Abstract
Caffeine is the most consumed pychostimulant in the world, and it is known to affect basic and fundamental human processes such as sleep, arousal, cognition and learning and memory. It works as a nonselective blocker of adenosine receptors (A1, A2a, A2b and A3) and has been related to the regulation of heart rate, the contraction/relaxation of cardiac and smooth muscles, and the neural signaling in the central nervous system (CNS). Since the late 1990s, studies using adenosine receptor antagonists, such as Caffeine, to block the A1 and A2a adenosine receptor subtypes have shown to reduce the physical, cellular and molecular damages caused by a spinal cord injury (SCI) or a stroke (cerebral infarction) and by other neurodegenerative diseases such as Parkinson's and Alzheimer's diseases. Interestingly, other studies using adenosine receptor agonists have also shown to provide a neuroprotective effect on various models of neurodegenerative diseases through the reduction of excitatory neurotransmitter release, apoptosis and inflammatory responses, among others. The seemingly paradoxical use of both adenosine receptor agonists and antagonists as neuroprotective agents has been attributed to differences in dosage levels, drug delivery method, extracellular concentration of excitatory neurotransmitters and stage of disease progression. We discuss and compare recent findings using both antagonists and agonists of adenosine receptors in animal models and patients that have suffered spinal cord injuries, brain strokes, and Parkinson's and Alzheimer's diseases. Additionally, we propose alternative interpretations on the seemingly paradoxical use of these drugs as potential pharmacological tools to treat these various types of neurodegenerative diseases.
Keywords: Adenosine receptors, Caffeine, Stroke, Spinal cord injury, Parkinson's disease, Alzheimer's disease
Spinal cord injury (SCI)
Spinal cord injury (SCI) is the main cause of disability worldwide producing mainly mechanical and physical damage, which may lead to inflammation and neuronal cell death (Palacios et al., 2012). Adenosine receptors have been shown to have a major role in regulating the inflammatory responses after a SCI (Song et al., 2009). For example, the blockade of the A1 adenosine receptor by caffeine has been involved in mediating neuroprotective effects against SCI, including reduction of hyperalgesia, which involves an attenuation of hypersensitivity to pain, usually caused by damage to nociceptors (pain receptors) and/or peripheral nerves after injury (Palacios et al., 2012; Stone et al., 2009). Also, daily caffeine intake in mice has been shown to inhibit the process of antinociception (increased tolerance to pain) by modulation of the A1 receptor as demonstrated using the specific A1 receptor antagonist DPCPX, which mimicked the effects of caffeine (Salvemini et al., 2013). In addition, caffeine application to the spinal cord of guinea pigs after a SCI induced an up-regulation of the A1 receptor and of tissue growth factor (TGF)-beta mRNAs, which has been shown to provide immune regulation of inflammation further supporting a neuroprotective role of caffeine (Butler and Prendergast, 2012; Chen et al., 2010; Salvemini et al., 2013). These findings suggest a role of the A1 adenosine receptor as a key target for the regulation of pain and the inflammatory response that ensues in patients after suffering a SCI (Fig. 1).
Fig. 1.
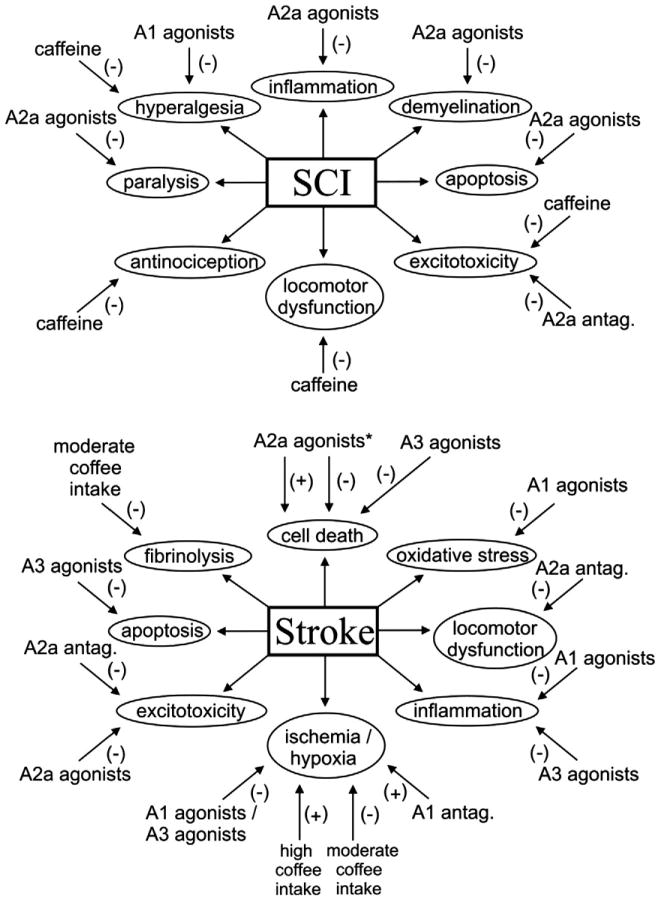
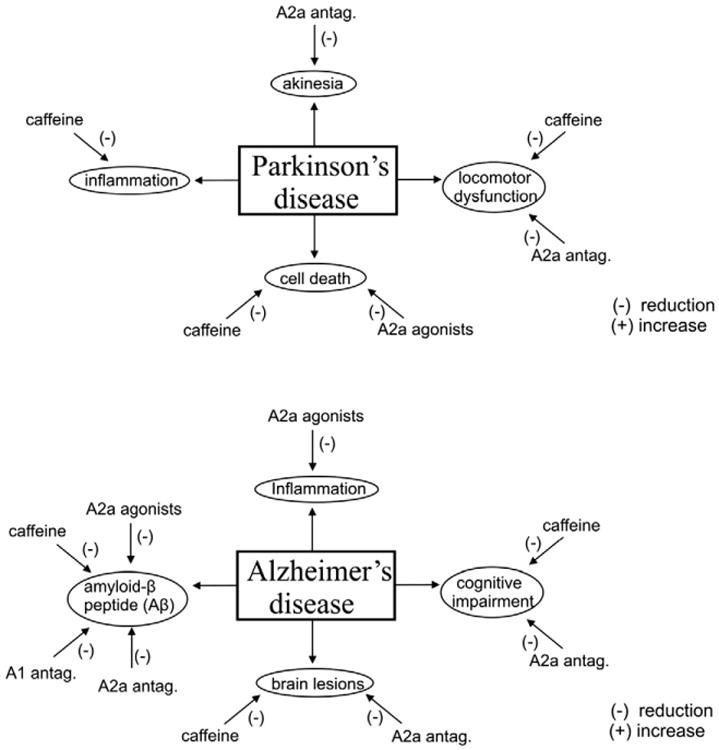
Summary of the reported results of using adenosine receptor agonists and antagonists to treat the main detrimental effects caused by a spinal cord injury, stroke, and Parkinson's and Alzheimer's diseases. *The effects of A2a receptor agonists on cell death caused by a stroke have been shown to include a neuroprotective role in acute experiments and a neurally detrimental role in chronic experiments after brain damage (Stone et al., 2009).
