIB | 10 – 20 μM | mDial [26] |
| FBS -- Cell Clustering with Cells on Top of Matrix | IIA | 2.5 – 5.0 μM | Rho Kinase [25] |
| FBS -- Matrix Flattening with Cells on Top of Matrix | Neither IIA or IIB | 20 – 40 μM | not tested |
See text for details
Human fibroblasts cultured in collagen matrices initially exhibit diffuse organization of the actin cytoskeleton. Overall cell morphology tends to be rounded (Rho-dependent) in serum-containing medium and dendritic (Rac-dependent) in PDGF-containing medium [27-29]. If the matrices are attached, then as matrix reorganization occurs in serum or PDGF-containing medium, cells develop tension indicated by formation of actin stress fibers and reorganization of the initially punctuate distribution of activated β1 integrins into focal adhesions that also contain vinculin [28].
In the current studies with siRNA-silenced human fibroblasts, we found that MyoIIA but not MyoIIB was required for formation of actin stress fibers and for global inward contractile force that resulted in rounded cell morphology in FBS-containing medium. We cannot exclude the possibility that absence of a MyoIIB effect occurred because of residual MyoIIB remaining after siRNA knockdown. However, others have reported a similar differential role of MyoIIA vs. MyoIIB in stress fiber formation and Rho-dependent rounding of cells interacting with 2D surfaces [23, 34-36].
Previous work established that stressed matrix contraction was a cell contraction process. Upon release of stressed matrices, cell shortening and stress fiber disruption occur [17] dependent on Rho kinase and myosin II [22, 37]. We found that MyoIIA was necessary for stressed matrix contraction. MyoIIA also was shown to be required for contraction of stressed fibrin matrices [35]. MyoIIA and MyoIIB both can contribute to cell tractional force [e.g., 38, 39]. However, differences in localization such as the enrichment of MyoIIA compared to MyoIIB is stress fibers [40] is consistent with the idea that cells can utilize these isoforms to generate force as part of different cellular functions. Examples include the differential role of MyoIIA vs. MyoIIB in stress fiber formation already discussed and the requirement for MyoIIA but not MyoIIB in focal adhesion formation by cells interacting with 2D surfaces [23, 35, 36]. Our findings suggest that collagen matrix contraction provides an additional case in which tractional force generated through different myosin isoforms is utilized differently.
Unlike stressed matrix contraction, floating collagen matrix contraction occurs with cells before tension develops (e.g., absence of stress fibers and focal adhesions). During matrix contraction, increased cell spreading rather than shortening occurs [17], contrary to what one would expect if cell contraction was the mechanism of matrix contraction. We found that either MyoIIA or MyoIIB was sufficient for PDGF-FMC since siRNA silencing of both myosin II isoforms was required to inhibit matrix contraction.
Differences in collagen matrix stiffness may explain the selective requirement for MyoIIA in stressed matrix contraction vs. the ability of MyoIIA or MyoIIB to function in PDGF-FMC. The stiffness of collagen matrices has been measured by many laboratories, typically using shear rheometry or uniaxial testing. Typical stiffness values reported for newly polymerized collagen matrices are 0.5-50 Pa (storage modulus) and 0.5-33 kPa (tensile modulus) [41]. Collagen density is the major determinant of stiffness, and the 1.5 mg/ml matrices that we use measure ~5 Pa (storage modulus) initially but increase to ~700 Pa when the matrices are fully contracted [42]. Therefore, the matrix stiffness encountered by fibroblasts is much higher for stressed matrix contraction (matrices pre-contracted overnight before release from the culture surface) compared to floating matrix contraction (matrices newly polymerized). An attractive hypothesis to explain our findings is that tractional force exerted by cells through myosin IIA is required to remodel stiff matrices, whereas myosin IIA or myosin IIB provide sufficient force to remodel matrices that are soft, at least under PDGF-containing medium conditions. Possible mechanisms of collagen translocation would include rearward force exerted during cell protrusion [43, 44] and/or hand-over-hand cycles of cell protrusion and retraction [45, 46].
Other evidence supports the idea that matrix stiffness affects MyoIIA and MyoIIB differently. In 3D collagen matrices, cells respond to increased stiffness by increasing expression of MyoIIA but not MyoIIB [24]. On soft, polyacrylamide 2D surfaces, MyoIIA but not MyoIIB becomes organized with cellular stress fibers as substrate stiffness increases [47].
