Abstract
PURPOSE:
To report the effect of intravitreal bevacizumab (Avastin; Genentech Inc, South San Francisco, California, USA) on visual acuity and macular thickness in patients with inflammatory choroidal neovascularization (CNV) or cystoid macular edema (CME).
DESIGN:
Retrospective, noncomparative, interventional case series.
METHODS:
Each eye received 1.25 mg of intravitreal bevacizumab at baseline. Follow-up examinations were scheduled at 1- to 2-month intervals, with additional injections at the discretion of the physician. Comprehensive evaluations, including Snellen best-corrected visual acuity (BVCA) and optical coherence tomography measurements, were performed at each visit. Main outcome measures were BCVA and central subfield thickness (CST), as measured by optical coherence tomography.
RESULTS:
Thirty-four eyes of 30 patients with inflammatory CNV (n = 21 eyes of 19 patients; 9 male, 10 female) or CME (n = 13 eyes of 11 patients; 4 male, 7 female) were identified. Median ages were 52 years (range, 7 to 83) and 67 years (range, 17 to 83) for the CNV and CME groups, respectively. The median length of follow-up for CNV eyes was 7 months (range, 1 to 28) while the median follow-up for CME eyes was 13 months (range, 1 to 20). Both groups received a median of two injections (range, 1 to 9 for CNV and 1 to 4 for CME). For eyes with CNV, BCVA improved significantly at follow-up month 1, but was not different from baseline thereafter; CST remained unchanged throughout follow-up. For eyes with CME, neither BCVA nor CST changed significantly over the course of follow-up.
CONCLUSIONS:
Bevacizumab appears to stabilize BCVA and CST for eyes with inflammatory CNV or CME.
Choroidal neovascularization (CNV) and Cystoid macular edema (CME) are well-recognized complications of inflammatory eye disease and important causes of vision loss in uveitis.1-9 Although the pathogenesis is incompletely understood, disruption of the inner and outer blood-ocular barriers, as well as the release of inflammatory mediators by leukocytes and macrophages, may trigger accumulation of intraretinal fluid or neovascularization. In neovascular age-related macular degeneration (AMD) and diabetic or pseudophakic macular edema, vascular endothelial growth factor (VEGF) is a principal mediator of angiogenesis and increased vascular permeability.10-12 Since similar mechanisms likely apply in inflammatory disease, treatments that are effective for CNV or CME associated with common retinal disorders may also be effective for CNV and CME in uveitis patients.
Clinicians have employed a variety of methods to treat uveitic CNV and CME. In the case of CNV, laser photocoagulation, photodynamic therapy (PDT), local or systemic corticosteroid administration, and surgical removal have been attempted.13-18 For CME, topical and systemic nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids (topical, local, or systemic), systemic carbonic anhydrase inhibitors, and somatostatin analogs have been reported.19,20 All of these therapies, however, are associated with potential limitations, such as patient un-responsiveness, or high recurrence rates.
Bevacizumab (Avastin; Genentech Inc, South San Francisco, California, USA), a monoclonal antibody to VEGF, has been successfully used to treat CNV and CME secondary to AMD, myopia, and central retinal vein occlusion.21-23 Its efficacy in these settings, as well as the established link between uveitis and increased intraocular VEGF levels,24 has prompted clinicians to use bevacizumab to manage uveitic CNV and CME. In order to expand the available literature on this subject,25-33 we report our experience.
METHODS
We performed a retrospective chart review of eyes treated with intravitreal bevacizumab for uveitic CNV or CME through February 1, 2008. A computerized search of billing codes was used to identify eligible patients at the Bascom Palmer Eye Institute. Inclusion criteria consisted of a diagnosis of inflammatory CNV or CME, treatment with at least one injection of intravitreal bevacizumab, and follow-up of at least 1 month. Eyes were excluded if they had received sub-Tenon or intravitreal corticosteroids during the 12 weeks preceding bevacizumab injection. Available demographic and ophthalmic data, including Snellen best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, indirect ophthalmoscopy, fluorescein angiography (FA), and optical coherence tomography (OCT), were collected at baseline and follow-up visits.
