Abstract
Purpose
To examine the degree to which shared risk factors explain the relationship of periodontitis (PD) with rheumatoid arthritis (RA) and to examine associations of PD and Porphyomonas gingivalis (Pg) with disease features.
Methods
RA cases (N=287) and controls (N=330) underwent a standardized periodontal examination. HLA-DRB1 status was imputed using SNPs from the extended MHC. Circulating anti-Pg antibody was measured using ELISA and subgingival plaque was assessed for the presence of Pg using PCR. Associations of PD with RA were examined using multivariable regression.
Results
PD was more common in RA (35%, p = 0.022) and aCCP positive RA (n=240; 37%; p = 0.006) vs. controls (26%). There were no RA-control differences in anti-Pg or the frequency of Pg positivity by PCR. Anti-Pg antibody showed weak but statistically significant associations with both anti-CCP (r=0.14, p=0.022) and RF (r=0.19, p=0.001). PD was associated with increased swollen joint counts (p=0.004), DAS-28-CRP (p=0.045), total Sharp scores (p=0.015), aCCP (p=0.011), and RF (p<0.001). Select anti-citrullinated peptide antibody (ACPA; including antibody to citrullinated filaggrin) were higher in patients with subgingival Pg and higher anti-Pg antibody levels irrespective of smoking. Associations of PD with established seropositive RA were independent of all covariates examined including evidence of Pg infection.
Conclusions
Both PD and Pg appear to shape RA-related autoreactivity in RA. In addition, PD demonstrates an independent relationship with established seropositive RA.
Keywords: rheumatoid arthritis, periodontitis, Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, anti-citrullinated protein antibody, disease severity
Periodontitis (PD) has emerged as a risk factor in a number of health conditions including rheumatoid arthritis (RA) (1). Sharing both morphologic and histopathologic similarities with RA (2), PD is an inflammatory disease initiated by bacterial infection resulting in soft and hard tissue destruction and ultimately leading to tooth loss. In addition to shared inflammatory pathways, PD and RA share risk factors for susceptibility and progression, most notably cigarette smoking and, possibly, shared epitope-containing HLA-DRB1 alleles, the latter associated with localized aggressive periodontitis (3–10). Although a causal link between these conditions has not been established, several reports have demonstrated an increased PD prevalence in RA patients compared to controls (11–18).
Growing evidence suggests that pathogens associated with PD could play a role in RA propagation. Chief among the organisms of interest is Porphyromonas gingivalis (P. gingivalis) (19). P. gingivalis is the only known pathogen expressing peptidylarginine deiminase (PPAD). Similar to its human counterpart, P. gingivalis-expressed PAD catalyzes the citrullination of arginine-containing peptides. This is noteworthy because citrullinated antigens are thought to drive adaptive immune responses that are nearly exclusive to RA. The potential role of P. gingivalis in RA pathogenesis has been borne out in epidemiologic investigations. Concentrations of circulating antibody to P. gingivalis have been demonstrated to be associated with the expression of anti-citrullinated peptide antibody (ACPA) (20–22). More recently, our group has shown that antibody to P. gingivalis is associated with the presence of RA-related autoantibody (a combination of rheumatoid factor [RF] and/or ACPA) among individuals at increased risk for disease but who have not yet developed RA symptoms (23), underscoring the potential role of this pathogen in RA development.
As part of the present study, we conducted a large case-control investigation to examine the relationship of PD with established RA. We sought to examine the degree to which this relationship is impacted by shared genetic and/or environmental factors. We also sought to elucidate the degree to which the relationship of PD with RA may be related to infection and/or colonization with P. gingivalis. By using a rigorously selected control population, we attempted to mitigate issues of bias or unmeasured confounding that may have impacted other efforts often using healthy volunteers as comparators (16–18). Finally, using a multiplex approach, we examined the associations of PD and P. gingivalis with autoreactivity to several citrullinated autoantigens that have been implicated in RA disease pathogenesis.
Materials and Methods
Study participants
RA cases and osteoarthritis controls were enrolled from rheumatology, orthopedic, and primary care clinics from four U.S. Veterans Affairs Medical Centers (Omaha, NE; Dallas, TX; Salt Lake City, UT; and Washington, DC) and a single academic coordinating center (University of Nebraska Medical Center). Cases satisfied the 1987 American College of Rheumatology (ACR) classification criteria for RA (24) (age of onset > 18 years). Informed with pilot data (12), we enrolled patients with osteoarthritis as disease controls with the expectation that these patients would share similar sociodemographic characteristics as cases. Osteoarthritis was confirmed through medical record review, based on medical documentation or imaging results consistent with ‘degenerative’ arthritis in the absence of inflammatory arthritis (e.g. systemic lupus erythematosus, ankylosing spondylitis, polymyalgia rheumatica, inflammatory bowel disease, acute gout, etc.). Additional inclusion criteria included the presence of ≥ 9 posterior teeth. Patients were excluded if any of the following were present: tetracycline or related antibiotic use in the previous 6 months; prior use of cyclosporine or dilantin; or a requirement for antibiotic prophylaxis prior to dental probing. This latter exclusion involved RA cases with a history of any total joint replacement or for controls, having received a total joint replacement within the previous 24 months (25, 26). This study was approved by the Institutional Review Board at each participating center and all study participants provided informed written consent prior to study enrollment.
Periodontal assessments
Periodontal assessments were completed by a single dentist or periodontist at each site, blinded to case diagnosis. Probing depth and gingival recession measurements were determined at six sites per tooth for all erupted teeth (except for 3rd molars). Prior to study initiation, periodontal assessors were calibrated against a gold-standard periodontist (JBP) by ensuring at least 85% of probing depth and gingival recession measurements taken were within ± 1 mm. PD was defined a priori according to the definition of Machtei et al as the presence of clinical attachment loss ≥ 6 mm on ≥ 2 teeth and one or more sites with probing depths ≥ 5 mm (27). Additional periodontal measurements included bleeding on probing, the presence of supragingival plaque (serving as an indicator of oral hygiene (28)), and missing teeth (out of 28; third molars excluded). Subgingival plaque was collected from up to four mesiobuccal sites. Following removal of visible supragingival plaque, subgingival plaque samples were collected using a single sterile endodontic paper point for each site (29) and frozen at −70° C until analysis.
