Abstract
Osteoporosis has been recognised as a public health concern for at least three decades but it has been relatively recent that the push has been for orthopaedic surgeons to take a more active role in the diagnosis and treatment of patients with decreased bone mineral density (BMD). Most often these patients are encountered after they have suffered a fracture making secondary prevention the area where orthopaedists may exert the greatest influence on patient care. The purpose of this article is to provide a succinct framework for the diagnosis and treatment of patients with decreased BMD. Patients are deemed to have decreased BMD if they have suffered a fragility fracture, a fracture caused by a low-energy traumatic event. These patients are often encountered in the emergency department and admitted for further treatment of their fractures or recommended for follow-up in the clinic. Regardless of treatment course these are opportunities for the orthopaedic surgeon to intervene in the osteoporotic disease process and positively affect a patient’s bone health. This article compiles the available literature on osteoporosis and presents it succinctly with the incorporation of both a diagnosis algorithm and treatment profile table. With the use of these two tools, orthopaedic surgeons everywhere should be able to take a more active role in their patients’ bone health.
Keywords: Osteoporosis, Orthopaedic surgeon, Early management, Screening, Fragility fracture
Introduction
Osteoporosis, defined as the breakdown in the micro-architectural structure of bone due to decreased bone mineral density (BMD), has become a significant public health issue that is approaching epidemic proportions in both developed and developing countries [1]. The US Surgeon General recognised this area of increasing morbidity as being particularly important within the USA due to the increasing population of senescent individuals. In 2004 the US Surgeon General issued a report to bring awareness to this situation. Part of the report detailed a lack of cooperation and communication between primary care physicians (PCP) and orthopaedic surgeons who were often the first health care provider to have objective evidence of a specific patient’s bone health following a low-energy fracture, termed a fragility fracture [2]. The American Orthopaedic Association (AOA) took up this charge in 2005 with the “Own the Bone” project. The goals of the project were to assess current practices within the orthopaedic community in preventing further low-energy fractures, to implement quality improvement measures and to assess what barriers might exist for the expansion of the project [3].
Many studies have highlighted that a previous fracture is highly correlated with an increased risk for a future fracture [4, 5]. As such it becomes important for orthopaedic surgeons to recognise that a patient with a low-energy fracture has poor bone stock and also to be proactive in the prevention of future fractures in the treatment of that patient [6]. To that end the purpose of this paper is to provide a basic framework for the work up of a patient with a primary fragility fracture and also to provide a treatment algorithm for easy reference (Fig. 1).
Fig. 1.

Work up of a patient with a primary fragility fracture and treatment algorithm
Background
Osteoporosis is characterised by the breakdown in the micro-architectural structure of bone leading to decreased bone mass and subsequently to increased bone fragility and fracture risk [7, 8]. The decreased BMD results in bones that are more brittle and are consequently more easily broken when subjected to deforming forces that would not injure a person with normal BMD. Fractures that are attributable to osteoporotic bone stock are termed fragility fractures and are associated with increased morbidity and mortality [9, 10]. There tends to be some debate surrounding exactly which fractures are to be included in the fragility fracture definition, with some suggesting that all fractures resulting from low-energy mechanisms should be included, while others feel that only certain fractures should be included in the definition [8, 11]. For the purposes of this review all fractures that result from a low-energy traumatic event will be included regardless of age, gender and race. Though this may lead to diagnostic testing of individuals who do not have decreased BMD, it should ensure that patients who do have decreased BMD are not missed.
Detecting patients with low BMD is vitally important due to the increased risk of a secondary fracture. Patients who have suffered a fragility fracture are almost twice as likely to suffer another fragility fracture as their age-matched peers who have not had a fragility fracture [4, 12]. Though all fractures have some degree of morbidity and mortality, the overarching concern with a second fracture in an osteoporotic patient is that the patient will suffer a hip fracture. Hip fractures are the most devastating consequence of osteoporosis, with 25 % of patients having to spend time in a nursing home following the fracture and 50 % of patients never reaching their pre-fracture activity levels. The mortality rate following a hip fracture is 25 % in the first year with men almost twice as likely to die as women [2]. It has been shown that close to 50 % of patients who have suffered a hip fracture had a previous fragility fracture suggesting that there is an opportunity for early diagnosis and treatment [13].