Regulation of the adenosine A2a receptor has been implicated in the modulation of the anti-inflammatory or proinflammatory responses having a protective role against tissue damage and locomotor dysfunction in animal models of SCI (Dai et al., 2010b; Pan and Chen, 2004). Pharmacological blockade of A2a receptors helps protect the CNS after a SCI by reducing excessive release of neurotransmitters caused by high levels of intracellular calcium ions, which can lead to neuronal death through increased excitability (excitotoxicity) (Pan and Chen, 2004). For example, enhanced release of the endogenous neurotransmitter adenosine soon after a SCI has been related to the development of many known functional motor and sensory deficits (Pan and Chen, 2004). Thus, the blockade of both A1 and A2a adenosine receptors has shown to provide a protective role against SCI-induced pain, inflammation and cell death caused by excessive neuronal activity.
The role of adenosine receptor agonists as potential neuroprotective agents against SCI has also been reported. A recent study showed that the intrathecal application of R(−)N6-(2-phenylisopropyl) adenosine (R-PIA), a selective A1 receptor agonist, inhibited SCI-induced hyperalgesia in rats (Higashi et al., 2002). On the contrary, the intrathecal application of CGS21680, a selective A2a receptor agonist, did not inhibit SCI-induced hyperalgesia, suggesting that adenosine inhibits hyperalgesia through the specific activation of A1 receptors (Higashi et al., 2002). Although there is a substantial body of work showing the use of A1 receptor agonists as therapeutic tools in many animal models of Central (CNS) pain (Sawynok and Reid, 2011), few studies have shown the use of A1 receptor agonists as neuroprotective agents after a SCI.
In contrast, multiple A2a adenosine receptor agonists have been demonstrated to protect against damage and locomotor dysfunction after a SCI (Reece et al., 2004). A study found that A2a receptor agonist ATL146e, given during rabbit spinal cord reperfusion, resulted in a time-dependent improvement in spinal cord function after ischemia and reduced paralysis and apoptosis (Cassada et al., 2002). In another rabbit model, the application of ALT146e also significantly improved motor function and neuronal viability after a SCI (Okonkwo et al., 2006). The intraperitoneal injection of the A2a receptor agonist CGS21680 onto mice where a SCI was induced by extradural compression at T5 to T8 displayed a neuroprotective effect by reducing tissue damage, locomotor dysfunction and inflammation (Genovese et al., 2010; Paterniti et al., 2011). In another study using a similar mouse model of SCI the combinatory use of the A2a receptor agonists ATL 313 and CGS 21680 administered after injury, reduced tissue damage, TUNEL staining, cytokine (TNF-alpha) expression, Bax, Fas-L and caspase-3 expression and annexin V staining while increasing Bcl-2 expression, which suggested a protective role against the onset of neuronal apoptosis, which is a significant source of secondary damage after a SCI (Gelber et al., 2011).
Results obtained from the use of adenosine receptor antagonists and agonists provided both similar and contrasting results. The use of A1 and A2a adenosine receptor blockers provided neuroprotection by decreasing pain and inflammatory responses triggered after a SCI, while the use of agonists was shown to mostly enhance cellular viability and motor function in the animal models. But there were also similar results regarding the reduction of hyperalgesia by both the A1 adenosine receptor antagonist and agonist. Also, the use of A2a adenosine receptor antagonists and agonists showed decreased neuronal death by the reduction of neurotransmitter release, thus avoiding neural excitotoxicity (A2a antagonist) and by the reduction of overall tissue damage and apoptosis (A2a agonist). Potential explanations for the seemingly paradoxical effects by using both agonists and antagonists of adenosine receptors will be discussed later.
Stroke
A brain stroke can lead to rapid neurological damage due to ischemia or blood-related neurotoxicity as in the case of hemorrhagic strokes. The inflammatory response triggered after a stroke plays an important role in the pathogenesis of this type of injury. Recent studies have shown dose-dependent effects of caffeine on human health, which were positively or negatively associated with the development of cardiovascular diseases such as hypercholesterolemia, hypertension and myocardial infarction (Arosio et al., 2011). For instance, a study showed that elevated coffee intake is associated with a transiently increased risk of ischemic stroke (Mostofsky et al., 2010). Another study showed that the long-term moderate consumption of coffee can provide protective effects (reducing the risk of both coronary heart disease and stroke by 10%–20%) in healthy individuals yet detrimental effects when intake was high (Bohn et al., 2012). An epidemiological study showed that neither the high (more than 4 cups a day) nor the low doses (less than 2 cups a day) has the most dangerous effect but is the intermediate consumption (2–4 cups a day) of coffee, which can be the most harmful by decreasing, for example, the process of fibrinolysis, which prevents blood clots from increasing in size (Montagnana et al., 2012). Additionally, studies performed in women without cardiovascular disease or cancer history show that low or no coffee consumption is associated with an increased risk of stroke (Larsson et al., 2011). Thus, the risk of ischemic stroke associated with caffeine consumption still demands further study since it can exert positive or negative effects depending on dosage (biphasic effects). These seemingly contrasting effects by different doses could be attributed to a differential expression of adenosine receptor subtypes from subject to subject, mainly A1 and A2a, in the basal ganglia including the striatum and globus pallidus (Xie et al., 2007).