RA measures of disease activity and severity
ACPA was measured using a second-generation anti-cyclic citrullinated peptide (anti-CCP2) ELISA (Diastat, Axis-Shield, Dundee, Scotland, UK; positive test ≥ 5 U/ml). Both rheumatoid factor (RF; positive ≥ 15 IU/ml) and high sensitivity C-reactive protein (hs-CRP, mg/L) were determined by nephelometry (Siemens Healthcare Diagnostics, Munich, Germany). Serum samples were also evaluated for 19 specific ACPA using a bead-based multiplex antigen array on the BioPlex platform (30), an array measuring disease-specific autoantibody reactivity to multiple citrullinated autoantigens. An ACPA score was calculated as the sum of normalized fluorescent values divided by 19 (the number of ACPA tested). Other disease characteristics assessed included tender and swollen joint counts (0 to 28) in addition to both provider and patient global well-being scores (0 to 100 mm visual analog scales or VAS). A 28-joint Disease Activity Score (DAS-28-CRP) was calculated (31). Standard posterior-anterior hand and wrist radiographs were obtained in RA cases and scored by a single investigator (AE) blinded to PD status using the modified Sharp scoring method (32).
Bacterial serologies & the detection of P. gingivalis in subgingival plaque
Serum concentrations of IgG antibody to P. gingivalis were measured using two separate but complementary ELISAs as previously described. The first utilized strain 381 of P. gingivalis (American Type Culture Collection, Bethesda, MD) and measured antibody responses to outer membrane antigen (OMA) (23). The second approach measures antibody responses to P. gingivalis-specific lipopolysaccharide (LPS) (20). Antibody (IgG) to OMA of both Prevotella intermedia (IgG anti-Pi) and Fusobacterium nucleatum were also evaluated as previously described (23). Both bacteria are specifically known to co-aggregate with P. gingivalis in PD-related bio-films (33). All bacterial antibody concentrations were determined in μg/ml extrapolated from a standard curve, and then log-transformed for analysis. Nested PCR was used for the detection of P. gingivalis from subgingival plaque samples (34).
Genotyping for HLA-DRB1 shared epitope
We utilized single nucleotide polymorphism (SNP)-based imputation of four-digit HLA-DRB1 alleles using the web interface HLA-IMP:02 as previously described (35, 36). SNP markers across the extended MHC (from 26MB to 34MB on chromosome 6) used for imputation were obtained from samples genotyped on the Illumina ImmunoChip array. Agreement of imputation with direct HLA-DRB1 sequencing was examined among 116 RA patients for whom these data were available as part of a separate study (37). The two methods were concordant in defining SE status (positive vs. negative) in 108 of 116 patients (93%; kappa = 0.79). Levels of agreement were similar among Caucasian (96% concordance; kappa = 0.86) and African American (89% concordance; kappa = 0.72) RA cases. Comparisons of SE status and multivariable models including HLA-DRB1 SE were thus limited to Caucasians and African Americans.
Statistical analysis
Participant characteristics, periodontal measures, and log-transformed bacterial values were compared separately between the RA cases or anti-CCP positive cases and controls. When a single measure was collected from one subject, the two sample t-test or Wilcoxon rank sum test was used for comparison of continuous data, and the Chi-square test was used for comparison of categorical data. When multiple measures (e.g. probing depths) were made from different oral sites of one subject, the data were categorized as binary data, and generalized estimating equations (GEE) models with logit link and compound symmetry variance structure were used for analyses in order to account for within-subject correlations. Similarly, case-control differences in bacterial measures and RA outcomes (limited to RA cases) based on the presence of PD were evaluated. Spearman correlations were used to quantify correlations with bacterial serologies.
Multivariate logistic regression was used to evaluate the association of PD with RA risk overall and the risk of anti-CCP antibody positive RA among Caucasian and African American subjects for whom imputed HLA-DRB1 SE data was available. Given their potential relevance (3–10), HLA-DRB1 SE status (positive vs. negative), and smoking (ever vs. never) were included in all models. Other potential confounders included age, gender, race/ethnicity, body mass index (BMI), oral hygiene (using the presence of supragingival plaque as a surrogate), self-reported diabetes mellitus, marital status, presence of patient-reported oral dryness, and education. Stepwise variable selection with a selection criterion of p < 0.1 for both entry and elimination was used to select the other important risk factors. A p-value < 0.05 was considered to be significant.
Significance analysis of microarrays (SAM, version 3.08) (38) was used to analyze multiplex ACPA data among anti-CCP2 antibody positive RA patients to identify potential differences in ACPA profiles associated with PD, smoking, or both. To explore whether the ACPA score might differ based on PD and/or smoking, we also compared these scores among current, former, and never smokers stratified by PD status using one-way analysis of variance (ANOVA). SAM was also used to assess whether there were differences in ACPA expression among anti-CCP2 positive RA cases based on tertiles of anti-P. gingivalis OMA antibody or PCR positivity of subgingival samples, accounting for the effects of smoking by using stratified analyses. SAM output was sorted based on false discovery rates (FDRs) in order to identify antigens with the greatest differences in autoantibody reactivity comparator groups. Hierarchical clustering software Cluster® 3.0 was used to arrange SAM results according to similarities among autoantibody specificities, and significant ACPA values were displayed as heatmaps using Java Treeview® (Version 1.1.3).