At baseline, diagnoses of CNV or CME were made by fundus biomicroscopy and FA or OCT. After obtaining informed consent, topical proparacaine hydrochloride 0.5% (Akorn Inc, Buffalo Grove, Illinois, USA), sterile-filtered 4% viscous lidocaine (Akten; Akorn Inc), and povidone-iodine 5% were instilled into the eye. A distance of 3.5 or 4.0 mm was measured from the corneoscleral limbus and the eye was injected intravitreally with 1.25 mg of bevacizumab (0.05 ml) using a sterile 30-gauge needle. Patients were instructed to instill topical moxifloxacin hydrochloride 0.5% (Vigamox; Alcon Laboratories Inc, Fort Worth, Texas, USA) onto the injected eye 4 times daily for 3 days. Patients were then examined (and OCT performed) at 1-month intervals, with additional ancillary tests or bevacizumab injections administered at the discretion of the treating physician. As some eyes subsequently received surgery or sub-Tenon or intravitreal corticosteroids, follow-up, for the purposes of this study, extended only to the visit immediately prior to implementation of the alternative, non-bevacizumab therapy.
The main outcome measures for this study were BCVA and central subfield thickness (CST), as measured by OCT; the rate of macular fluid resolution was a secondary outcome measure. Descriptive statistics are presented. Follow-up visual acuity (VA) was compared to baseline acuity with the Wilcoxon signed-rank test while follow-up CST was compared to baseline with the paired t test. χ2 and t tests were used to examine possible predictive factors for favorable visual or CST outcomes. Kaplan-Meier survival analysis was used to describe the time from the first injection of bevacizumab to the time of resolution of macular fluid (or to the time of the last follow-up visit for those patients who did not resolve), with the log-rank test used to compare time to resolution between CNV and CME patients. Time to treatment failure was analyzed in a similar way.
RESULTS
Tables 1 and 2 summarize demographic and ophthalmic results. Thirty-four eyes of 30 patients met inclusion criteria; an additional 4 eyes were excluded because they had received sub-Tenon or intravitreal corticosteroids during the 12 weeks preceding bevacizumab injection. Twenty-one eyes of 19 patients (9 male, 10 female) with a median age of 52 years (range, 7 to 83) demonstrated CNV while 13 eyes of 11 patients (4 male, 7 female) with a median age of 67 years (range, 17 to 83) demonstrated CME. The median length of follow-up for CNV eyes was 7 months (range, 1 to 28) while the median follow-up for CME eyes was 13 months (range, 1 to 20). Both groups received a median of two injections (range, 1 to 9 for CNV and 1 to 4 for CME). Phakic status, history of prior corticosteroid injections (sub-Tenon or intravitreal), uveitic activity, systemic diabetic status, concurrent topical or systemic anti-inflammatory treatment, history of prior vitrectomy, and CNV location did not statistically influence visual or CST outcomes for either CNV or CME eyes.
TABLE 1.
Demographic and Ophthalmic Data for Patients with Choroidal Neovascularization
| Patient | Age | Gender | Eye | Diagnosis | Follow-up (months) |
Number of Injections |
Phakic Statusa | Prior Injectionsa,b |
Active Uveitisa |
Diabetica,c | Concurrent Topical Treatmenta,b |
Concurrent Systemic Treatmenta,b |
Prior Vitrectomya |
CNV Locationa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | F | OS | MCP | 2 | 1 | Pseudophakic | STTA ×2 | Yes | No | Prednisolone | CSA | Yes | Peripapillary |
| 2 | 24 | M | OS | VKH | 14 | 1 | Phakic | None | Yes | No | Prednisolone | None | No | Peripapillary |
| 3 | 53 | F | OD | PIC | 20 | 2 | Pseudophakic | IVTA ×2 | No | No | None | None | No | Juxtafoveal |
| 4 | 55 | F | OD | Idiopathic panuveitis | 10 | 5 | Phakic | None | Yes | No | None | MTX, prednisone | No | Peripapillary |
| 5 | 52 | M | OD | MCP | 5 | 4 | Phakic | STTA ×1 | Yes | No | None | Mycophenolate | No | Peripapillary |
| 6 | 46 | F | OS | PIC | 9 | 2 | Phakic | None | No | No | None | None | No | Subfoveal |
| 7 | 54 | M | OD | Serpiginous choroidopathy |
28 | 3 | Phakic | None | Yes | No | None | AZA, CSA, predisone | No | Juxtafoveal |
| 8 | 56 | F | OD | Sarcoid panuveitis | 7 | 2 | Phakic | IVTA ×4 | Yes | Yes | None | Mycophenolate | No | Peripapillary |