In addition to the mortality associated with a fragility fracture, the financial costs of treatment and rehabilitation continue to increase. Multiple estimates have suggested that the annual cost hovers around 18–20 billion dollars annually in the USA alone and is only going to increase [12]. Despite its increasing financial costs and dramatic consequences, the recognition, diagnosis and treatment of osteoporosis continues to be an area needing further improvement and education within the health care community [3, 14–17]. It is unacceptable for close to 80 % of patients with a hip fracture to never have received medication to treat their pre-fracture osteoporosis, particularly given that osteoporosis treatment decreases the rate of fragility fractures and mortality by up to 30 % [18–21]. The presence of fragility fractures should lead to the consideration of treatment or at least exploration of bone metabolism. The orthopaedic surgeon is routinely involved in cases of fragility fractures, and many studies have shown that early osteoporosis screening by the orthopaedic surgeon, as part of the treatment plan, leads to better disease management [3, 22–25].
Who are the osteoporotic patients and what can I do for them?
Orthopaedic surgeons are typically only involved in the osteoporotic patient’s care as a consequence of a fracture and with the single biggest risk factor for a future fracture being a previous fragility fracture, it therefore follows that the area of focus for the orthopaedist should be on the secondary prevention of future fractures [4, 26, 27]. Though there has been a historical reluctance to get involved in the osteoporosis treatment of patients seen with fragility fractures that reticence has changed [28]. With the instigation of the Own the Bone program by the AOA, the idea of the orthopaedist being a key component in the care of a patient’s bone health, beyond the acute fracture care, has gained a great deal of traction [16]. Due to this interest there has been a great deal of research undertaken regarding which patients can most benefit from the interventions of the orthopaedic surgeon in the acute phase of fracture care and how the surgeon can effect a positive change in BMD.
As previously stated there should be a high degree of suspicion for low BMD in any patient that suffers a fracture from a low-energy mechanism. Though there is debate about which fractures should be considered fragility type fractures, we advocate for assuming all fractures should be considered fragility fractures, except for fractures of the facial bones, skull, feet, hands, digits and patella [8]. Areas of fracture outside of the above exceptions have been correlated with decreased BMD and therefore are deemed fragility fractures [29]. These patients should all be considered osteoporotic until proven otherwise by diagnostic testing.
Interestingly enough, one of the fractures that are the most common in the osteoporotic individual is also the most often missed: vertebral body fractures. They are most often missed due to a lack of inclusion in the differential diagnosis of patients with back pain and are thus overlooked [30]. A vertebral body fracture should be suspected in any patient at risk for osteoporosis with back pain or kyphosis. Due to their potentially asymptomatic presentation, the National Osteoporosis Foundation recommends screening by vertebral imaging of the following patients:
Women >70 years old and men >80 years old
BMD measurements compatible with osteopenia in women aged 65–69 and men aged 75–79
In postmenopausal women aged 50–64 and men aged 50–69 with a low-energy traumatic vertebral body fracture, actual height loss (prospective 0.8 inches or historical of 1.5 inches) or corticosteroid use [31]
Though the full work up of patients with presumed decreased BMD should include tests designed to tease out if their decreased BMD is due to a primary or secondary pathology, this work up is best left for a patient’s PCP, geriatrician or endocrinologist. Areas where orthopaedic surgeons can be most effective are in the initial consultation with the patient where a conversation takes place regarding the patient’s modifiable and non-modifiable risk factors (Fig. 2), the initiation of pharmacological treatment if appropriate (Fig. 3) and obtaining or recommending a bone mineral densitometric analysis using dual-energy X-ray absorptiometry (DXA) [14, 23]. In addition to diagnostic tests, the FRAX tool can also be used to assess a patient’s ten year risk of fracture based on lab values and DXA scores if available [32]. Studies have demonstrated that with well-developed communication systems between the orthopaedic surgeon and the PCP of patients where the results of the above-outlined tests can be communicated there is increased BMD assessment using DXA scans, increased pharmacological treatment and decreased secondary fractures [12, 20, 33].
Fig. 2.
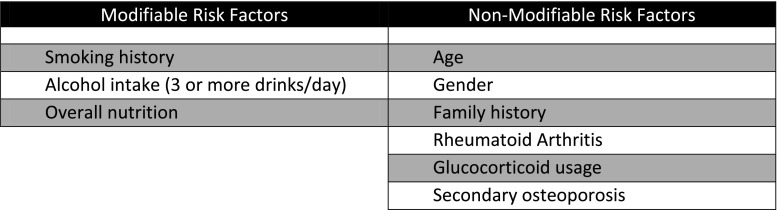
Modifiable and non-modifiable risk factors
Fig. 3.