Results
Participants
There were 617 study participants, including 287 RA cases and 330 controls. Characteristics of the 617 study participants are shown in Table 1. There were no group differences in sociodemographics. Consistent with known disease epidemiology (39), RA cases were more likely to be ever smokers and were more likely to be positive for HLA-DRB1 SE. Controls demonstrated a higher mean BMI than RA cases and a higher frequency of self-reported hypertension.
Table 1.
Study participant characteristics.
| Total (N = 617) |
RA (N = 287) |
Controls (N = 330) |
P-value | |
|---|---|---|---|---|
|
|
||||
| Sociodemographics | ||||
| Age, years | 59 (11) | 59 (12) | 59 (11) | 0.867 |
| Male gender | 62 | 63 | 60 | 0.384 |
| Race | ||||
| Caucasian | 75 | 77 | 72 | 0.174 |
| African American | 20 | 17 | 23 | |
| Other | 5 | 6 | 5 | |
| Education, years | 14 (2) | 14 (2) | 14 (2) | 0.113 |
| Married | 65 | 68 | 62 | 0.135 |
| Comorbidity and Health Factors | ||||
| Body mass index, kg/m2 | 31 (7) | 30 (7) | 32 (7) | 0.001 |
| Smoking status | ||||
| Never | 46 | 38 | 54 | < 0.001 |
| Former | 39 | 43 | 35 | |
| Current | 15 | 19 | 11 | |
| Diabetes mellitus | 22 | 18 | 25 | 0.053 |
| Hypertension | 52 | 45 | 57 | 0.004 |
| Cardiovascular disease | 11 | 13 | 10 | 0.259 |
| Osteoporosis | 13 | 11 | 15 | 0.111 |
| HLA-DRB1 SE positivity* | 59 | 76 | 45 | < 0.001 |
Comparison limited to Caucasians and African Americans (n = 530); continuous values expressed as means and standard deviations and categorical data are expressed as a percentage.
Periodontal assessments
Results from blinded periodontal assessments are summarized in Table 2. Compared to controls (26%), a higher proportion of RA cases overall (35%; ORunadj 1.49; 95% CI 1.06, 2.11; p = 0.02) and ACPA positive cases (37%; ORunadj 1.65; 95% CI 1.15, 2.36; p = 0.006) satisfied criteria for PD. Relative to controls, RA cases overall (p = 0.026) and anti-CCP positive cases (p = 0.005) demonstrated a higher percentage of sites with probing depths ≥ 5 mm with a non-significant trend towards a higher proportion of sites with attachment loss ≥ 5 mm among anti-CCP positive cases (p = 0.060). Other individual periodontal measures did not differ significantly by group with the exception that controls were more likely than RA cases to exhibit any bleeding on probing, although there were no differences in the mean proportion of sites bleeding per subject.
Table 2.
Periodontal measures in rheumatoid arthritis (RA) cases, anti-CCP antibody positive RA cases, and controls
| RA (N = 287) |
Anti-CCP+ RA (N = 240) |
Controls (N = 330) |
|
|---|---|---|---|
|
|
|||
|
Periodontitis (% patients) |
34.8 P = 0.022 |
37.1 P = 0.006 |
26.4 Ref. |
|
Missing teeth (0 to 28, mean, SD) |
3.2 (3.1) P = 0.877 |
3.3 (3.1) P = 0.838 |
3.3 (3.5) Ref. |
|
Any plaque present (% patients) |
95.3 P = 0.157 |
96.7 P = 0.675 |
97.3 Ref. |
|
Proportion sites with plaque (mean % sites / patient ) |
42.0 P = 0.320 |
42.7 P = 0.202 |
39.9 Ref. |
|
Any bleeding with probing (% patients) |
87.1 P = 0.001 |
87.9 P = 0.005 |
94.6 Ref. |
|
Proportion sites bleeding with probing (mean % sites / patient) |
21.0 P = 0.383 |
21.2 P = 0.450 |
21.0 Ref. |
|
Any probing depth≥ 5mm (% patients) |
51.8 P=0.208 |
53.8 P=0.095 |
46.7 Ref. |
|
Proportion sites with probing depth ≥ 5mm (mean % sites / patient ) |
3.6 P = 0.026 |
4.1 P = 0.005 |
2.4 Ref. |
|
Any attachment loss ≥ 5mm (% patients) |
71.7 P = 0.833 |
73.3 P = 0.525 |
70.9 Ref. |
|
Proportion sites with attachment loss ≥ 5mm (mean % sites / patient) |
9.3 P = 0.135 |
10.0 0.060 |
7.5 Ref. |
After multivariable adjustment, including adjustments for smoking and HLA-DRB1 SE, PD remained significantly more frequent among anti-CCP antibody positive RA cases (OR 1.59; 95% CI 1.01, 2.49; p = 0.043) than controls, an association that was attenuated and not significant when all RA cases including anti-CCP antibody negative patients were evaluated (OR 1.36; 95% CI 0.89, 2.06; p = 0.153) (Table 3). Additional adjustments for anti-P. gingivalis antibodies (both OMA and LPS sequentially) and the presence of subgingival P. gingivalis (PCR) did not alter these results (data not shown). To examine for evidence of residual confounding, we then limited these analyses to never smokers. Although not reaching statistical significance given the reduced sample size, associations of PD with RA (OR 1.37; 95% CI 0.65, 2.89) and anti-CCP antibody positive RA (OR 1.65; 95% CI 0.75, 3.63) were similar in models limited to never smokers (data not shown). We observed no evidence of interaction of PD with either cigarette smoking or HLA-DRB1 SE positivity (data not shown).
Table 3.