In contrast to PDGF-FMC, FBS-FMC appeared to be independent of MyoIIA and MyoIIB since blocking either myosin isoform alone or both together did not inhibit matrix contraction. Other data also shows that PDGF-FMC and FBS-FMC depend on different mechanisms. Rho kinase was reported to be the Rho effector required for PDGF-FMC, whereas mDia1 is the Rho effector required for FBS-FMC [22, 26]. Also, in the current studies we found that the focal adhesion kinase inhibitor blocked PDGF-FMC but not FBS-FMC.
The possibility that fibroblasts might utilize a MyoIIA and MyoIIB-independent motor mechanism to translocate collagen during FBS-FMC was surprising so we tested a second, experimental model -- morphogenic cell clustering, which occurs when fibroblasts are cultured on the surfaces of collagen matrices. Morphogenic cell clustering requires fibronectin matrix assembly [25], which takes place at the cell surface through a cell-contraction dependent mechanism [48-50]. We found that blocking MyoIIA but not MyoIIB prevented fibronectin matrix assembly that was observed during cell clustering but, similar to the findings for FBS-FMC, silencing either MyoIIA or MyoIIB or both together did not inhibit flattening of collagen matrices, a contraction-like process that occurs as fibroblasts surface-seeded on collagen matrices undergo cell spreading [32, 33]. Although others have reported that MyoIIB but not MyoIIA played a role in FBS-FMC by mouse fibroblasts [46], those studies were carried out with lower cell concentrations than we use, conditions that would permit cell spreading to precede matrix contraction.
One might argue theoretically that even less force is required for FBS-FMC compared to PDGF-FMC so that residual MyoIIA and/or MyoIIB remaining after siRNA knockdown would be sufficient to mediate contraction. This interpretation seems unlikely since the concentration of blebbistatin required to inhibit FBS-FMC was much higher than the concentration of blebbistatin required to inhibit PDGF-FMC. A higher blebbistatin concentration also was required to inhibit contraction of attached collagen matrices during cell clustering. Although blebbistatin is specific for myosin II amongst the different types of myosin [51], this drug also has been shown to inhibit some myosin-independent cell motile processes [52]. Therefore, a different motor mechanism besides myosin II might be involved in FBS-FMC. Possibilities worth investigating in the future include a role for kinesin family members that could generate force through microtubule depolymerizaton [53, 54] or a role for a dynein-dependent reward transport mechanism [55, 56]. That microtubules are required for FBS-FMC was shown before [57, 58], but the role of microtubules in matrix contraction has never been clear. Once cell tension develops, disrupting microtubules stimulates rather than inhibits matrix contraction [59].
Ironically, although the motor mechanism remains to be determined, serum-dependent floating matrix contraction is the longest studied and original model introduced to mimic wound contraction in vitro [18]. As described in the Introduction, two different mechanisms of wound closure have been described that occur by different mechanisms depending on mechanical tension within the wound. We suggest that FBS-stressed matrix contraction resembles the myofibroblast/granulation tissue mechanism, which depends on mechanosensing and cell contraction; and that FBS-floating matrix contraction resembles the fibroblast/wound margin mechanism, which occurs independently of mechanosensing and cell contraction.
Supplementary Material
Highlights.
We tested the role of myosin II isoforms in fibroblast interactions with 3D collagen matrices.
MyoIIA was required for stressed matrix contraction and morphogenic cell clustering.
Either MyoIIA or MyoIIB was sufficient for PDGF-floating matrix contraction.
Neither MyoIIA or MyoIIB was necessary for serum-floatin matrix contraction.
Motor mechanisms for collagen contraction depend on cell tension and growth factor stimulation.