| 9 | 69 | M | OS | Sympathetic ophthalmia |
15 | 9 | Pseudophakic | STTA ×1 | Yes | Yes | Prednisolone | Mycophenolate, prednisone |
No | Subfoveal |
| 10a | 7 | F | OD | Idiopathic panuveitis | 2 | 2 | Phakic | STTA ×1 | Yes | No | None | None | No | Extrafoveal |
| 10b | 7 | F | OS | Idiopathic panuveitis | 2 | 2 | Phakic | None | Yes | No | Prednisolone | None | No | Subfoveal |
| 11 | 32 | F | OS | Toxocariasis | 27 | 5 | Phakic | None | No | No | None | None | No | Subfoveal |
| 12 | 12 | F | OS | Idiopathic panuveitis | 4 | 1 | Phakic | None | No | Yes | Prednisolone | MTX | No | Peripapillary |
| 13 | 61 | M | OD | Serpiginous choroidopathy |
16 | 2 | Phakic | None | No | No | None | AZA, CSA, prednisone |
No | Peripapillary |
| 14 | 48 | M | OS | Toxoplasmosis | 12 | 5 | Phakic | None | No | No | None | None | No | Subfoveal |
| 15a | 58 | F | OD | Inflammatory papillitis |
6 | 4 | Phakic | None | No | No | None | Prednisone | No | Peripapillary |
| 15b | 58 | F | OS | Inflammatory papillitis |
6 | 5 | Phakic | None | No | No | None | Prednisone | No | Peripapillary |
| 16 | 29 | F | OS | POHS | 1 | 1 | Phakic | None | No | No | None | None | No | Subfoveal |
| 17 | 38 | M | OD | Idiopathic retinal vasculitis |
7 | 1 | Phakic | None | No | No | None | MTX, mycophenolate | No | Extrafoveal |
| 18 | 83 | M | OD | CMVR | 2 | 1 | Pseudophakic | None | No | Yes | Prednisolone | None | No | Extrafoveal |
| 19 | 53 | M | OD | Pars planitis/reactive arthritis |
12 | 3 | Aphakic | None | Yes | No | Prednisolone | Prednisone | Yes | Extrafoveal |
AZA = azathioprine; CMVR = cytomegalovirus retinitis; CNV = choroidal neovascularization; CSA = cyclosporine A; IVTA = intravitreal triamcinolone; MCP = multifocal choroiditis and panuveitis; MTX = methotrexate; OD = right eye; OS = left eye; PIC = punctuate inner choroiditis; POHS = presumed ocular histoplasmosis syndrome; STTA = sub-Tenon triamcinolone; VKH = Vogt-Koyanagi-Harada syndrome.
None of these variables statistically influenced outcomes with respect to visual acuity or macular thickness.
Limited to anti-inflammatory therapies.
Refers to the presence or absence of diabetes mellitus; no patients had diabetic retinopathy.
TABLE 2.
Demographic and Ophthalmic Data for Patients with Cystoid Macular Edema
| Patient | Age | Gender | Eye | Diagnosis | Follow-up (months) |
Number of Injections |
Phakic Statusa | Prior Injectionsa,b |
Active Uveitisa |
Diabetica,c | Concurrent Topical Treatmenta,b |
Concurrent Systemic Treatmenta,b |
Prior Vitrectomya |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 17 | F | OS | Pars planitis | 13 | 4 | Phakic | None | Yes | No | Prednisolone | CSA, MTX | No |
| 21 | 58 | F | OS | Idiopathic panuveitis | 1 | 1 | Pseudophakic | None | Yes | No | Prednisolone | Prednisone, MTX | No |
| 22 | 81 | F | OD | Toxoplasmosis | 3 | 1 | Aphakic | None | No | No | None | None | No |
| 23 | 57 | F | OD | Fuchs uveitis syndrome | 1 | 1 | Pseudophakic | IVTA ×3 | Yes | No | MP, ketorolac | None | No |
| 24 | 68 | F | OS | Idiopathic chronic iridocyclitis/vitreitis |
8 | 2 | Pseudophakic | None | Yes | No | Prednisolone, nepafenac |
None | No |
| 25 | 72 | M | OS | Idiopathic retinal vasculitis |
13 | 1 | Pseudophakic | None | Yes | Yes | Loteprednol, diclofenac |
None | Yes |
| 26 | 44 | M | OD | CMVR/immune-recovery uveitis |
10 | 2 | Phakic | STTA ×3 | Yes | No | None | None | No |
| 27a | 33 | M | OD | Pars planitis | 16 | 2 | Pseudophakic | None | No | No | Prednisolone, nepafenac |
Mycophenolate | Yes |
| 27b | 33 | M | OS | Pars planitis | 14 | 2 | Pseudophakic | None | Yes | No | Prednisolone, nepafenac |
Mycophenolate | No |
| 28 | 67 | M | OS | Sarcoid panuveitis | 9 | 2 | Pseudophakic | STTA ×1 | Yes | No | None | None | Yes |
| 29 | 79 | F | OS | Chronic idiopathic anterior/intermediate uveitis |
13 | 2 | Pseudophakic | None | Yes | No | Nepafenac | MTX | No |
| 30a | 83 | F | OD | Idiopathic occlusive vasculitis |
20 | 4 | Pseudophakic | None | Yes | No | None | Prednisone | No |
| 30b | 83 | F | OS | Idiopathic occlusive vasculitis |
20 | 1 | Pseudophakic | None | Yes | No | None | Prednisone | No |
CMVR = cytomegalovirus retinitis; CSA = cyclosporine A; IVTA = intravitreal triamcinolone; MP = methylprednisolone; MTX = methotrexate; OD = right eye; OS = left eye; STTA = sub-Tenon triamcinolone.