Multivariable associations of periodontitis with rheumatoid arthritis *
| Odds Ratio (95% Confidence Interval) | ||
|---|---|---|
| All RA | Anti-CCP Positive RA | |
|
| ||
| Periodontitis | 1.36 (0.89, 2.06) P = 0.153 |
1.59 (1.01, 2.49) P = 0.043 |
| HLA-DRB1 SE positive | 3.95 (2.68, 5.83) P < 0.001 |
5.32 (3.44, 8.22) P < 0.001 |
| Ever Smoking | 1.93 (1.31, 2.83) P = 0.001 |
1.97 (1.29, 2.99) P = 0.002 |
Additional factors accounted for in multivariable models included age, gender, race/ethnicity, body mass index, self-reported diabetes mellitus, marital status, presence of oral dryness, and education; multivariable models limited to participants reporting either Caucasian or African American race/ethnicity
Bacterial serologies and subgingival P. gingivalis
Circulating antibody concentrations to P. gingivalis OMA and LPS were higher in participants with PD compared to those without, differences not seen with P. intermedia or F. nucleatum antibody. We observed no differences in anti-P. gingivalis or P. intermedia antibody concentration between RA cases and controls. In contrast, antibody concentrations to F. nucleatum were significantly higher in RA cases than controls (4.37 ± 0.85 units vs. 4.21 ± 0.87 units; p = 0.018). Subgingival colonization with P. gingivalis was common, observed in two-thirds (67%) of study participants. Although more common among those with PD vs. those without, there were no RA-control differences in the frequency of detectable subgingival P. gingivalis (Table 4). We observed no significant differences for any of the aforementioned bacterial measures between cases and controls after limiting the analysis to those with PD (data not shown).
Table 4.
Log-transformed bacterial IgG antibody concentrations based on cases status (rheumatoid arthritis [RA] and anti-CCP positive RA vs. Controls) and the presence of periodontitis (PD)
| RA | Anti-CCP+ RA |
Controls | PD (all) | No PD | |
|---|---|---|---|---|---|
| (n = 287) | (n = 240) | (n = 330) | (n = 187) | (n = 430) | |
|
|
|||||
| Anti-P. gingivalis | |||||
| Outer membrane | 4.30 (0.94) P = 0.819 |
4.32 (0.93) P = 0.943 |
4.32 (0.93) Ref. |
4.57 (0.87) P < 0.001 |
4.20 (0.94) Ref. |
| LPS | 5.32 (0.36) P = 0.544 |
5.32 (0.35) P = 0.411 |
5.30 (0.33) Ref. |
5.36 (0.31) P = 0.008 |
5.28 (0.36) Ref. |
| Anti-F. nucleatum | 4.37 (0.85) P = 0.018 |
4.40 (0.85) P = 0.008 |
4.21 (0.87) Ref. |
4.35 (0.82) P = 0.205 |
4.26 (0.88) Ref. |
| Anti-P. intermedia | 4.83 (0.59) P = 0.883 |
4.84 (0.57) P = 0.766 |
4.82 (0.63) Ref. |
4.88 (0.53) P = 0.155 |
4.80 (0.64) Ref. |
| Subgingival P. gingivalis PCR Positive | 65% P = 0.185 |
65% P = 0.197 |
70% Ref. |
84% P < 0.001 |
60% Ref. |
Periodontitis and RA disease characteristics
The frequency of HLA-DRB1 SE positivity did not differ between RA cases with PD (78%) and those without (74%) (p = 0.442) (data not shown). Compared to RA cases without PD, those with PD had significantly higher swollen joint counts, DAS-28-CRP, and radiographic damage (Table 5). Likewise, patients with PD were more likely to be RF positive and demonstrated significantly higher concentrations of circulating RF and anti-CCP2 compared to cases without periodontitis. Although not reaching statistical significance, there was a trend towards higher prevalence of anti-CCP2 positivity (p = 0.066) and higher tender joint counts (p = 0.069) in patients with PD.
Table 5.
Rheumatoid arthritis (RA) related measures based on the presence of periodontitis
| All RA (n = 287) |
RA with Periodontitis (n = 100) |
RA without Periodontitis (n = 187) |
P-value | |
|---|---|---|---|---|
|
|
||||
| Disease duration, years | 12.6 (9.9) | 12.0 (10.0) | 12.9 (9.9) | 0.380 |
| Swollen joint count (0–28) | 3.6 (4.3) | 4.5 (4.5) | 3.1 (4.1) | 0.004 |
| Tender joint count (0–28) | 3.2 (4.6) | 3.8 (4.9) | 2.8 (4.4) | 0.069 |
| High sensitivity CRP, mg/L | 8.3 (21.1) | 10.6 (30.8) | 7.1 (13.1) | 0.420 |
| Patient global (0–10) | 4.2 (2.7) | 4.3 (2.7) | 4.2 (2.6) | 0.610 |
| DAS-28-CRP* | 3.2 (1.3) | 3.5 (1.4) | 3.1 (1.2) | 0.045 |
| Total Sharp score | 19.5 (23.1) | 24.5 (27.7) | 16.9 (19.8) | 0.015 |
| Joint space narrowing | 15.1 (17.1) | 18.6 (19.6) | 13.3 (15.3) | 0.011 |
| Erosions | 4.4 (8.2) | 5.8 (10.6) | 3.6 (6.4) | 0.289 |
| Anti-CCP positivity, % | 85 | 90 | 82 | 0.066 |
| Anti-CCP, U/ml | 145 (128) | 170 (128) | 131 (126) | 0.011 |
| RF positivity, % | 77 | 86 | 72 | 0.006 |
| RF, IU/ml | 256 (491) | 390 (633) | 185 (379) | <0.001 |
| Methotrexate, % | 62 | 61 | 62 | 0.864 |
| Prednisone, % | 30 | 33 | 28 | 0.359 |
| Biologic, % | 31 | 37 | 27 | 0.089 |
P-value for DAS-28-CRP was generated by comparing log-transformed values and using two-sample t-test; actual calculated values shown for each group; continuous values expressed as means and standard deviations and categorical data are expressed as a percentage
Associations of serologies in patients with RA
There were weak but statistically significant correlations of anti-P. gingivalis OMA antibody concentration with anti-CCP2 (r = 0.14, p = 0.022), RF (r = 0.19, p = 0.001), and hs-CRP (r = 0.15, p = 0.011). Although we observed similarly weak correlations of anti-P. gingivalis LPS antibody with RF (r = 0.14, p = 0.018), there were no associations of this bacterial LPS antibody or alternative bacterial serologies with anti-CCP2 or hs-CRP concentrations nor were there correlations of alternative bacterial serologies with RF concentration (r < 0.1, p > 0.1).