Pharmacological treatment
The osteoporotic patient in my clinic…who should I call?
Osteoporosis diagnosis and treatment requires a multidisciplinary approach. The management of this condition highlights the patient-centred medical home concept. Part of the Own the Bone concept was to impassion orthopaedic surgeons to make systematic changes in their practices to facilitate a more open flow of communication with a patient’s primary care provider [16]. Studies in many different countries have demonstrated that with increased communication between the orthopaedist, patient and patient’s PCP there is increased usage of pharmacotherapeutics, calcium and vitamin D supplementation, and BMD assessment with DXA scan [34]. There is also good evidence that the use of calcium, vitamin D and pharmaceutical interventions results in a decreased risk of fragility fractures [31, 35].
A concept that has been implemented in a few health care systems in the USA and has shown good results in increasing communication between the orthopaedist and a patient’s PCP is the Fracture Liaison Service (FLS) [36, 37]. The FLS functions by identifying patients who have suffered fragility fractures in the emergency department, orthopaedics ward and outpatient clinics. The service can then ensure patients are appropriately diagnosed as being osteoporotic or at risk of osteoporosis. The diagnosis can then facilitate further assessment of bone density and ensure proper treatment has been initiated. The FLS can also coordinate the communication between the orthopaedist and the PCP and make sure that all information is passed on to the PCP [38].
Early interventions that can be driven by the orthopaedist
Besides pharmacological treatments, there are also certain general interventions listed below that can be recommended to most patients to reduce fracture risk.
Ensure adequate calcium and vitamin D intake
Decrease alcohol, caffeine and tobacco consumption
Encourage regular physical activity [39]
Fall prevention, with a home safety evaluation, if indicated [31, 40]
Recommending the use of calcium and vitamin D supplementation is an easy and relatively inexpensive intervention that can be applied to the vast majority of people encountered after a fragility fracture. There are good meta-analyses on the fracture risk reduction of calcium and vitamin D supplementation, with the corollary that the benefit might be seen more in institutionalised individuals more so than in community-dwelling individuals [41–43]. Although there is some controversy regarding calcium and vitamin D supplementation [44], there are many clinical representative bodies that support the use of calcium and vitamin D for fracture prevention including the National Osteoporosis Foundation, the Institute of Medicine and the American Association of Clinical Endocrinologists [31, 45, 46]. Figure 4 outlines the current dietary recommendations for both calcium and vitamin D taken from the above references.
Fig. 4.
Current dietary recommendations for calcium and vitamin D
Excessive alcohol or tobacco consumption also needs to be addressed, and if needed a referral to a substance abuse specialist should be made [31]. It has long been thought that alcohol in any amount has a negative effect on bone health, but researchers are now concluding that alcohol consumption in excess of three average drinks per day does indeed have a deleterious effect on BMD but low to moderate alcohol consumption, one to three drinks per week, in fact seems to be beneficial for bone health [47]. Smoking on the other hand should be avoided altogether [48].
Weight-bearing exercise should also be encouraged. Two meta-analyses have shown improvements in the BMD at the lumbar spine and femoral neck in postmenopausal women involved in weight-bearing exercises [49, 50]. The American College of Sports Medicine recommends weight-bearing exercise three to five times a week and resistance exercise two to three times a week for 30–60 minutes to improve bone health [51]. These guidelines may seem to be unrealistic, particularly for a busy populace, but any type of physical activity needs to be encouraged [52]. At a minimum, a daily 30- to 40 minutes walk as suggested by the American Association of Clinical Endocrinologists should certainly be encouraged [45]. In addition to its positive effects on BMD, physical activity may also show a benefit in fall prevention. A recent Cochrane Review looked at exercise as part of a fall prevention strategy and the authors’ conclusions were that exercises that incorporated multiple different types of exercises were successful at reducing the fall risk in both individuals who were classified as “high-risk” and “low-risk” for falls [53]. And with the best predictor of future falls being previous falls [27, 54], it is of paramount importance to halt the fall cycle as early as possible with referrals to physical therapy and occupational therapy for general physical exercise recommendations and also for home safety evaluations. With the close relationship between orthopaedic surgeons and the physical and occupational therapists, it should be relatively effortless to provide this referral while seeing patients with fragility fractures either in the clinical setting or in the emergency department.