Adenosine is a biological mediator with an important role in signal transduction, which increases drastically when brain ischemia is caused by a stroke (Wei et al., 2011). Thus, it is important to consider the use of adenosine receptor agonists and antagonists as pharmacological tools to promote normal CNS function after a stroke. Several reports have confirmed the neuroprotective role of A2a receptor antagonists in different models of ischemia. The selective A2a receptor antagonist SCH58261 reduced ischemic brain damage in an adult rat model of focal cerebral ischemia (Melani et al., 2003; Monopoli et al., 1998a, 1998b). The same antagonist, subchronically administered, was protective against both brain damage and neurological deficits (Melani et al., 2006; Pedata et al., 2005). Also, the selective A2A antagonist ZM241385 reduced hippocampal injury and improved performance in the Morris water maze after four-vessel occlusion in the rat (Higashi et al., 2002). Other studies have benefited by the recent generation and characterization of genetic knockout models for all the four adenosine receptors (A1, A2a, A2b and A3), which further supported the potential neuromodulatory role of these receptors in the control of normal and abnormal CNS function after a stroke (Wei et al., 2011). A later study extended these findings by using selective inactivation of A2a receptors on bone marrow-derived cells (BMDCs) to demonstrate that A2a receptor loss on either BMDCs or non-BMDCs was sufficient to protect against a traumatic brain injury (Dai et al., 2010a). Notably, using this same paradigm, A2a receptors were found capable of producing contrasting effects on brain injury responses and outcomes in a manner that depended on local glutamate concentrations (Dai et al., 2010b). A2a receptor agonists attenuated the morphologic, behavioral, cellular and cytokine changes induced by brain injury when glutamate levels were low. In contrast, when glutamate levels were high (as when following a stroke), A2a receptor antagonists (not agonists) were found to have a protective effect as well. These results suggest that the seemingly paradoxical use of both an A2a antagonist and agonist to provide cellular protection after a stroke can be dependent on the concentration of excitatory neurotransmitters such as glutamate and possibly other neurotransmitter systems known to be critical in the regulation of motor responses such as adenosine and dopamine (Collins et al., 2010; Ferré, 2008).
Regarding the role of the A1 adenosine receptor in stroke, a recent study found that the activation of this receptor provided neuroprotection after transient middle cerebral artery occlusion and that blocking this receptor with DPCPX (an A1 adenosine receptor antagonist) eliminates the neuroprotective effects (Hu et al., 2012). Additionally, the activation of the A1 receptor subtype after a cerebral ischemic injury was shown to be neuroprotective by inducing decreases in oxidative stress and inflammation (Hu et al., 2012; Stone et al., 2009). These findings support that the activation of the A1 adenosine receptor, and not its inhibition, could be beneficial to stroke patients.
The activation of the A2a receptor has also been shown to have a potential therapeutic role against stroke. This adenosine receptor subtype is highly sensitive to neuromodulation after brain insults and related inflammatory responses (Chen et al., 2006). A2a receptor agonists have been found protective in the global ischemia model in the gerbil (Von Lubitz et al., 1995), and an A2a receptor knockout neonatal mouse model showed aggravated hypoxic ischemic injury in comparison to wild-type mice (Adén et al., 2003). Another study showed contrasting effects of A2a activation, which include a neuroprotective role in acute experiments and a neurally detrimental role in chronic experiments after brain damage (Stone et al., 2009). Therefore, it is very important to consider that the duration of exposure to some of these drugs could be critical on the final outcome. Additionally, we again see a protective role against the inflammatory processes induced by neural tissue damage (as seen after a SCI) when using either antagonists (A2a) or agonists (A1) of adenosine receptors after a stroke (Fig. 1).
Parkinson's disease
Neurodegenerative diseases, such as Parkinson's disease, are characterized by progressive nervous system dysfunction and neuroinflammation (Morelli et al., 2012; Peterson et al., 2012; Stone et al., 2009). Parkinson's disease induces dyskinesia, a progressive degeneration of the CNS, which includes symptoms such as rigidity, shaking and slowed movement. Recent studies provide evidence of the significant beneficial effects of caffeine intake in improving motor activity, through neuroprotection and neurorestoration by thropic proteins, such as TGF-beta, glial cell-derived neurotrophic factor, neurturin, bone morphogenic proteins and others (Airavaara et al., 2012; Chen and Chern, 2011; Postuma et al., 2012; Rosim et al., 2011). Studies performed using rat models for Parkinson's disease showed that the administration of caffeine to rats a week after the start of the progression of the disease prevented the loss of nigral dopaminergic neurons, indicating a role for caffeine in delaying neuronal degeneration (Li et al., 2008; Morelli et al., 2012; Sonsalla et al., 2012). In addition, caffeine has been shown to be a “down-regulator” of neuroinflammatory responses and nitric oxide (NO) production, which, together with prostaglandins, is believed to underlie the initiation of many pathological processes including cell death (Salvemini et al., 2013; Tsutsui et al., 2004; Yaday et al., 2012). These studies further support the use of caffeine and thus the blockade of adenosine receptors as a potential mechanism to protect against inflammatory damage produced after an injury to the CNS and/or during the progression of a neurodegenerative disease.
Recent studies support the strategy that blocking adenosine receptors might also confer a disease-modifying benefit against Parkinson's disease. For example, the blockade of A2a adenosine receptors has been shown to be an effective symptomatic treatment in Parkinson's patients without provoking marked impairment in the ability to control movements and thus avoid the onset of spasmodic (convulsive) or repetitive motions or lack of coordination (such as dyskinesia) at very early stages of the disease (Jenner et al., 2009). A study using 6-hydroxydopamine (OHDA)-lesioned rats showed that the application of 8-(3-chlorostryryl) caffeine (CSC), a selective A2a adenosine receptor antagonist, inhibited the levodopa-induced motor fluctuations through a downstream DARPP-32 and ERK1/2 signaling pathway (Song et al., 2009). Also, the use of Istradefylline (KW-6002), another A2a receptor antagonist, was shown to not only slow the onset of motor deficits related to the disease but also induce neurogeneration and reduce neuroplasticity (changes in neural pathways and synapses) that ensues when treating patients with other standard dopamine replacement strategies, like l-DOPA (Schwarzschild et al., 2006). Additionally, in animal models for Parkinson's disease, which displayed significant akinesia (the loss or impairment of voluntary movements), the application of caffeine and the A2a receptor antagonists 8-(3-chlorostyryl) caffeine (CSC) and SCH-58261 but not an A1 antagonist (8-cyclopentyltheophylline) or an A1 receptor agonist (N(6)-cyclopentyladenosine) was shown to provide a therapeutic effect by reducing the onset of akinesia in these animals (Coccurello et al., 2004; Kelsey et al., 2009). Thus, blockade of adenosine receptors, especially the A2a receptor subtype, can confer a beneficial effect by reducing some of the side effects experienced by other traditional pharmacological treatments for Parkinson's patients (Fig. 1).