ACKNOWLEDGMENTS
We thank Dr. da Rocha-Azevedo for helpful suggestions. This work was supported by NIH grant GM 031321.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR, Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972;135:719–34. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787–96. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross J. Getting to mammalian wound repair and amphibian limb regeneration: a mechanistic link in the early events. Wound Repair Regen. 1996;4:190–202. doi: 10.1046/j.1524-475X.1996.40205.x. [DOI] [PubMed] [Google Scholar]
- 4.Hinz B. The myofibroblast: Paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 6.Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136–43. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 7.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doillon CJ, Hembry RM, Ehrlich HP, Burke JF. Actin filaments in normal dermis and during wound healing. Am J Pathol. 1987;126:164–70. [PMC free article] [PubMed] [Google Scholar]
- 9.Berry DP, Harding KG, Stanton MR, Jasani B, Ehrlich HP. Human wound contraction: collagen organization, fibroblasts, and myofibroblasts. Plast Reconstr Surg. 1998;102:124–31. doi: 10.1097/00006534-199807000-00019. discussion 132-4. [DOI] [PubMed] [Google Scholar]
- 10.Nedelec B, Ghahary A, Scott PG, Tredget EE. Control of wound contraction. Basic and clinical features. Hand Clin. 2000;16:289–302. [PubMed] [Google Scholar]
- 11.Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, Sakakura T, Yoshida T. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin P. Wound healing and inflammation studies in genetically tractable organisms. International Congress Series. 2007;1302:3–16. [Google Scholar]
- 13.Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci U S A. 2001;98:11295–300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 15.Schober M, Raghavan S, Nikolova M, Polak L, Pasolli HA, Beggs HE, Reichardt LF, Fuchs E. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J Cell Biol. 2007;176:667–80. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, Kuang AA, Longaker MT, Gurtner GC. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2011;18:148–52. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–4. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proceedings of the National Academy of Sciences U S A. 1979;76:1274–8. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 2007;17:178–86. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–90. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heissler SM, Manstein DJ. Nonmuscle myosin-2: mix and match. Cell Mol Life Sci. 2013;70:1–21. doi: 10.1007/s00018-012-1002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe M, Ho CH, Kamm KE, Grinnell F. Different molecular motors mediate platelet-derived growth factor and lysophosphatidic acid-stimulated floating collagen matrix contraction. J Biol Chem. 2003;278:47707–12. doi: 10.1074/jbc.M306228200. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, van Grunsven LA, Van Rossen E, Schroyen B, Timmermans JP, Geerts A, Reynaert H. Blebbistatin inhibits contraction and accelerates migration in mouse hepatic stellate cells. Br J Pharmacol. 2010;159:304–15. doi: 10.1111/j.1476-5381.2009.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bond JE, Ho TQ, Selim MA, Hunter CL, Bowers EV, Levinson H. Temporal spatial expression and function of non-muscle myosin II isoforms IIA and IIB in scar remodeling. Lab Invest. 2010;91:499–508. doi: 10.1038/labinvest.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da Rocha-Azevedo B, Ho CH, Grinnell F. Fibroblast cluster formation on 3D collagen matrices requires cell contraction dependent fibronectin matrix organization. Exp Cell Res. 2013;319:546–555. doi: 10.1016/j.yexcr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee S, Grinnell F. P21-activated kinase 1: convergence point in PDGF- and LPA-stimulated collagen matrix contraction by human fibroblasts. J Cell Biol. 2006;172:423–32. doi: 10.1083/jcb.200505175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang H, Rhee S, Ho C-H, Grinnell F. Distinguishing fibroblast promigratory and procontractile growth factor environments in 3D collagen matrices. FASEB J. 2008;22:2151–2160. doi: 10.1096/fj.07-097014. [DOI] [PubMed] [Google Scholar]
- 28.Tamariz E, Grinnell F. Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Mol Biol Cell. 2002;13:3915–3929. doi: 10.1091/mbc.E02-05-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grinnell F, Ho CH, Tamariz E, Lee DJ, Skuta G. Dendritic fibroblasts in three-dimensional collagen matrices. Mol Biol Cell. 2003;14:384–95. doi: 10.1091/mbc.E02-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grinnell F, Ho CH. The effect of growth factor environment on fibroblast morphological response to substrate stiffness. Biomaterials. 2013;34:965–74. doi: 10.1016/j.biomaterials.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sevilla CA, Dalecki D, Hocking DC. Extracellular matrix fibronectin stimulates the self-assembly of microtissues on native collagen gels. Tissue Eng Part A. 2010;16:3805–19. doi: 10.1089/ten.tea.2010.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grinnell F, Lamke CR. Reorganization of hydrated collagen lattices by human skin fibroblasts. J Cell Sci. 1984;66:51–63. doi: 10.1242/jcs.66.1.51. [DOI] [PubMed] [Google Scholar]