Periodontitis, anti-P. gingivalis antibody, and ACPA expression
Based on the observed associations with anti-CCP2 values (associations that were not observed with the alternative bacterial serologies), we subsequently examined the associations of PD and anti-P. gingivalis OMA antibody with specific ACPA subtypes limiting analyses to anti-CCP2 positive RA cases (n = 240). Given its potential effects on ACPA expression, all analyses accounted for smoking status. Compared to never smokers without PD, we observed minimal differences in ACPA expression with either smoking or PD alone (Figure 1). In contrast, RA patients with both PD and a history of smoking demonstrated greater expression of ACPA targeting several citrullinated antigens including those derived from clusterin, enolase, filaggrin, and fibrinogen (Figure 1). Among seropositive RA cases with a history of smoking, higher concentrations of anti-P. gingivalis OMA antibody were similarly associated with increased expression of ACPA targeting clusterin, enolase, filaggrin, and fibrinogen (Supplementary Figure 1, top panel). Among seropositive never-smokers, higher concentrations of anti-P. gingivalis antibody were associated with increased expression of ACPA targeting filaggrin, histone 2A, and vimentin (both recombinant protein and citrulline-containing peptide 58–77) (Supplementary Figure 1, bottom panel). Similar associations were observed among anti-CCP2 positive patients based on the presence of subgingival P. gingivalis (Supplementary Figure 2). Among seropositive cases with a history of smoking, the presence of subgingival P. gingivalis was associated with increased expression of several ACPA including autoantibodies recognizing citrullinated forms of clusterin, histone 2B, apolipoprotein E, fibrinogen, and filaggrin (Supplementary Figure 2, top panel). Among anti-CCP2 positive never smokers, the presence of subgingival P. gingivalis was associated with increased expression of ACPA targeting filaggrin, histone 2B, and apolipoprotein E (Supplementary Figure 2, bottom panel). In analyses stratified by the presence of PD, current smokers without PD demonstrated a significantly higher ACPA score than never smokers (p = 0.043) with a non-significant trend towards a higher ACPA score than former smokers (p = 0.083) (Figure 2). In contrast, among those with PD, there were no observed differences in ACPA score based on smoking status.
Figure 1.
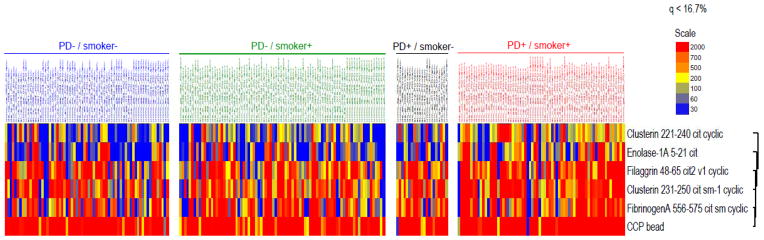
Heat map demonstrating increased levels of several antigen-specific ACPAs in anti-CCP2 antibody positive RA cases with both periodontitis and a history of ever smoking (only significant ACPA shown); compared to those without periodontitis and never smoking, there were no significant differences in ACPA expression seen among those with only periodontitis or only smoking.
Figure 2.
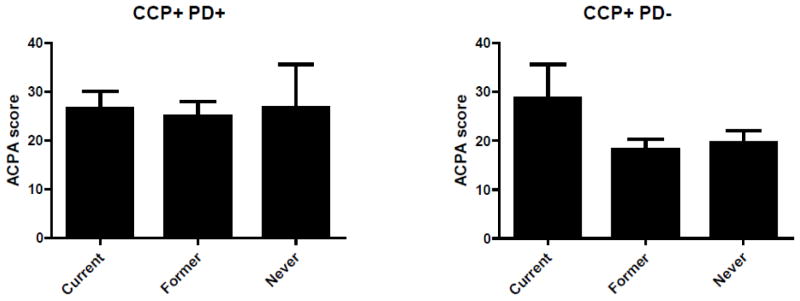
ACPA score among anti-CCP2 positive RA cases categorized as never, former, or current smokers; ACPA scores shown for patients with periodontitis (top panel) and without periodontitis (bottom panel). Among RA cases without periodontitis, ACPA scores significantly higher in current smokers compared to both never smokers (p = 0.043) with a non-significant trend (p = 0.083) in comparisons of current vs. former smokers. There were no differences in ACPA score based on smoking status among those with periodontitis (p = NS).
Discussion
Our observations both confirm and extend prior reports demonstrating a higher frequency of PD in the context of RA and in particular seropositive RA. In lending support that this relationship is most relevant to ACPA-positive disease, our results further demonstrate that this association is independent of several factors that have been previously hypothesized to act as confounders or mediators. Of covariates examined in this study, cigarette smoking has perhaps been speculated to serve as the single greatest confounder in assessments of the relationship of PD with RA. Estimated to account for approximately 1 in 6 new RA cases, the attributable risk of smoking in the development of PD is even greater, accounting for ~1 in 2 incident cases (40). Using periodontal surgery as a disease surrogate, yielding a sensitivity of only 25% for the identification of severe PD (41), separate investigators observed positive trends that were evident only in smokers, a relationship that was absent among never smokers (42). This contrasts with results from our study in addition to recent results from Scher and colleagues, both of which included full-mouth periodontal examinations, with the latter showing a significantly higher prevalence of PD among RA patients with new-onset treatment-naïve disease, the majority of whom had never smoked (17).