Alzheimer's disease
Alzheimer's disease (AD) is characterized by synaptic loss and neuronal cell death as well as the presence of extracellular amyloid plaques, composed of the amyloid-β (Aβ) protein and intracellular neurofibrillary tangles. According to the ‘amyloid hypothesis,’ increased levels of Aβ occur in AD, and Aβ leads to synaptic dysfunction, neuronal cell death and, ultimately, impairment of higher cortical activity, including memory and cognition (Morley and Farr, 2014). Recent studies have been conducted in order to understand how the modulation of the adrenergic systems can contribute to the reduction or stoppage of the neurodegenerative processes related to Alzheimer's disease. In mice, it has been shown that both caffeine and adenosine receptor antagonists prevent the accumulation of amyloid-β-peptide (Aβ) in and around cerebral blood vessels, which, if untreated, could result in cognitive deficits (Cupino and Zabel, 2013; Gahr et al., 2013). Recent studies have shown that chronic caffeine consumption reverses cognitive impairment and decreases brain Aβ levels in AD mice (Arendash et al., 2009; Cao et al., 2009; Chu et al., 2012).
A possible mechanism for the caffeine-induced effects is through the stimulation of pro-survival cascades and inhibition of pro-apoptotic pathways in the striatum and/or cortex, as shown in a recent study by Zeitlin et al. (2011), where caffeine treatment in a transgenic model of AD was shown to stimulate PKA activity, to increase phospho-CREB levels and to decrease phospho-JNK and phospho-ERK expression in the striatum, all of which are thought to be beneficial changes for brain function. In the frontal cortex, caffeine did not significantly increase phospho-CREB and PKA activity but significantly reduced phospho-JNK and phospho-ERK expression in both transgenic and non-transgenic mice. These results suggest that caffeine promotes neuronal survival and reduces the process of neurodegeneration in the striatum and/or cortex, which may contribute to its beneficial effects against AD (Zeitlin et al., 2011).
Inflammation is known to be a crucial feature related to AD (Haskó et al., 2004, 2008), and the pathogenesis of neurodegeneration has been at least in part attributed to the release of proinflammatory cytokines from brain resident cells (Fredholm et al., 2000, 2001) and, although less consistently, from peripheral cells (Erdmann et al., 2005; Lappas et al., 2005a). As previously mentioned, adenosine is a naturally occurring metabolite that is ubiquitously distributed throughout the body as a metabolic intermediary. It has been shown to accumulate in the extracellular space at the site of inflammation (Lappas et al., 2005b) in response to metabolic stress and cell damage, and there is evidence that it could play a key role in preserving homeostasis (Canas et al., 2009). The physiological responses to adenosine take place as a result of its binding to adenosine receptors, and recent studies show that the activation of the adenosine system (specifically the A2a adenosine receptor subtype) can lead to the down-regulation of the inflammatory response (Csóka and Haskó, 2011; Querfurth and LaFerla, 2010; Tarkowski et al., 1999, 2001; Wyss-Coray, 2006) and also the prevention of beta amyloid (Aβ)-induced synaptotoxicity (Bermejo et al., 2008) by inducing the production of interleukin-10 (IL-10), the major anti-inflammatory cytokine (Bonotis et al., 2008). Also, IL-10 is largely correlated to the expression of adenosine A2A receptors in peripheral blood mononuclear cells (PBMCs). The PBMCs are usually disrupted in patients with mild cognitive impairment and Alzheimer's disease. Recent experiments show that there was a significant linear increase in adenosine A2a receptor mRNA levels and receptor density in patients with mild cognitive impairment but only partially in patients with AD (Arosio et al., 2011). In summary, the above studies suggest that there is a significant positive correlation between the modulation of the adenosine system and the regulation of the inflammatory response in the central and peripheral nervous systems.
Aging is another factor that has been correlated to the onset and further progression of AD. What happens if we could control neuronal aging? The effects of the modulation of gene expression on normal aging and in pathological conditions (as Alzheimer's disease) are still unclear, but the use of caffeine to treat AD-related cognitive deficits is showing promising results. Caffeine is emerging as a protective agent against Alzheimer's disease by blocking the A2a adenosine receptor whose expression and function become aberrant throughout aging and in age-related pathologies such as AD (Marques et al., 2011). In a recent “Cardiovascular Risk Factors, Aging and Dementia” (CAIDE) study, coffee drinking of 3–5 cups per day at midlife was associated with a decreased risk of dementia/AD by about 65% in later life, suggesting that coffee drinking may be associated with a decreased risk of dementia/AD (Eskelinen and Kivipelto, 2010). Another study in humans showed that there were no significant associations between coffee or caffeine intake and risk of cognitive impairment, overall dementia, AD, vascular dementia (VaD) or moderate/high levels of the individual neuropathologic lesion types. However, men in the highest quartile of caffeine intake were less likely than men in the lowest quartile to have any of the lesion types (Gaoand Phillis, 1994). These findings might open possibilities for the use of caffeine and other adenosine receptor antagonists for preventing or postponing the onset of age-related deficits that could lead to AD (Fig. 1).
Potential interpretations for the seemingly paradoxical use of adenosine receptor antagonists and agonists to treat the effects of various neurodegenerative diseases
A potential explanation of the seemingly paradoxical use of both adenosine receptor agonists and antagonists to treat neurological diseases was recently addressed by Dai and Zhou (2011). The authors attributed what they called the “bidirectional effect” of A2a adenosine receptor activation and inhibition to the following: (1) different developmental stages of the animal subjects since the use of a A2a knockout aggravated hypoxic ischemic brain injury in neonatal mice but reduced injury progression in mature mice (Adén et al., 2003); (2) different stages of pathological processes after injury since neuroprotection by A2a receptor agonists was observed during the early stages of SCI, whereas neuroprotection by inhibiting of the A2a receptor in bone marrow cells or by using an A2a receptor knockout animal model was observed during the later stage of SCI (Li et al., 2006); and (3) different routes and times of A2a receptor drug administration since a study showed that peripheral administration of the A2a receptor agonist, CGS21680, protects the hippocampus against kainate-induced excitotoxicity. However, the direct injection of CGS21680 into the hippocampus failed to provide protection, whereas the direct injection of the A2a antagonist ZM241385 into the hippocampus reduced kainate-induced neuronal damage (Jones et al., 1998a, 1998b). The authors also added that different A2a receptor drug treatment methods could affect the regulation of neurotransmitter release (e.g. glutamate) and the onset of inflammatory processes. In these cases, protection could result from the anti-inflammatory effect of A2a receptor activation, whereas cellular damage could be mediated by glutamate excitotoxicity and neuroinflammation enhanced by A2a receptor activation depending on the timing and dosage of the adenosine receptor agonists or antagonists used.