None of these variables statistically influenced outcomes with respect to visual acuity or macular thickness.
Limited to anti-inflammatory therapies.
Refers to the presence or absence of diabetes mellitus; no patients had diabetic retinopathy.
Table 3 summarizes VA and macular thickness results for eyes with CNV (see Supplemental Figures 1 and 2 available at AJO.com). At month 1 (n = 19 eyes), 8 eyes (42%) had improved VA, 7 (37%) had unchanged VA, and 4 (21%) had worse VA; 8 (42%) improved by a halving of the visual angle and 1 (5%) worsened by a doubling of the visual angle. For all eyes, the median change between baseline and month 1 was 0. At month 6 (n = 12 eyes), 2 eyes (17%) had improved VA, 4 (33%) had unchanged VA, and 6 (50%) had worse VA; 2 (17%) improved by a halving of the visual angle and 2 (17%) worsened by a doubling of the visual angle. For all eyes, the median change between baseline and month 6 was 0.
TABLE 3.
Visual Acuities and Macular Thicknesses at Baseline and Follow-up Visits for Patients with Choroidal Neovascularization
| Visit | Median BCVA (range) | Number of Eyes |
P valuea | Mean Central Subfield Thicknessb ± SD μm (range) |
Number of Eyes |
P valuec |
|---|---|---|---|---|---|---|
| Baseline | 20/80 (20/20 to HM) | 21 | — | 292 ± 114 (157 to 592) | 19 | — |
| Month 1 | 20/40 (20/20 to CF) | 19 | .034 | 249 ± 65 (162 to 389) | 17 | .12 |
| Month 3 | 20/60 (20/70 to 20/300) | 14 | .84 | 285 ± 84 (84 to 437) | 12 | .27 |
| Month 6 | 20/50 (20/25 to 4/200) | 12 | .67 | 264 ± 55 (187 to 333) | 9 | .35 |
| Month 12 | 20/60 (20/20 to 20/300) | 6 | .23 | 220 ± 29 (202 to 271) | 5 | .44 |
BCVA = best-corrected visual acuity; CF = count fingers; HM = hand motion; SD = standard deviation.
P value comparing baseline to follow-up BCVA (Wilcoxon signed-rank test).
Measured by optical coherence tomography.
P value comparing baseline to follow-up central subfield thickness (paired t test).
Table 4 summarizes VA and macular thickness results for eyes with CME (see Supplemental Figures 3 and 4 available at AJO.com). At month 1 (n = 13 eyes), 5 eyes (38%) had improved VA, 2 (15%) had unchanged VA, and 6 (46%) had worsened VA; 3 (23%) improved by a halving of the visual angle and 2 (15%) worsened by a doubling of the visual angle. For all eyes, the median change between baseline and month 1 was 0. At month 6 (n = 10 eyes), 4 eyes (40%) had improved VA, 2 (20%) had unchanged VA, and 4 (40%) had worsened VA; 2 (20%) improved by a halving of the visual angle and 4 (40%) worsened by a doubling of the visual angle. For all eyes, the median change between baseline and month 6 was 0.
TABLE 4.