What happens to patients that I refer for evaluation?
Though orthopaedic surgeons are willing and interested in being an integral part of the diagnosis and initial treatment of patients with decreased BMD, previous studies have demonstrated that the gap in the treatment of osteoporotic individuals occurs between the orthopaedist and the patient’s primary care provider [33, 55, 56]. In an effort to rectify this situation, the FLS has been proposed to function as the clearing house and manager for a patient’s BMD work up and treatment [34, 57]. This concept has been in place for many years in various countries throughout the world and with continued analysis has become more efficacious with time and experience. When the FLS is designed such that there is an independent practitioner or team of practitioners that function to identify, assist with assessment and coordinate information and treatment with a patient’s PCP it has shown excellent success at reducing the rates of a second fracture in patients [58–60].
Once patients have been referred to the FLS or other similar service, the FLS can then focus on this area of patient treatment. This can be particularly useful for patients who are encountered in the emergency department or in the clinic who will need specific follow-up and who might not have the opportunity acutely to obtain a DXA scan and the other recommended blood work.
Another area to consider with regards to the FLS is the push towards quality outcomes measurement in the USA. The Centers for Medicare & Medicaid Services (CMS) uses the Physician Quality Reporting System (PQRS) to measure and track the diagnosis and treatment of osteoporosis in patients. The PQRS currently assesses the appropriate treatment of osteoporotic individuals, among other measures, and passes that information on to CMS to use as a determination of health care reimbursement rates [61]. It has also been suggested that soon the Joint Commission will be using similar quality measures during its assessment of hospitals for accreditation [23].
ABC’s of pharmacological treatment
In addition to a diagnostic work up of patients with fragility fractures, studies have also demonstrated decreased rates of secondary fractures when osteoporosis treatment is initiated at the time of the initial fracture [31, 62]. There also appears to be better long-term compliance with medication administration when the orthopaedic surgeon or FLS prescribe the medication. As part of the treatment of osteoporosis, communication with the patient’s PCP should be carried out to not only inform the provider of the new medication but also how to assess the efficacy of the medication and what the follow-up should be [38].
Currently it is recommended by most clinical organisations dealing with osteoporosis that patients be treated subsequent to their fracture [31, 45]. There are many different drugs for the treatment of osteoporosis and often it can be confusing when and for whom a specific drug should be used. A brief description of the different categories is presented below with more in-depth treatment parameters presented in Fig. 3 [31].
- Bisphosphonates
Bind to calcium and are then resorbed by osteoclasts during bone remodelling and cause osteoclast cell death which results in decreased resorption of bone.
- Calcitonin
Causes decreased resorption of bone by osteoclasts.
- Oestrogen agonist/antagonists
The only US Food and Drug Administration (FDA) approved drug in this class is raloxifene, which is an oestrogen receptor agonist in bone which causes decreased bone resorption by osteoclasts.
- Teriparatide
This is recombinant human parathyroid hormone and is an anabolic agent that causes an increase in BMD through greater absorption of calcium and stimulating osteoblastic activity.
- Receptor activator of nuclear factor kappa-B (RANK) ligand inhibitor
This drug is a monoclonal antibody with activity against RANK ligand. RANK ligand causes increased osteoclast activity; by binding the ligand with an antibody, osteoclastic activity is decreased.