Additionally, we propose several explanations which could explain the seemingly paradoxical actions of both antagonists and agonists of adenosine receptors in the treatment of the neurodegenerative disease models discussed in this review. Caffeine has been described to have the ability to influence dopaminergic neurotransmission and potentiate dopamine receptor-mediated behavioral responses, such as locomotion and cognitive functions (Ferré, 2008; Xie et al., 2007). The adenosine A2A and the dopamine D2 receptors as well as the adenosine A1 and dopamine D1 receptors have been shown to have antagonistic effects supported by their anatomical co-localization (dimerization) and pharmacological interactions (Chen et al., 2001; Ferré, 2008). Studies in the nucleus accumbens and caudate putamen have demonstrated that A2A receptor activation inhibits the ability of dopamine to bind to the D2 receptor, thus functionally antagonizing it, resulting in behavioral effects such as depressed locomotor activity, low attention and concentration (Collins et al., 2010; Ferré, 2008). This seemingly “intimate” anatomical, pharmacological and physiological relationship between the adenosine and dopamine systems also influences glutamate release from presynaptic terminals as they have been shown to express A1 and A2a adenosine receptors (Xie et al., 2007). This highly complex relationship between the adenosine, dopamine and glutamate systems could explain why the use of adenosine receptor antagonists, such as caffeine, and agonists at various instances during the progression of a neurodegenerative disease and at different concentrations could produce these seemingly paradoxical results.
We propose that the most effective course of action when administering any of these modulators of adenosine receptors to potential patients during clinical trials will have to include: (1) the cell-specific pharmacological agents since caffeine and other modulators of the adenosine system have been shown to have effects on neurons and non-neurons, (2) the dosage and time of drug application will be critical since studies suggest that it is crucial not only to know the state of disease progression but also to choose the proper exposure period (acute versus chronic) and concentration and (3) the knowledge of the levels of the main neurotransmitter systems present in the patients is of paramount importance since it has been established that the modulatory effects of these drugs on adenosine receptors can be influenced by the levels of excitatory neurotransmitters such as glutamate. Thus, blood testing for peripheral levels of these signaling molecules as well as imaging techniques, such as magnetic resonance spectroscopy (Hall et al., 2012), to measure central levels of glutamate and dopamine will be needed in order to effectively treat the patient.
Conclusions
Recent experimental evidence suggests that the primary target for the neuroprotective effects of caffeine (Glade, 2010; Snel and Lorist, 2011), the most consumed psychostimulant in the world (Nehlig et al., 1992), is mostly through either the activation or inhibition of the A1 and A2a adenosine receptor subtypes (Doré et al., 2011; Sebastião and Ribeiro, 2009) (Table 1; includes earlier findings not discussed in this review). The use of adenosine receptor antagonists, such as caffeine, and agonists has been shown to protect against neurological diseases such as spinal cord injury, stroke, Alzheimer's and Parkinson's diseases (Fig. 1; Federico and Spalluto, 2012; Glade, 2010; Marjo and Miia, 2010; Müller and Jacobson, 2011; Ojeda-López et al., 2012; Paul et al., 2011; Ramlackhansingh et al., 2011; Rosim et al., 2011; Schenone et al., 2010). Experimental evidence supports the use of caffeine and other adenosine receptor antagonists as well as adenosine receptor agonists in the reduction of hyperalgesia, antinociception, excitotoxicity, inflammatory response, dyskinesia, akinesia, sensory and motor deficits and neuronal cell death related to the pathophysiology of the neurodegenerative diseases discussed (Camilo and Goldstein, 2004; Ding et al., 2007; Horiuchi et al., 2010; Kowaluk, 1998; Kitta et al., 2012; Tomić et al., 2006; Xiao et al., 2011). The seemingly paradoxical use of adenosine receptor agonists and antagonists to treat similar diseases suggests that factors such as dosage, drug delivery method, state of disease progression, extracellular concentrations of potential excitotoxic transmitters and known anatomical and pharmacological relationship between adenosine and dopamine receptors as they influence glutamate release must be taken into consideration when designing treatment strategies. Finally, the neuroprotective effects and the reduction in side effects produced in comparison to current treatments support the use of agonists and antagonists of adenosine receptors as potential therapeutic tools to treat neural degeneration such as that induced by spinal cord injury, stroke, Parkinson's and Alzheimer's diseases as well as other known diseases of the CNS.
Table 1.
Effects of adenosine receptor antagonists and agonists on neurodegenerative diseases.
| Drug type (adenosine receptor subtype) | Known effects on each of the following types of neurodegenerative diseases | |||
|---|---|---|---|---|
|
| ||||
| Spinal cord injury (SCI) | Stroke | Parkinson's disease | Alzheimer's disease | |
| Caffeine (broad adenosine receptor antagonist) |
|
|
|
|
| Agonists (A1) | - Inhibits hyperalgesia (Higashi et al., 2002) |
|
N/A | |
| Agonists (A2a) |
|
|
- Reduces toxic effects in 6-OHDA lesioned rats (Agnati et al., 2004) | - Down-regulates the inflammatory response (Csóka and Haskó, 2011; Querfurth and LaFerla, 2010; Tarkowski et al., 1999, 2001; Wyss-Coray, 2006) and also prevents beta amyloid (Aβ)-induced synaptotoxicity (Bermejo et al., 2008) by inducing the production of interleukin-10 (IL-10), the major anti-inflammatory cytokine (Bonotis et al., 2008) |
| Agonists (A2b) | - Activated under pathophysiological conditions; potential therapeutic role in the recovery of motor function after a SCI (Agnati et al., 2004) | N/A | N/A | |
| Agonists (A3) | N/A |
|
N/A | |
| Antagonists (A1) | N/A | - Enhanced ischemia-evoked injury (Melani et al., 2006) | - Prevents the accumulation of amyloid-β-peptide (Aβ) in and around cerebral blood vessels (Cupino and Zabel, 2013; Gahr et al., 2013) | |
| Antagonists (A2a) | - Reduces excessive release of neurotransmitters (excitotoxicity) (Pan and Chen, 2004) |
|
|
|
Acknowledgments
We would like to thank Jeidiel De Leon, Ernesto Cabezas, Jean Marie Acevedo-Rosario and Nikol Matos-Vergara for their helpful comments regarding the manuscript.
This work was supported bythe Craig Neilsen Foundation (grant no. 124554), the National Science Foundation (grant no. 1026061), the RCMI-UPR-MSC (grant no. G12RR03051) and a Louis Stokes Alliances for Minority Participation Pre-doctoral Fellowship (NSF grant no. 1139888) to M. Rivera-Oliver.