Visual Acuities and Macular Thicknesses at Baseline and Follow-up Visits for Patients with Cystoid Macular Edema
| Visit | Median BCVA (range) | Number of Eyes |
P valuea | Mean Central Subfield Thicknessb ± SD μm (range) |
Number of Eyes |
P valuec |
|---|---|---|---|---|---|---|
| Baseline | 20/100 (20/20 to CF) | 13 | — | 447 ± 269 (186 to 1154) | 13 | — |
| Month 1 | 20/100 (20/25 to CF) | 13 | .89 | 405 ± 229 (186 to 986) | 13 | .15 |
| Month 3 | 20/100 (20/20 to CF) | 9 | .23 | 462 ± 159 (265 to 621) | 4 | .30 |
| Month 6 | 20/90 (20/30 to 4/200) | 10 | .40 | 364 ± 160 (200 to 677) | 9 | .23 |
| Month 12 | 20/200 (20/20 to CF) | 7 | .69 | 465 ± 171 (237 to 749) | 6 | .78 |
BCVA = best-corrected visual acuity; CF = count fingers; SD = standard deviation.
P value comparing baseline to follow-up BCVA (Wilcoxon signed-rank test).
Measured by optical coherence tomography.
P value comparing baseline to follow-up central subfield thickness (paired t test).
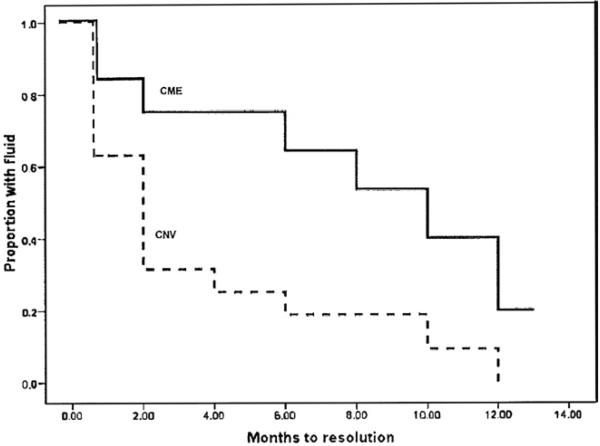
The Figure illustrates results of the Kaplan-Meier survival analysis comparing resolution rates of macular edema (ME) between the CNV and CME groups. ME was more likely to resolve in the CNV group; 89% (17/19) of those in the CNV group vs 54% (7/13) of those in the CME group resolved (P = .038; Fisher exact test). Resolution of edema also occurred earlier in the course of treatment in the CNV group (P = .017, log-rank test).
There were no adverse events, including endophthalmitis, hypotony, retinal detachment, or drug reaction, for any eye in this review. Five eyes—3 with CNV and 2 with CME— received alternative therapy after undergoing bevacizumab treatment, as they were considered to be treatment failures. For the CNV eyes, Eye 1 received intravitreal triamcinolone (IVTA) at 2 months, Eye 15b received IVTA at 6 months, and Eye 8 underwent pars plana vitrectomy at 7 months. For the CME eyes, Eye 21 received sub-Tenon triamcinolone at 1 month and Eye 23 received IVTA at 1 month. Kaplan-Meier survival analysis indicated that time to failure between CNV and CME eyes was not statistically significantly different (data not shown).
DISCUSSION
Although case reports have described favorable outcomes in cases of inflammatory CNV and CME treated with intravitreal bevacizumab,25-28 larger series contain less consistent results. Adan and associates, for example, retrospectively reviewed the outcomes of 9 eyes treated with intravitreal bevacizumab for uveitic CNV.29 Over a mean follow-up of 7.1 months, they found that BCVA improved in 8 eyes (88.8%) and stabilized in 1 (11.2%); foveal thickness decreased in all eyes, a result that reached statistical significance; and CNV resolved angiographically in all cases. In a prospective, nonrandomized study, Chan and associates administered 3 monthly intravitreal injections of bevacizumab to 4 patients with CNV secondary to punctate inner choroidopathy.23 By 6 months, all eyes had gained at least 1 line of VA and 3 (75%) had gained at least 2 lines. None had angiographic evidence of CNV at 3 months and none demonstrated a recurrence by 6 months. More recently, Chang and associates reported the results of 39 eyes that received intravitreal bevacizumab for CNV related to both inflammatory and noninflammatory disorders, including 12 eyes with multifocal choroiditis and panuveitis (MCP).30 Overall VA improved in 31% of eyes, worsened in 8%, and remained stable in 62% after a median follow-up of 60 weeks, results that were statistically significant and unrelated to the underlying diagnosis. Cordero and associates retrospectively reviewed 13 patients with uveitic CME.31 They reported an improvement in VA of 2≥2 lines in 5 patients (38.4%) and a decrease in foveal thickness in 6 patients (46.15%). Though the change in mean retinal thickness over the course of follow-up was significant, the change in mean logarithm of the minimal angle of resolution (logMAR) was not. Survival analysis, however, revealed that the probability of improvement in VA began to increase progressively at 6 weeks and reached 81% at 14 weeks. Ziemssen and associates reported their experiences with 6 patients who received intravitreal bevacizumab for uveitic CME. Despite marked improvement in VA and central retinal thickness in 2 patients at week 1, none of their patients displayed significant improvement in either parameter at 1 month.32 Finally, Mackensen and associates reported the results of 11 eyes of 10 patients treated with a single injection of intravitreal bevacizumab for refractory CME.33 At 4 weeks postinjection, the mean foveal thickness had decreased significantly, but VA had improved in only 4 patients—a change that was not statistically significant. Apart from rupture of a retinal cyst in one of Ziemssen’s patients and cataract formation in one of Mackensen’s patients, no ocular or systemic adverse events were reported in any of the mentioned articles.