Conclusion
With the 2004 position paper from the US Surgeon General regarding fragility fractures, there has been a great deal of interest generated in osteoporotic fractures, also termed fragility fractures. In general, patients who suffered a fracture from a low-energy mechanism that would not cause a fracture in a normal person should be considered osteoporotic until proven otherwise. With the suspicion of osteoporosis in a patient, it now behoves the orthopaedic surgeon to initiate a diagnostic work up and initiate a referral to either the FLS or analogous position if available or generate a letter to the patient’s primary care provider that provides the results of the diagnostic testing performed while the patient was under the care of the orthopaedic service. This testing is spelled out in the algorithm presented in Fig. 1. Once testing has been accomplished, immediate treatment for the patient should be initiated with calcium, vitamin D and depending on the patient often a bisphosphonate or other anti-resorptive pharmaceutical treatment. The treatment options currently available are listed in Fig. 3 including individual drug indications. With these tools readily at hand, diagnosis and treatment of osteoporotic patients should be more straightforward and efficacious.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Office of the Surgeon General (2004) Bone health and osteoporosis: a report of the Surgeon General [PubMed]
- 3.Tosi LL, Gliklich R, Kannan K, Koval KJ. The American Orthopaedic Association's “own the bone” initiative to prevent secondary fractures. J Bone Joint Surg Am. 2008;90(1):163–173. doi: 10.2106/JBJS.G.00682. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35(2):375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Wustrack R, Seeman E, Bucci-Rechtweg C, et al. Predictors of new and severe vertebral fractures: results from the HORIZON Pivotal Fracture Trial. Osteoporos Int. 2012;23(1):53–58. doi: 10.1007/s00198-011-1664-4. [DOI] [PubMed] [Google Scholar]
- 6.Sorbi R, Aghamirsalim MR. Knowledge of orthopaedic surgeons in managing patients with fragility fracture. Int Orthop. 2012;36(6):1275–1279. doi: 10.1007/s00264-012-1482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.No authors listed (1993) Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94 (6):646–650 [DOI] [PubMed]
- 8.Ström O, Borgström F, Kanis JA, et al. Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2011;6(1–2):59–155. doi: 10.1007/s11657-011-0060-1. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamsen B, van Staa T, Ariely R, et al. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20(10):1633–1650. doi: 10.1007/s00198-009-0920-3. [DOI] [PubMed] [Google Scholar]
- 10.Bass E, French DD, Bradham DD, Rubenstein LZ. Risk-adjusted mortality rates of elderly veterans with hip fractures. Ann Epidemiol. 2007;17(7):514–519. doi: 10.1016/j.annepidem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ, 3rd, Thamer M, Ray NF, et al. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12(1):16–23. doi: 10.1359/jbmr.1997.12.1.16. [DOI] [PubMed] [Google Scholar]
- 12.Akesson K, Marsh D, Mitchell PJ, et al. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int. 2013;24(8):2135–2152. doi: 10.1007/s00198-013-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards BJ, Bunta AD, Simonelli C, et al. Prior fractures are common in patients with subsequent hip fractures. Clin Orthop Relat Res. 2007;461:226–230. doi: 10.1097/BLO.0b013e3180534269. [DOI] [PubMed] [Google Scholar]
- 14.Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am. 2008;90(Suppl 4):188–194. doi: 10.2106/JBJS.H.00628. [DOI] [PubMed] [Google Scholar]
- 15.Giangregorio L, Papaioannou A, Cranney A, et al. Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum. 2006;35(5):293–305. doi: 10.1016/j.semarthrit.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 16.American Orthopaedic Association (2005) Leadership in orthopaedics: taking a stand to own the bone. American Orthopaedic Association position paper. J Bone Joint Surg Am 87 (6):1389–1391 [DOI] [PubMed]
- 17.Borovecki F, Pecina-Slaus N, Vukicevic S. Biological mechanisms of bone and cartilage remodelling–genomic perspective. Int Orthop. 2007;31(6):799–805. doi: 10.1007/s00264-007-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolland MJ, Grey AB, Gamble GD, Reid IR. Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab. 2010;95(3):1174–1181. doi: 10.1210/jc.2009-0852. [DOI] [PubMed] [Google Scholar]