In contrast to our a priori hypothesis, the association of PD with established RA did not appear to be dependent on evidence of prior infection or subgingival colonization with P. gingivalis. Although the presence of subgingival bacteria and antibody responses to P. gingivalis were higher in those with PD as expected, we found no difference in either antibody concentrations or the presence of subgingival P. gingivalis in RA cases compared to controls. These results parallel recent reports, including one study examining patients with new-onset disease (17) and another focused on established RA (18). In the latter study, investigators found no significant differences in IgG anti-P. gingivalis OMA antibody concentrations or cultivatable subgingival bacteria in RA cases compared to controls (although case-control differences in IgM overall and both IgM and IgG anti-P. gingivalis antibody concentrations were observed among those with severe PD). Using pyrosequencing techniques, Scher and colleagues found no significant case-control differences in the presence of subgingival P. gingivalis or in the frequency of circulating bacterial antibody (17). While unexpected, we found significantly higher levels of circulating antibody to F. nucleatum among RA cases than in controls, the first time to our knowledge that this has been reported. In contrast to P. gingivalis, F. nucleatum is less virulent and has less robust evidence of an etiologic link with oral disease; a fact that may explain its lack of association with PD in our study. In experimental PD, co-infection of P. gingivalis and F. nucleatum has been shown to have a synergistic effect on PD-related soft tissue destruction compared to mono-infection with either of the bacteria alone (43). Moreover, recent studies of experimental PD have shown that co-infection attenuates immune responses to P. gingivalis while having no effect on immune responses to F. nucleatum (44). Expressing a variety of surface adhesins, F. nucleatum is speculated to act as a ‘bridging’ organism in the complex bio-film that characterizes PD, linking early colonizers (often Gram positive anaerobes) to later colonizers (Gram negative anaerobes including P. gingivalis) (44). Whether co-aggregation of F. nucleatum with P. gingivalis or alternative oral pathogens explains the observed association of this particular bacterial species with RA remains to be defined.
Our results demonstrate that PD is positively associated with several measures of RA disease activity and severity. The associations of PD with higher serum concentrations of RF and anti-CCP2 autoantibody are perhaps most noteworthy, given the robust associations of these measures with poor long-term outcomes in RA (45). Indeed, the presence of PD was associated with increased joint damage as reflected in higher radiographic Sharp scores. Similar to the recent study of de Smit and colleagues (18), we observed weak but statistically significant correlations of anti-P. gingivalis OMA antibody with anti-CCP2 concentrations. Importantly, these correlations appear to be unique to P. gingivalis as they were not observed with the other bacterial serologies examined. Whether there are specific bacterial antigens that drive this association are unknown, recognizing recent work focusing on putative antigens expressed by P. gingivalis including bacterially expressed PAD (46) and intra-cellular alpha-enolase (47).
In the study by de Smit et al, investigators observed no associations of PD with ACPA fine specificity (18), although this study examined autoreactivity to only 5 citrullinated peptides. We also found no association of PD alone with ACPA fine specificity among anti-CCP2 positive patients. However, PD and smoking appeared to serve as co-factors in shaping ACPA specificity, suggesting a synergistic effect in promoting autoantibody reactivity to multiple citrullinated antigens that included clusterin, enolase, filaggrin, and fibrinogen. Using the ACPA score as a surrogate for the magnitude of epitope spreading present, we examined associations of smoking status with autoantibody expression in those with and without PD. Although only the difference between current and never smokers reached statistical significance, both former and never smokers demonstrated numerically lower ACPA scores compared to current smokers, but only in the absence of PD. These data suggest that ACPA responses could be modifiable with smoking cessation, but only if PD is absent (or possibly treated), and that PD exerts an independent effect on ACPA expression. Additional longitudinal studies with even larger numbers of patients will be essential in understanding whether interventions focused on smoking cessation and/or PD treatment may alter the course of RA.
In addition to the potential effect of PD on ACPA expression, our results suggest that P. gingivalis may help to shape autoantibody specificity in RA independent of smoking status. We observed increased ACPA among both smokers and non-smokers with higher serum concentrations of IgG anti-P. gingivalis antibody. Similarly, we observed overexpression of ACPA targeting both citrullinated filaggrin and histone in patients with evidence of subgingival P. gingivalis, regardless of smoking status. To our knowledge, this is the first time that autoreactivity to these antigens has been examined in the context of RA and PD. These results contrast with a report showing associations of cultivatable subgingival P. gingivalis with higher serum concentrations of ACPA targeting only citrullinated fibrinogen (18). Although we also observed associations of subgingival P. gingivalis with anti-citrullinated fibrinogen antibody, this was present only in smokers and not in never-smokers, suggesting that the impact of smoking may at least partially account for these previously reported findings. Whether the diseased periodontium acts as a ‘reservoir’ for autoantigen in RA remains to be defined. Filaggrin, ubiquitous in skin epithelium, is also highly expressed in the oral mucosa and its expression is up-regulated with smoking (48). Both PD and P. gingivalis have been linked to neutrophil activation (49, 50), a possible source of deiminated histone (51).
In summary, these results demonstrate an independent relationship between PD and established ACPA positive RA. Importantly, these data suggest that PD may act in concert with cigarette smoking to shape the autoantibody reactivity that characterizes RA, with supporting evidence P. gingivalis infection impacts disease-specific autoantibody responses independent of cigarette smoking.
Supplementary Material
Acknowledgments
Grant Support: Funding for this project was provided by the Rheumatology Research Foundation Disease Targeted Research Initiative (PI, Mikuls). Dr. Mikuls is also supported by the Nebraska Arthritis Outcomes Research Center and by grants from the Veterans Affairs Office of Research & Development (VA Merit) and NIH/NIAMS.