Our review yielded similarly mixed results. Eyes with CNV, for example, demonstrated a statistically significant improvement in VA at follow-up month 1, but were not different from baseline thereafter. These findings suggest that bevacizumab promotes stability of VA after initial improvement, possibly reflecting the time-dependent impact of photoreceptor disruption or fibrosis deposition on vision. However, this result could be spurious, since it was not consistent over time. Macular thickness remained unchanged at all time points, further indicating a possible stabilizing effect of bevacizumab. Overall, bevacizumab may be most effective when administered immediately after development of CNV.
Eyes with CME displayed comparable outcomes: Neither VA nor macular thickness changed significantly over the course of follow-up, suggesting that bevacizumab exerts a stabilizing influence on CME. Although photoreceptor or retinal pigment epithelial disruption may contribute to the absence of visual improvement, the general lack of responsiveness to bevacizumab among eyes with CME may be a consequence of the high proportion of eyes with active uveitis at the time of initial injection (85%), though our statistical analysis did not reveal a relationship between uveitic activity and visual outcome. However, bevacizumab’s lack of efficacy may also reflect the natural history of the disease process or VEGF’s limited role in mediating CME.
Unexpectedly, neither prior vitrectomy nor concurrent adjunctive anti-inflammatory therapy influenced visual or CST outcomes for either CNV or CME eyes. However, the lack of a control group and the small number of patients in our review may not have permitted detection of a statistical difference.
Statistical comparison of ME resolution in bevacizumab-treated CNV and CME eyes yielded two interesting results: ME was more likely to resolve—and to resolve earlier in the treatment course—in eyes with CNV than in eyes with CME. These findings suggest that bevacizumab may be a more effective treatment in patients with inflammatory CNV than in patients with inflammatory CME.
Although our study was limited by its retrospective design, small number of patients, lack of a control group, brief follow-up, and losses to follow-up, our results suggest that bevacizumab deserves consideration as a treatment for patients with inflammatory CNV or CME. Intravitreal bevacizumab offers some clear advantages over intravitreal corticosteroids: It is unlikely to increase intraocular pressure, trigger symptoms of floaters, or cause cataracts. As such, it may serve as a useful primary or adjunct therapy, especially for patients who are phakic or who have a history of glaucoma or steroid-related ocular hypertension. Efforts to identify prognostic factors for improvement and to collect further data regarding bevacizumab’s efficacy in treating inflammatory CNV and CME, including prospective trials comparing intravitreal bevacizumab to intravitreal corticosteroids in cases of CME, are warranted.
Supplementary Material
FIGURE.
Kaplan-Meier plots of the probability of fluid resolution in bevacizumab-treated patients with uveitic choroidal neovascularization (CNV) or cystoid macular edema (CME). Macular edema resolved earlier in the CNV group than in the CME group (P = .017, log-rank test).
Acknowledgments
This study was supported by the National Eye Institute (No. EY014801), National Institutes of Health, Bethesda, Maryland (Dr Schiffman); and Research to Prevent Blindness Inc, New York, New York (Dr Schiffman)
Footnotes
Supplemental Material available at AJO.com.
The authors indicate no financial conflict of interest. Involved in design and conduct of study (M.N.L., J.L.D.); collection, management, analysis, and interpretation of data (M.N.L., J.C.S., J.L.D.); and preparation, review, or approval of the manuscript (M.N.L., J.C.S., J.L.D.). The study was approved by the Human Subjects Research Committee of the University of Miami Miller School of Medicine. It complied with the Health Information Portability and Accountability Act and conformed to the tenets of the Declaration of Helsinki.