- 33.Guidry C, Grinnell F. Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci. 1985;79:67–81. doi: 10.1242/jcs.79.1.67. [DOI] [PubMed] [Google Scholar]
- 34.Sandquist JC, Swenson KI, Demali KA, Burridge K, Means AR. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J Biol Chem. 2006;281:35873–83. doi: 10.1074/jbc.M605343200. [DOI] [PubMed] [Google Scholar]
- 35.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 36.Jorrisch MH, Shih W, Yamada S. Myosin IIA deficient cells migrate efficiently despite reduced traction forces at cell periphery. Biol Open. 2011;2:368–72. doi: 10.1242/bio.20133707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-Promoted Myofibroblast Contraction: Role of Rho, Myosin Light Chain Kinase, and Myosin Light Chain Phosphatase. Exp Cell Res. 2000;254:210–220. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- 38.Lo CM, Buxton DB, Chua GC, Dembo M, Adelstein RS, Wang YL. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol Biol Cell. 2004;15:982–9. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai Y, Biais N, Giannone G, Tanase M, Jiang G, Hofman JM, Wiggins CH, Silberzan P, Buguin A, Ladoux B, Sheetz MP. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys J. 2006;91:3907–20. doi: 10.1529/biophysj.106.084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saitoh T, Takemura S, Ueda K, Hosoya H, Nagayama M, Haga H, Kawabata K, Yamagishi A, Takahashi M. Differential localization of non-muscle myosin II isoforms and phosphorylated regulatory light chains in human MRC-5 fibroblasts. FEBS Lett. 2001;509:365–9. doi: 10.1016/s0014-5793(01)03186-6. [DOI] [PubMed] [Google Scholar]
- 41.Billiar KL. In: The mechanical environment of cells in collagen gel models. In “Cellular and Biomolecular Mechanics and Mechanobiology”. Gefen A, editor. Springer; Berlin: 2011. [Google Scholar]
- 42.Miron-Mendoza M, Seemann J, Grinnell F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 2010;31:6425–35. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy P, Petroll WM, Cavanagh HD, Chuong CJ, Jester JV. An in vitro force measurement assay to study the early mechanical interaction between corneal fibroblasts and collagen matrix. Exp Cell Res. 1997;232:106–17. doi: 10.1006/excr.1997.3511. [DOI] [PubMed] [Google Scholar]
- 44.Petroll WM, Ma L, Kim A, Ly L, Vishwanath M. Dynamic assessment of fibroblast mechanical activity during Rac-induced cell spreading in 3-D culture. J Cell Physiol. 2008;217:162–171. doi: 10.1002/jcp.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glaser BM, Cardin A, Biscoe B. Proliferative vitreoretinopathy. The mechanism of development of vitreoretinal traction. Ophthalmology. 1987;94:327–32. doi: 10.1016/s0161-6420(87)33443-8. [DOI] [PubMed] [Google Scholar]
- 46.Meshel AS, Wei Q, Adelstein RS, Sheetz MP. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol. 2005;7:157–64. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- 47.Raab M, Swift J, Dingal PC, Shah P, Shin JW, Discher DE. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J Cell Biol. 2012;199:669–83. doi: 10.1083/jcb.201205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8:1415–25. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–51. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoneda A, Ushakov D, Multhaupt HA, Couchman JR. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol Biol Cell. 2007;18:66–75. doi: 10.1091/mbc.E06-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25:337–41. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 52.Shu S, Liu X, Korn ED. Blebbistatin and blebbistatin-inactivated myosin II inhibit myosin II-independent processes in Dictyostelium. Proc Natl Acad Sci U S A. 2005;102:1472–7. doi: 10.1073/pnas.0409528102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wordeman L. Microtubule-depolymerizing kinesins. Curr Opin Cell Biol. 2005;17:82–8. doi: 10.1016/j.ceb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Daire V, Pous C. Kinesins and protein kinases: key players in the regulation of microtubule dynamics and organization. Arch Biochem Biophys. 2011;510:83–92. doi: 10.1016/j.abb.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Dujardin DL, Barnhart LE, Stehman SA, Gomes ER, Gundersen GG, Vallee RB. A role for cytoplasmic dynein and LIS1 in directed cell movement. J Cell Biol. 2003;163:1205–11. doi: 10.1083/jcb.200310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10:854–65. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellows CG, Melcher AH, Aubin JE. Association between tension and orientation of periodontal ligament fibroblasts and exogenous collagen fibers in collagen gels in vitro. J. Cell Sci. 1982;58:125–38. doi: 10.1242/jcs.58.1.125. [DOI] [PubMed] [Google Scholar]
- 58.Ehrlich HP, Buttle DJ, Bernanke DH. Physiological variables affecting collagen lattice contraction by human dermal fibroblasts. Exp Mol Pathol. 1989;50:220–9. doi: 10.1016/0014-4800(89)90033-6. [DOI] [PubMed] [Google Scholar]
- 59.Lee DJ, Ho CH, Grinnell F. LPA-stimulated fibroblast contraction of floating collagen matrices does not require Rho kinase activity or retraction of fibroblast extensions. Exp Cell Res. 2003;289:86–94. doi: 10.1016/s0014-4827(03)00254-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.