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- Adén U, Halldner L, Lagercrantz H, Dalmau I, Ledent C, Fredholm BB. Aggravated brain damage after hypoxic ischemia in immature adenosine A2A knockout mice. Stroke. 2003;34:739–44. doi: 10.1161/01.STR.0000060204.67672.8B. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Voutilainen MH, Wang Y, Hoffer B. Neurorestoration. Parkinsonism Relat Disord. 2012;1:s143–6. doi: 10.1016/S1353-8020(11)70045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati LF, Leo G, Vergoni AV, Martínez E, Hockemeyer J, Lluis C, et al. Neuroprotective effect of l-DOPA co-administered with the adenosine A2A receptor agonist CGS 21680 in an animal model of Parkinson's disease. Brain Res Bull. 2004;64(2):155–64. doi: 10.1016/j.brainresbull.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, et al. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer's disease mice. J Alzheimers Dis. 2009;17(3):661–80. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- Arosio B, Mastronardi L, Gussago C, Nicolini P, Casè A, Ziglioli E, et al. Adenosine A(2A) receptor and IL-10 in peripheral blood mononuclear cells of patients with mild cognitive impairment. Int J Alzheimers Dis. 2011:484021. doi: 10.4061/2011/484021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo P, Martín-Aragón S, Benedí J, Susín C, Felici E, Gil P, et al. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer's disease. Immunol Lett. 2008;177(2):198–202. doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Bohn SK, Ward NC, Hodgson JM, Croft KD. Effects of tea and coffee on cardiovascular disease risk. Food Funct. 2012;3(6):575–91. doi: 10.1039/c2fo10288a. [DOI] [PubMed] [Google Scholar]
- Bonotis K, Krikki E, Holeva V, Aggouridaki C, Costa V, Baloyannis S. Systemic immune aberrations in Alzheimer's disease patients. J Neuroimmunol. 2008;193(1–2):183–7. doi: 10.1016/j.jneuroim.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Butler TR, Prendergast MA. Neuroadaptations in adenosine receptor signaling following long-term ethanol exposure and withdrawal. Alcohol Clin Exp Res. 2012;36(1):4–13. doi: 10.1111/j.1530-0277.2011.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilo F, Goldstein LB. Seizures and epilepsy after ischemic stroke. Stroke. 2004;35(7):1769–75. doi: 10.1161/01.STR.0000130989.17100.96. [DOI] [PubMed] [Google Scholar]
- Canas PM, Porciúncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, et al. Adenosine A receptor blockade prevents synaptotoxicity and memory dysfunction caused by β-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci. 2009;29(47):14741–51. doi: 10.1523/JNEUROSCI.3728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Cirrito JR, Lin X, Wang L, Verges DK, Dickson A, et al. Caffeine suppresses amyloid-beta levels in plasma and brain of Alzheimer's disease transgenic mice. J Alzheimers Dis. 2009;17(3):681–97. doi: 10.3233/JAD-2009-1071. Erratum in: J Alzheimers Dis 18 (3):727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada DC, Tribble CG, Young JS, Gangemi JJ, Gohari AR, Butler PD, et al. Adenosine A2A analogue improves neurologic outcome after spinal cord trauma in the rabbit. J Trauma. 2002;53:225–9. doi: 10.1097/00005373-200208000-00005. discussion 229–231. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Chen YY, Wang XS, Wu SZ, Yang HM, Xu HQ, et al. Chronic caffeine treatment attenuates experimental autoimmune encephalomyelitis induced by guinea pig spinal cord homogenates in Wistar rats. Brain Res. 2010;1309:116–25. doi: 10.1016/j.brainres.2009.10.054. [DOI] [PubMed] [Google Scholar]
- Chen JF, Chern Y. Impacts of methylxanthines and adenosine receptors on neurodegeneration: human and experimental studies. Handb Exp Pharmacol. 2011;200:267–310. doi: 10.1007/978-3-642-13443-2_10. [DOI] [PubMed] [Google Scholar]
- Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res. 2006;84(8):1848–55. doi: 10.1002/jnr.21071. [DOI] [PubMed] [Google Scholar]
- Chen JF, Pedata F. Modulation of ischemic brain injury and neuroinflammation by adenosine A2A receptors. Curr Pharm Des. 2008;14(15):1490–9. doi: 10.2174/138161208784480126. [DOI] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, et al. The role of the D(2) dopamine receptor (D(2)R) in A(2A) adenosine receptor (A(2A)R)-mediated behavioral and cellular responses as revealed by A(2A) and D(2) receptor knockout mice. Proc Natl Acad Sci U S A. 2001;98(4):1970–5. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu YF, Chang WH, Black RM, Liu JR, Sompol P, Chen Y, et al. Crude caffeine reduces memory impairment and amyloid β (1–42) levels in an Alzheimer's mouse model. Food Chem. 2012;135(3):2095–102. doi: 10.1016/j.foodchem.2012.04.148. [DOI] [PubMed] [Google Scholar]
- Coccurello R, Breysse N, Amalric M. Simultaneous blockade of adenosine A2A and metabotropic glutamate mGlu5 receptors increase their efficacy in reversing Parkinsonian deficits in rats. Neuropsychopharmacology. 2004;29(8):1451–61. doi: 10.1038/sj.npp.1300444. [DOI] [PubMed] [Google Scholar]
- Collins LE, Galtieri DJ, Collins P, Jones SK, Port RG, Paul NE, et al. Interactions between adenosine and dopamine receptor antagonists with different selectivity profiles: effects on locomotor activity. Behav Brain Res. 2010;211(2):148–55. doi: 10.1016/j.bbr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Csóka B, Haskó G. Adenosine, inflammation pathways and therapeutic challenges. Joint Bone Spine. 2011;78(1):4–6. doi: 10.1016/j.jbspin.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Cupino TL, Zabel MK. Alzheimer's silent partner: cerebral amyloid angiopathy. Transl Stroke Res. 2013 doi: 10.1007/s12975-013-0309-7. Nov 19. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Dai SS, Li W, An JH, Wang H, Yang N, Chen XY, et al. Adenosine A2A receptors in both bone marrow cells and non-bone marrow cells contribute to traumatic brain injury. J Neurochem. 2010a;113(6):1536–44. doi: 10.1111/j.1471-4159.2010.06716.x. [DOI] [PubMed] [Google Scholar]
- Dai SS, Zhou YG, Li W, An JH, Li P, Yang N, et al. Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J Neurosci. 2010b;30(16):5802–10. doi: 10.1523/JNEUROSCI.0268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SS, Zhou YG. Adenosine A2 receptor: a crucial neuromodulators with bidirectional effect in neuroinflammation and brain injury. Rev Neurosci. 2011;22(2):231–9. doi: 10.1515/RNS.2011.020. [DOI] [PubMed] [Google Scholar]