REFERENCES
- 1.Watzke RC, Packer AJ, Folk JC, Benson WE, Burgess D, Ober RR. Punctate inner choroidopathy. Am J Ophthalmol. 1984;98:572–584. doi: 10.1016/0002-9394(84)90243-5. [DOI] [PubMed] [Google Scholar]
- 2.Olk RJ, Burgess DB, McCormick PA. Subfoveal and juxtafoveal subretinal neovascularization in the presumed ocular histoplasmosis syndrome. Visual prognosis. Ophthalmology. 1984;91:1592–1602. doi: 10.1016/s0161-6420(84)34113-6. [DOI] [PubMed] [Google Scholar]
- 3.Chew EY, Crawford J. Sympathetic ophthalmia and choroidal neovascularization. Case report. Arch Ophthalmol. 1988;106:1507–1508. doi: 10.1001/archopht.1988.01060140675015. [DOI] [PubMed] [Google Scholar]
- 4.Rothova A, Suttorp-van Schulten MS, Frits TW, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–336. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown J, Jr, Folk JC, Reddy CV, Kimura AE. Visual prognosis of multifocal choroiditis, punctate inner choroidopathy, and the diffuse subretinal fibrosis syndrome. Ophthalmology. 1996;103:1100–1105. doi: 10.1016/s0161-6420(96)30561-7. [DOI] [PubMed] [Google Scholar]
- 6.Rothova A, Berendschot TT, Probst K, van Kooij B, Baarsma GS. Birdshot chorioretinopathy: long-term manifestations and visual prognosis. Ophthalmology. 2004;111:954–959. doi: 10.1016/j.ophtha.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology. 2004;111:2299–2306. doi: 10.1016/j.ophtha.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Markomichelakis NN, Halkiadakis I, Pantelia E, et al. Patterns of macular edema in patients with uveitis: qualitative and quantitative assessment using optical coherence tomography. Ophthalmology. 2004;111:946–953. doi: 10.1016/j.ophtha.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 9.Lim WK, Buggage RR, Nussenblatt RB. Serpiginous choroiditis. Surv Ophthalmol. 2005;50:231–244. doi: 10.1016/j.survophthal.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Aiello LP. Vascular endothelial growth factor and the eye: biochemical mechanisms of action and implications for novel therapies. Ophthalmic Res. 1997;29:354–362. doi: 10.1159/000268033. [DOI] [PubMed] [Google Scholar]
- 11.Fine HF, Baffi J, Reed GF, Csaky KG, Nussenblatt RB. Aqueous humor and plasma vascular endothelial growth factor in uveitis-associated cystoid macular edema. Am J Ophthalmol. 2001;132:794–796. doi: 10.1016/s0002-9394(01)01103-5. [DOI] [PubMed] [Google Scholar]
- 12.Van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. JAMA. 2005;293:1509–1513. doi: 10.1001/jama.293.12.1509. [DOI] [PubMed] [Google Scholar]
- 13.Martidis A, Miller DG, Ciulla TA, Danis RP, Moorthy RS. Corticosteroids as an antiangiogenic agent for histoplasmo-sis-related subfoveal choroidal neovascularization. J Ocul Pharmacol Ther. 1999;15:425–428. doi: 10.1089/jop.1999.15.425. [DOI] [PubMed] [Google Scholar]
- 14.Rogers AH, Duker JS, Nichols N, Baker BJ. Photodynamic therapy of idiopathic and inflammatory choroidal neovascularization in young adults. Ophthalmology. 2003;110:1315–1320. doi: 10.1016/S0161-6420(03)00466-4. [DOI] [PubMed] [Google Scholar]
- 15.Wachtlin J, Heimann H, Behme T, Foerster MH. Long-term results after photodynamic therapy with verteporfin for choroidal neovascularizations secondary to inflammatory chorioretinal diseases. Graefes Arch Clin Exp Ophthalmol. 2003;241:899–906. doi: 10.1007/s00417-003-0734-5. [DOI] [PubMed] [Google Scholar]
- 16.Leslie T, Lois N, Christopoulou D, Olson JA, Forrester JV. Photodynamic therapy for inflammatory choroidal neovascularisation unresponsive to immunosuppression. Br J Ophthalmol. 2005;89:147–150. doi: 10.1136/bjo.2004.046623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowilaty SR, Bouhaimed M. Photodynamic therapy for subfoveal choroidal neovascularisation in Vogt-Koyanagi-Harada disease. Br J Ophthalmol. 2006;90:982–986. doi: 10.1136/bjo.2006.091538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim JI, Flaxel CJ, LaBree L. Photodynamic therapy for choroidal neovascularisation secondary to inflammatory chorioretinal disease. Ann Acad Med Singapore. 2006;35:198–202. [PubMed] [Google Scholar]