References
- 1.Pizzo G, Guiglia R, Lo Russo L, Campisi G. Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur J Intern Med. 2010;21(6):496–502. doi: 10.1016/j.ejim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Snyderman R, McCarty GA. Analogous mechanism of tissue destruction in rheumatoid arthritis and periodontal disease. In: Genco R, Mergenhagen S, editors. Host-Parasite Interactions in Periodontal Diseases. Washington, DC: American Society for Microbiology; 1982. pp. 354–62. [Google Scholar]
- 3.Albandar JM, Streckfus CF, Adesanya MR, Winn DM. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J Periodontol. 2000;71(12):1874–81. doi: 10.1902/jop.2000.71.12.1874. [DOI] [PubMed] [Google Scholar]
- 4.Bonfil JJ, Dillier FL, Mercier P, Reviron D, Foti B, Sambuc R, et al. A ‘case control’ study on the role of HLA DR4 in severe periodontitis and rapidly progressive periodontitis. Identification of types and subtypes using molecular biology (PCR.SSO) J Clin Periodontal. 1999;26:77–84. doi: 10.1034/j.1600-051x.1999.260203.x. [DOI] [PubMed] [Google Scholar]
- 5.Calsina G, Ramon JM, Echeverria JJ. Effects of smoking on periodontal tissues. J Clin Periodontol. 2002;29(8):771–6. doi: 10.1034/j.1600-051x.2002.290815.x. [DOI] [PubMed] [Google Scholar]
- 6.Criswell LA, Merlino LA, Cerhan JR, Mikuls TR, Mudano AS, Burma M, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;15:465–71. doi: 10.1016/s0002-9343(02)01051-3. [DOI] [PubMed] [Google Scholar]
- 7.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30(11):1205–13. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 8.Karlson EW, Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. A retrospective cohort study of cigarette smoking and risk of rheumatoid arthritis in female health professionals. Arthritis Rheum. 1999;42(5):910–7. doi: 10.1002/1529-0131(199905)42:5<910::AID-ANR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Nepom GT, Nepom BS. Prediction of susceptibility to rheumatoid arthritis by human leukocyte antigen genotyping. Rheum Dis Clin North Am. 1992;18(4):785–92. [PubMed] [Google Scholar]
- 10.Wordsworth P, Pile KD, Buckely JD, Lanchbury JS, Ollier B, Lathrop M, et al. HLA heterozygosity contributes to susceptibility to rheumatoid arthritis. Am J Hum Genet. 1992;51(3):585–91. [PMC free article] [PubMed] [Google Scholar]
- 11.de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35(1):70–6. [PubMed] [Google Scholar]
- 12.Dissick A, Redman RS, Jones M, Rangan BV, Reimold A, Griffiths GR, et al. Association of periodontitis with rheumatoid arthritis - a pilot study. J Periodontol. 2010;81:223–9. doi: 10.1902/jop.2009.090309. [DOI] [PubMed] [Google Scholar]
- 13.Kasser UR, Gleissner C, Dehne F, Michel A, Willershausen-Zonnchen B, Bolten WW. Risk for periodontal disease in patients with longstanding rheumatoid arthritis. Arthritis Rheum. 1997;40:2248–51. doi: 10.1002/art.1780401221. [DOI] [PubMed] [Google Scholar]
- 14.Malmstrom M, Calonius PE. Teeth loss and the inflammation of teeth-supporting tissues in rheumatoid disease. Scand J Rheumatol. 1975;4:49–55. doi: 10.3109/03009747509095615. [DOI] [PubMed] [Google Scholar]
- 15.Pischon N, Pischon T, Kroger J, Gulmez E, Kleber BM, Bernimoulin JP, et al. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol. 2008;79:979–86. doi: 10.1902/jop.2008.070501. [DOI] [PubMed] [Google Scholar]
- 16.Potikuri D, Dannana KC, Kanchinadam S, Agrawal S, Kancharla A, Rajasekhar L, et al. Periodontal disease is significantly higher in non-smoking treatment-naive rheumatoid arthritis patients: results from a case-control study. Ann Rheum Dis. 2012;71(9):1541–4. doi: 10.1136/annrheumdis-2011-200380. [DOI] [PubMed] [Google Scholar]
- 17.Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64(10):3083–94. doi: 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Smit MD, Westra J, Vissink A, Doornbos-van der Meer B, Brouwer E, van Winkelhoff AJ. Periodontitis in established rheumatoid arthritis patients: a cross-sectional clinical, microbiological and serological study. Arthritis Res Ther. 2012;14(5):R222. doi: 10.1186/ar4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation. 2004;28:311–8. doi: 10.1007/s10753-004-6641-z. [DOI] [PubMed] [Google Scholar]
- 20.Hitchon CA, Chandad F, Ferucci ED, Willemze A, Ioan-Facsinay A, Van der Woude D, et al. Antibodies to Porphyromonas Gingivalis are associated with anti-citrullinated protein antibodies in RA patients and their relatives. J Rheumatol. 2010;37:1105–12. doi: 10.3899/jrheum.091323. [DOI] [PubMed] [Google Scholar]
- 21.Mikuls TR, Payne JB, Reinhardt RA, Thiele GM, Maziarz E, Cannella AC, et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol. 2009;9:38–42. doi: 10.1016/j.intimp.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogrendik M, Kokino S, Ozdemir F, Bird PS, Hamlet S. Serum antibodies to oral anaerobic bacteria in patients with rheumatoid arthritis. MedGenMed. 2005;7:2. [PMC free article] [PubMed] [Google Scholar]
- 23.Mikuls TR, Thiele GM, Deane KD, Payne JB, O’Dell JR, Yu F, et al. Porphyromonas gingivalis and disease-related autoantibodies in Individuals at increased risk for future rheumatoid arthritis. Arthritis Rheum. 2012;64:3522–30. doi: 10.1002/art.34595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Little JW, Jacobson JJ, Lockhart PB. The dental treatment of patients with joint replacements: a position paper from the American Academy of Oral Medicine. J Am Dent Assoc. 2010;141(6):667–71. doi: 10.14219/jada.archive.2010.0255. [DOI] [PubMed] [Google Scholar]