- 19.Grey A, Bolland MJ. The effect of treatments for osteoporosis on mortality. Osteoporos Int. 2013;24(1):1–6. doi: 10.1007/s00198-012-2176-6. [DOI] [PubMed] [Google Scholar]
- 20.Dell RM, Greene D, Anderson D, Williams K. Osteoporosis disease management: what every orthopaedic surgeon should know. J Bone Joint Surg Am. 2009;91(Suppl 6):79–86. doi: 10.2106/JBJS.I.00521. [DOI] [PubMed] [Google Scholar]
- 21.Herrera A, Martinez AA, Ferrandez L, et al. Epidemiology of osteoporotic hip fractures in Spain. Int Orthop. 2006;30(1):11–14. doi: 10.1007/s00264-005-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogoch ER, Elliot-Gibson V, Beaton DE, et al. Effective initiation of osteoporosis diagnosis and treatment for patients with a fragility fracture in an orthopaedic environment. J Bone Joint Surg Am. 2006;88(1):25–34. doi: 10.2106/JBJS.E.00198. [DOI] [PubMed] [Google Scholar]
- 23.Edwards BJ, Koval K, Bunta AD, et al. Addressing secondary prevention of osteoporosis in fracture care: follow-up to “own the bone”. J Bone Joint Surg Am. 2011;93(15):e87. doi: 10.2106/JBJS.I.00540. [DOI] [PubMed] [Google Scholar]
- 24.Queally JM, Kiernan C, Shaikh M, et al. Initiation of osteoporosis assessment in the fracture clinic results in improved osteoporosis management: a randomised controlled trial. Osteoporos Int. 2013;24(3):1089–1094. doi: 10.1007/s00198-012-2238-9. [DOI] [PubMed] [Google Scholar]
- 25.Gaboury I, Corriveau H, Boire G, et al. Partnership for fragility bone fracture care provision and prevention program (P4Bones): study protocol for a secondary fracture prevention pragmatic controlled trial. Implement Sci. 2013;8:10. doi: 10.1186/1748-5908-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Helden S, Cals J, Kessels F, et al. Risk of new clinical fractures within 2 years following a fracture. Osteoporos Int. 2006;17(3):348–354. doi: 10.1007/s00198-005-2026-x. [DOI] [PubMed] [Google Scholar]
- 27.Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA. 2007;297(4):387–394. doi: 10.1001/jama.297.4.387. [DOI] [PubMed] [Google Scholar]
- 28.Skedros JG, Holyoak JD, Pitts TC. Knowledge and opinions of orthopaedic surgeons concerning medical evaluation and treatment of patients with osteoporotic fracture. J Bone Joint Surg Am. 2006;88(1):18–24. doi: 10.2106/JBJS.D.02949. [DOI] [PubMed] [Google Scholar]
- 29.Delmas PD, Marin F, Marcus R, et al. Beyond hip: importance of other nonspinal fractures. Am J Med. 2007;120(5):381–387. doi: 10.1016/j.amjmed.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Lems WF. Clinical relevance of vertebral fractures. Ann Rheum Dis. 2007;66(1):2–4. doi: 10.1136/ard.2006.058313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Osteoporosis Foundation (2013) Clinician’s guide to prevention and treatment of osteoporosis. Washington: National Osteoporosis Foundation. http://www.nof.org/hcp/clinicians-guide. 2013
- 32.Kanis JA (2014) FRAX WHO fracture risk assessment tool. http://www.shef.ac.uk/FRAX/
- 33.Mitchell PJ. Best practices in secondary fracture prevention: fracture liaison services. Curr Osteoporos Rep. 2013;11(1):52–60. doi: 10.1007/s11914-012-0130-3. [DOI] [PubMed] [Google Scholar]
- 34.Ganda K, Puech M, Chen JS, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int. 2013;24(2):393–406. doi: 10.1007/s00198-012-2090-y. [DOI] [PubMed] [Google Scholar]
- 35.Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85(11):4118–4124. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- 36.Dell R. Fracture prevention in Kaiser Permanente Southern California. Osteoporos Int. 2011;22(Suppl 3):457–460. doi: 10.1007/s00198-011-1712-0. [DOI] [PubMed] [Google Scholar]
- 37.Newman ED. Perspectives on pre-fracture intervention strategies: the Geisinger Health System Osteoporosis Program. Osteoporos Int. 2011;22(Suppl 3):451–455. doi: 10.1007/s00198-011-1695-x. [DOI] [PubMed] [Google Scholar]
- 38.Curtis JR, Silverman SL. Commentary: the five Ws of a fracture liaison service: why, who, what, where, and how? In osteoporosis, we reap what we sow. Curr Osteoporos Rep. 2013;11(4):365–368. doi: 10.1007/s11914-013-0177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White MK, Martin RB, Yeater RA, et al. The effects of exercise on the bones of postmenopausal women. Int Orthop. 1984;7(4):209–214. doi: 10.1007/BF00266829. [DOI] [PubMed] [Google Scholar]