- 19.Rothova A. Medical treatment of cystoid macular edema. Ocul Immunol Inflamm. 2002;10:239–246. doi: 10.1076/ocii.10.4.239.15589. [DOI] [PubMed] [Google Scholar]
- 20.Papadaki T, Zacharopoulos I, Iaccheri B, Fiore T, Foster CS. Somatostatin for uveitic cystoid macular edema (CME) Ocul Immunol Inflamm. 2005;13:469–470. doi: 10.1080/09273940691001964. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–335. [PubMed] [Google Scholar]
- 22.Bashshur ZF, Bazarbachi A, Schakal A, Haddad ZA, El Haibi CP, Noureddin BN. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006;142:1–9. doi: 10.1016/j.ajo.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 23.Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (Avastin) for choroidal neovascularization secondary to central serous chorioretinopathy, secondary to punctate inner choroidopathy, or of idiopathic origin. Am J Ophthalmol. 2007;143:977–983. doi: 10.1016/j.ajo.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 24.Fine HF, Baffi J, Reed GF, Csaky KG, Nussenblatt RB. Aqueous humor and plasma vascular endothelial growth factor in uveitis-associated cystoid macular edema. Am J Ophthalmol. 2001;132:794–796. doi: 10.1016/s0002-9394(01)01103-5. [DOI] [PubMed] [Google Scholar]
- 25.Seth RK, Stoessel KM, Adelman RA. Choroidal neovascularization associated with West Nile virus chorioretinitis. Semin Ophthalmol. 2007;22:81–84. doi: 10.1080/08820530701418375. [DOI] [PubMed] [Google Scholar]
- 26.Margolis R, Lowder CY, Sears JE, Kaiser PK. Intravitreal bevacizumab for macular edema due to occlusive vasculitis. Semin Ophthalmol. 2007;22:105–108. doi: 10.1080/08820530701420074. [DOI] [PubMed] [Google Scholar]
- 27.Adan A, Navarro M, Casaroli-Marano RP, Ortiz S, Molina JJ. Intravitreal bevacizumab as initial treatment for choroidal neovascularization associated with presumed ocular histoplasmosis syndrome. Graefes Arch Clin Exp Ophthalmol. 2007;245:1873–1875. doi: 10.1007/s00417-007-0637-y. [DOI] [PubMed] [Google Scholar]
- 28.Kurup S, Lew J, Byrnes G, Yeh S, Nussenblatt R, Levy-Clarke G. Therapeutic efficacy of intravitreal bevacizumab on posterior uveitis complicated by neovascularization. Acta Ophthalmol. 2009;87:349–352. doi: 10.1111/j.1755-3768.2008.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adan A, Mateo C, Navarro R, Bitrian E, Casaroli-Marano RP. Intravitreal bevacizumab (Avastin) injection as primary treatment of inflammatory choroidal neovascularization. Retina. 2007;27:1180–1186. doi: 10.1097/IAE.0b013e31815e9834. [DOI] [PubMed] [Google Scholar]
- 30.Chang LK, Spaide RF, Brue C, Freund KB, Klancnik JM, Jr, Slakter JS. Bevacizumab treatment for subfoveal choroidal neovascularization from causes other than age-related macular degeneration. Arch Ophthalmol. 2008;126:941–945. doi: 10.1001/archopht.126.7.941. [DOI] [PubMed] [Google Scholar]
- 31.Cordero Coma M, Sobrin L, Onal S, Christen W, Foster CS. Intravitreal bevacizumab for treatment of uveitic macular edema. Ophthalmology. 2007;114:1574–1579. doi: 10.1016/j.ophtha.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 32.Ziemssen F, Deuter CM, Stuebiger N, Zierhut M. Weak transient response of chronic uveitic macular edema to intravitreal bevacizumab (Avastin) Graefes Arch Clin Exp Ophthalmol. 2007;245:917–918. doi: 10.1007/s00417-006-0512-2. [DOI] [PubMed] [Google Scholar]
- 33.Mackensen F, Heinz C, Becker MD, Heiligenhaus A. Intravitreal bevacizumab (Avastin) as a treatment for refractory macular edema in patients with uveitis: a pilot study. Retina. 2008;28:41–45. doi: 10.1097/IAE.0b013e318156db75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.