- 26.Watters W, 3rd, Rethman MP, Hanson NB, Abt E, Anderson PA, Carroll KC, et al. Prevention of orthopaedic implant infection in patients undergoing dental procedures. J Am Acad Orthop Surg. 2013;21(3):180–9. doi: 10.5435/JAAOS-21-03-180. [DOI] [PubMed] [Google Scholar]
- 27.Machtei EE, Christersson LA, Grossi SG, Dunford R, Zambon JJ, Genco RJ. Clinical criteria for the definition of “established periodontitis”. J Periodontol. 1992;63(3):206–14. doi: 10.1902/jop.1992.63.3.206. [DOI] [PubMed] [Google Scholar]
- 28.Htoon HM, Peng LL, Huak CY. Assessment criteria for compliance with oral hygiene: application of ROC analysis. Oral Health Prev Dent. 2007;5(2):83–8. [PubMed] [Google Scholar]
- 29.Walker C, Puumala S, Golub LM, Stoner JA, Reinhardt RA, Lee HM, et al. Subantimicrobial dose doxycycline effects on osteopenic bone loss: microbiologic results. J Periodontol. 2007;78(8):1590–601. doi: 10.1902/jop.2007.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hueber W, Tomooka BH, Zhao X, Kidd BA, Drijfhout JW, Fries JF, et al. Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with up regulation of proinflammatory cytokines. Ann Rheum Dis. 2007;66(6):712–9. doi: 10.1136/ard.2006.054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 32.van der Heijde D, Dankert T, Nieman F, Rau R, Boers M. Reliability and sensitivity to change of a simplification of the Sharp/van der Heijde radiological assessment in rheumatoid arthritis. Rheumatology (Oxford) 1999;38:941–7. doi: 10.1093/rheumatology/38.10.941. [DOI] [PubMed] [Google Scholar]
- 33.Dzink JL, Socransky SS, Haffajee AD. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol. 1988;15(5):316–23. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 34.Maeda H, Fujimoto C, Haruki Y, Maeda T, Kokeguchi S, Petelin M, et al. Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol Med Microbiol. 2003;39(1):81–6. doi: 10.1016/S0928-8244(03)00224-4. [DOI] [PubMed] [Google Scholar]
- 35.Dilthey AT, Moutsianas L, Leslie S, McVean G. HLA*IMP--an integrated framework for imputing classical HLA alleles from SNP genotypes. Bioinformatics. 2011;27(7):968–72. doi: 10.1093/bioinformatics/btr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leslie S, Donnelly P, McVean G. A statistical method for predicting classical HLA alleles from SNP data. Am J Hum Genet. 2008;82(1):48–56. doi: 10.1016/j.ajhg.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikuls TR, Gould KA, Bynote KK, Yu F, Levan TD, Thiele GM, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione s-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12(6):R213. doi: 10.1186/ar3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98(9):5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kallberg H, Ding B, Padyukov L, Bengtsson C, Ronnelid J, Klareskog L, et al. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70:508–11. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71(5):743–51. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- 41.Genco RJ, Falkner KL, Grossi S, Dunford R, Trevisan M. Validity of self-reported measures for surveillance of periodontal disease in two western New York population-based studies. J Periodontol. 2007;78(7 Suppl):1439–54. doi: 10.1902/jop.2007.060435. [DOI] [PubMed] [Google Scholar]
- 42.Arkema EV, Karlson EW, Costenbader KH. A prospective study of periodontal disease and risk of rheumatoid arthritis. J Rheumatol. 2010;37(9):1800–4. doi: 10.3899/jrheum.091398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polak D, Wilensky A, Shapira L, Halabi A, Goldstein D, Weiss EI, et al. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J Clin Periodontol. 2009;36(5):406–10. doi: 10.1111/j.1600-051X.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- 44.Polak D, Shapira L, Weiss EI, Houri-Haddad Y. The role of coaggregation between Porphyromonas gingivalis and Fusobacterium nucleatum on the host response to mixed infection. J Clin Periodontol. 2012;39(7):617–25. doi: 10.1111/j.1600-051X.2012.01889.x. [DOI] [PubMed] [Google Scholar]
- 45.Miriovsky BJ, Michaud K, Thiele GM, O’Dell J, Cannon GW, Kerr G, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease burden in U.S. veterans with rheumatoid arthritis. Ann Rheum Dis. 2010;69:1292–7. doi: 10.1136/ard.2009.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quirke AM, Lugli EB, Wegner N, Hamilton BC, Charles P, Chowdhury M, et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202726. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62(9):2662–72. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reno F, Rocchetti V, Migliario M, Rizzi M, Cannas M. Chronic exposure to cigarette smoke increases matrix metalloproteinases and Filaggrin mRNA expression in oral keratinocytes: role of nicotine stimulation. Oral Oncol. 2011;47(9):827–30. doi: 10.1016/j.oraloncology.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Delbosc S, Alsac JM, Journe C, Louedec L, Castier Y, Bonnaure-Mallet M, et al. Porphyromonas gingivalis participates in pathogenesis of human abdominal aortic aneurysm by neutrophil activation. Proof of concept in rats. PLoS One. 2011;6(4):e18679. doi: 10.1371/journal.pone.0018679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott DA, Krauss J. Neutrophils in periodontal inflammation. Front Oral Biol. 2012;15:56–83. doi: 10.1159/000329672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dwivedi N, Upadhyay J, Neeli I, Khan S, Pattanaik D, Myers L, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982–92. doi: 10.1002/art.33432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