- Ding Y, Restrepo J, Won L, Hwang DY, Kim KS, Kang UJ. Chronic 3,4-dihydroxyphenylalanine treatment induces dyskinesia in aphakia mice, a novel genetic model of Parkinson's disease. Neurobiol Dis. 2007;27(1):11–23. doi: 10.1016/j.nbd.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré AS, Robertson N, Errey JC, Ng I, Hollenstein K, Tehan B, et al. Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure. 2011;19(9):1283–93. doi: 10.1016/j.str.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic N, Delic V, Cao C, Copes N, Lin X, Mamcarz M, et al. Caffeine increases mitochondrial function and blocks melatonin signaling to mitochondria in Alzheimer's mice and cells. Neuropharmacology. 2012;63(8):1368–79. doi: 10.1016/j.neuropharm.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Erdmann AA, Gao ZG, Jung U, Foley J, Borenstein T, Jacobson KA, et al. Activation of Th1 and Tc1 cell adenosine A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105(12):4707–14. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen MH, Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer's disease. J Alzheimers Dis. 2010;20(Suppl. 1):S167–74. doi: 10.3233/JAD-2010-1404. http://dx.doi.org/10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- Federico S, Spalluto G. Therapeutic potential of A2 and A3 adenosine receptor: a review of novel patented ligands. Expert Opin Ther Pat. 2012;22(4):369–90. doi: 10.1517/13543776.2012.669375. [DOI] [PubMed] [Google Scholar]
- Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–79. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–52. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(4–5):364–74. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Gahr M, Nowak DA, Connemann BJ, Schönfeldt-Lecuona C. Cerebral amyloidal angiopathy— a disease with implications for neurology and psychiatry. Brain Res. 2013;26(1519):19–30. doi: 10.1016/j.brainres.2013.04.052. [DOI] [PubMed] [Google Scholar]
- Gao Y, Phillis JW. CGS 15943, an adenosine A2 receptor antagonist, reduces cerebral ischemic injury in the Mongolian gerbil. Life Sci. 1994;55:PL61–5. doi: 10.1016/0024-3205(94)00889-2. [DOI] [PubMed] [Google Scholar]
- Gelber RP, Petrovitch H, Masaki KH, Ross GW, White LR. Coffee intake in midlife and risk of dementia and its neuropathologic correlates. J Alzheimers Dis. 2011;23(4):607–15. doi: 10.3233/JAD-2010-101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Melani A, Esposito E, Mazzon E, Di Paola R, Bramanti P, et al. The selective adenosine A2A receptor agonist CGS 21680 reduces JNK MAPK activation in oligodendrocytes in injured spinal cord. Shock. 2009;32:578–85. doi: 10.1097/SHK.0b013e3181a20792. [DOI] [PubMed] [Google Scholar]
- Genovese T, Melani A, Esposito E, Paterniti I, Mazzon E, Di Paola R, et al. Selective adenosine A(2a) receptor agonists reduce the apoptosis in an experimental model of spinal cord trauma. J Biol Regul Homeost Agents. 2010;24:73–86. [PubMed] [Google Scholar]
- Glade MJ. Caffeine—not just a stimulant. Nutrition. 2010;(10):932–8. doi: 10.1016/j.nut.2010.08.004. Review. [DOI] [PubMed] [Google Scholar]
- Hall H, Cuellar-Baena S, Dahlberg C, In't Zandt R, Denisov V, Kirik D. Magnetic resonance spectroscopic methods for the assessment of metabolic functions in the diseased brain. Curr Top Behav Neurosci. 2012;11:169–98. doi: 10.1007/7854_2011_166. [DOI] [PubMed] [Google Scholar]
- Haskó G, Sitkovsky MV, Szabó C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25(3):152–7. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H, Meno JR, Marwaha AS, Winn HR. Hippocampal injury and neurobehavioral deficits following hyperglycemic cerebral ischemia: effect of theophylline and ZM241385. J Neurosurg. 2002;96:117–26. doi: 10.3171/jns.2002.96.1.0117. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Ogata T, Morino T, Yamamoto H. Adenosine A1 receptor agonists reduce hyperalgesia after spinal cord injury in rats. Spinal Cord. 2010;48(9):685–90. doi: 10.1038/sc.2009.194. [DOI] [PubMed] [Google Scholar]
- Hu S, Dong H, Zhang H, Wang S, Hou L, Chen S, et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. 2012;1459:81–90. doi: 10.1016/j.brainres.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Jenner P, Mori A, Hauser R, Morelli M, Fredholm BB, Chen JF. Adenosine, adenosine A2A antagonists, and Parkinson's disease. Parkinsonism Relat Disord. 2009;15(6):406–13. doi: 10.1016/j.parkreldis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Jones PA, Smith RA, Stone TW. Protection against hippocampal kainate excitotoxicity by intracerebral administration of an adenosine A2A receptor antagonist. Brain Res. 1998a;800:328–35. doi: 10.1016/s0006-8993(98)00540-x. [DOI] [PubMed] [Google Scholar]
- Jones PA, Smith RA, Stone TW. Protection against kainate-induced excitotoxicity by adenosine A2A receptor agonists and antagonists. Neuroscience. 1998b;85:229–37. doi: 10.1016/s0306-4522(97)00613-1. [DOI] [PubMed] [Google Scholar]
- Kelsey JE, Langelier NA, Oriel BS, Reedy C. The effects of systemic, intrastriatal, and intrapallidal injections of caffeine and systemic injections of A2A and A1 antagonists on forepaw stepping in the unilateral 6-OHDA-lesioned rat. Psychopharmacology (Berl) 2009;201(4):529–39. doi: 10.1007/s00213-008-1319-0. [DOI] [PubMed] [Google Scholar]
- Kowaluk EA. Adenosine modulation: a novel approach to analgesia and inflammation. Expert Opin Investig Drugs. 1998;7(4):535–43. doi: 10.1517/13543784.7.4.535. [DOI] [PubMed] [Google Scholar]
- Kitta T, Chancellor MB, de Groat WC, Kuno S, Nonomura K, Yoshimura N. Suppression of bladder overactivity by adenosine A2a receptor antagonist in a rat model of Parkinson's disease. J Urol. 2012;187(5):1890–7. doi: 10.1016/j.juro.2011.12.062. [DOI] [PubMed] [Google Scholar]