- 40.Nachtigall MJ, Nazem TG, Nachtigall RH, Goldstein SR. Osteoporosis risk factors and early life-style modifications to decrease disease burden in women. Clin Obstet Gynecol. 2013;56(4):650–653. doi: 10.1097/GRF.0b013e3182aa1daf. [DOI] [PubMed] [Google Scholar]
- 41.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 42.Chung M, Lee J, Terasawa T, et al. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(12):827–838. doi: 10.7326/0003-4819-155-12-201112200-00005. [DOI] [PubMed] [Google Scholar]
- 43.Bauer DC. Clinical practice. Calcium supplements and fracture prevention. N Engl J Med. 2013;369(16):1537–1543. doi: 10.1056/NEJMcp1210380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moyer VA, U.S. Preventive Services Task Force Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158(9):691–696. doi: 10.7326/0003-4819-158-9-201305070-00603. [DOI] [PubMed] [Google Scholar]
- 45.Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Suppl 3):1–37. doi: 10.4158/EP.16.S3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross AC, Taylor CL, Yaktine AL, et al., editors. Dietary reference intakes for calcium and vitamin D. Washington: National Academies Press (US) National Academy of Sciences; 2011. The National Academies collection: reports funded by National Institutes of Health. [PubMed] [Google Scholar]
- 47.Sommer I, Erkkilä AT, Järvinen R, et al. Alcohol consumption and bone mineral density in elderly women. Public Health Nutr. 2013;16(4):704–712. doi: 10.1017/S136898001200331X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward KD, Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int. 2001;68(5):259–270. doi: 10.1007/BF02390832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelley GA, Kelley KS, Tran ZV. Exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis of individual patient data. J Gerontol A Biol Sci Med Sci. 2002;57(9):M599–M604. doi: 10.1093/gerona/57.9.M599. [DOI] [PubMed] [Google Scholar]
- 50.Kelley GA, Kelley KS, Kohrt WM. Effects of ground and joint reaction force exercise on lumbar spine and femoral neck bone mineral density in postmenopausal women: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2012;13:177. doi: 10.1186/1471-2474-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohrt WM, Bloomfield SA, Little KD, et al. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36(11):1985–1996. doi: 10.1249/01.MSS.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 52.Henderson SA, Graham HK, Mollan RA, et al. Calcium homeostasis and exercise. Int Orthop. 1989;13(1):69–73. doi: 10.1007/BF00266727. [DOI] [PubMed] [Google Scholar]
- 53.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergland A, Jarnlo GB, Laake K. Predictors of falls in the elderly by location. Aging Clin Exp Res. 2003;15(1):43–50. doi: 10.1007/BF03324479. [DOI] [PubMed] [Google Scholar]
- 55.Leslie WD, Giangregorio LM, Yogendran M, et al. A population-based analysis of the post-fracture care gap 1996–2008: the situation is not improving. Osteoporos Int. 2012;23(5):1623–1629. doi: 10.1007/s00198-011-1630-1. [DOI] [PubMed] [Google Scholar]
- 56.Giammattei F, Giammattei J, Howland V. Physician follow-up care for osteoporosis after fragility fractures. Phys Sportsmed. 2009;37(4):62–67. doi: 10.3810/psm.2009.12.1743. [DOI] [PubMed] [Google Scholar]
- 57.Marsh D, Akesson K, Beaton DE, et al. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int. 2011;22(7):2051–2065. doi: 10.1007/s00198-011-1642-x. [DOI] [PubMed] [Google Scholar]
- 58.Cooper MS, Palmer AJ, Seibel MJ. Cost-effectiveness of the Concord Minimal Trauma Fracture Liaison service, a prospective, controlled fracture prevention study. Osteoporos Int. 2012;23(1):97–107. doi: 10.1007/s00198-011-1802-z. [DOI] [PubMed] [Google Scholar]
- 59.McLellan AR, Wolowacz SE, Zimovetz EA, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int. 2011;22(7):2083–2098. doi: 10.1007/s00198-011-1534-0. [DOI] [PubMed] [Google Scholar]
- 60.Sander B, Elliot-Gibson V, Beaton DE, et al. A coordinator program in post-fracture osteoporosis management improves outcomes and saves costs. J Bone Joint Surg Am. 2008;90(6):1197–1205. doi: 10.2106/JBJS.G.00980. [DOI] [PubMed] [Google Scholar]
- 61.Centers for Medicare & Medicaid Services (2014) MEDICARE “PAY FOR PERFORMANCE (P4P)” INITIATIVES. http://www.cms.gov/apps/media/press/release.asp?counter=1343
- 62.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–1541. doi: 10.1016/S0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]