- Lappas CM, Rieger JM, Linden J. A adenosine receptor induction inhibits IFN-γ production in murine CD4 T cells. J Immunol. 2005a;174(2):1073–80. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- Lappas CM, Sullivan GW, Linden J. Adenosine A2A agonists in development for the treatment of inflammation. Expert Opin Investig Drugs. 2005b;14(7):797–806. doi: 10.1517/13543784.14.7.797. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Virtamo J, Wolk A. Coffee consumption and risk in women. Stroke. 2011;42(4):908–12. doi: 10.1161/STROKEAHA.110.603787. [DOI] [PubMed] [Google Scholar]
- Li Y, Oskouian RJ, Day YJ, Rieger JM, Liu L, Kern JA, et al. Mouse spinal cord compression injury is reduced by either activation of the adenosine A2A receptor on bone marrow-derived cells or deletion of the A2A receptor on non-bone marrow-derived cells. Neuroscience. 2006;141:2029–39. doi: 10.1016/j.neuroscience.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Li W, Dai S, An J, Li P, Chen X, Xiong R, et al. Chronic but not acute treatment with caffeine attenuates traumatic brain injury in the mouse cortical impact model. Neuroscience. 2008;151(4):1198–207. doi: 10.1016/j.neuroscience.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Marjo HE, Miia K. Caffeine as a protective factor in dementia and Alzheimer's disease. J Alzheimers Dis. 2010;20:167–74. doi: 10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- Marques S, Batalha VL, Lopes LV, Outeiro TF. Modulating Alzheimer's disease through caffeine: a putative link to epigenetics. J Alzheimers Dis. 2011;24(Suppl. 2):161–71. doi: 10.3233/JAD-2011-110032. [DOI] [PubMed] [Google Scholar]
- Melani A, Pantoni L, Bordoni F, Gianfriddo M, Bianchi L, Vannucchi MG, et al. The selective A2A receptor antagonist Sch 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res. 2003;959:243–50. doi: 10.1016/s0006-8993(02)03753-8. [DOI] [PubMed] [Google Scholar]
- Melani A, Gianfriddo M, Vannucchi MG, Cipriani S, Baraldi PG, Giovannini MG, et al. The selective A(2A) receptor antagonist Sch 58261 protects from neurological deficit, brain damage and activation of p38 MAPK in rat focal cerebral ischemia. Brain Res. 2006;1073–1074:470–80. doi: 10.1016/j.brainres.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Monopoli A, Casati C, Lozza G, Forlani A, Ongini E. Cardiovascular pharmacology of the A2A adenosine receptor antagonist, Sch 58261, in the rat. J Pharmacol Exp Ther. 1998a;285:9–15. [PubMed] [Google Scholar]
- Monopoli A, Lozza G, Forlani A, Mattavelli A, Ongini E. Blockade of adenosine A2A receptors by Sch 58261 results in neuroprotective effects in cerebral ischemia in rats. Neuroreport. 1998b;9:3955–9. doi: 10.1097/00001756-199812010-00034. [DOI] [PubMed] [Google Scholar]
- Montagnana M, Favaloro EJ, Lippi G. Coffee intake and cardiovascular disease: virtue does not take center stage. Semin Thromb Hemost. 2012;28(2):164–77. doi: 10.1055/s-0032-1301414. [DOI] [PubMed] [Google Scholar]
- Morelli M, Blandini F, Simola N, Hauser RA. A(2A) receptor antagonism and dyskinesia in Parkinson's disease. Park Dis. 2012;2012:489853. doi: 10.1155/2012/489853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Farr SA. The role of amyloid-beta in the regulation of memory. Biochem Pharmacol. 2014 doi: 10.1016/j.bcp.2013.12.018. Jan 4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Mostofsky E, Schlaug G, Mukamal KJ, Rossamond WD, Mittleman MA. Coffee and acute ischemic stroke onset: the stroke onset study. Neurology. 2010;75(18):1583–8. doi: 10.1212/WNL.0b013e3181fb443d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CE, Jacobson KA. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta. 2011;1808(5):1290–308. doi: 10.1016/j.bbamem.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992;17(2):139–70. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- Ojeda-López C, Cervantes-Arriaga A, Rodríguez-Violante M, Corona T. Caffeine drinking, cigarette smoking, and dopaminergic replacement therapy dose in Parkinson's disease. Neurol Sci. 2012;34(6):979–83. doi: 10.1007/s10072-012-1180-0. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO, Reece TB, Laurent JJ, Hawkins AS, Ellman PI, Linden J, et al. A comparison of adenosine A2A agonism and methylprednisolone in attenuating neuronal damage and improving functional outcome after experimental traumatic spinal cord injury in rabbits. J Neurosurg Spine. 2006;4(1):64–70. doi: 10.3171/spi.2006.4.1.64. [DOI] [PubMed] [Google Scholar]
- Palacios N, Gao X, McCullough ML, Schwarzschild MA, Shah R, Gapstur S, et al. Caffeine and risk of Parkinson's disease in a large cohort of men and women. Mov Disord. 2012;27(10):1276–82. doi: 10.1002/mds.25076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HL, Chen SR. Sensing tissue ischemia: another new function for capsaicin receptors? Circulation. 2004;110(13):1826–31. doi: 10.1161/01.CIR.0000142618.20278.7A. [DOI] [PubMed] [Google Scholar]
- Paterniti I, Melani A, Cipriani S, Corti F, Mello T, Mazzon E, et al. Selective adenosine A2A receptor agonists and antagonists protect against spinal cord injury through peripheral and central effects. J Neuroinflammation. 2011;12:8–31. doi: 10.1186/1742-2094-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Khanapur S, Rybczynska AA, Kwizera C, Sijbesma JW, Ishiwata K, et al. Small-animal PET study of adenosine A(1) receptors in rat brain: blocking receptors and raising extracellular adenosine. J Nucl Med. 2011;52(8):1293–300. doi: 10.2967/jnumed.111.088005. [DOI] [PubMed] [Google Scholar]
- Pedata F, Gianfriddo M, Turchi D, Melani A. The protective effect of adenosine A2A receptor antagonism in cerebral ischemia. Neurol Res. 2005;27:169–74. doi: 10.1179/016164105X21913. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Goldberg JA, Surmeier DJ. Adenosine A2a receptor antagonist attenuate striatal adaptations following dopamine depletion. Neurobiol Dis. 2012;45(1):409–16. doi: 10.1016/j.nbd.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Lang AE, Munhoz RP, Charland K, Pelletier A, Moscovich M, et al. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012;79(7):651–8. doi: 10.1212/WNL.0b013e318263570d. Erratum in:Neurology 2012;79(16):1744.[Oct 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanthi JR, Dasari B, Marwarha G, Larson T, Chen X, Geiger JD, et al. Caffeine protects against oxidative stress and Alzheimer's disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic Biol Med. 2010;49(7):1212–20. doi: 10.1016/j.freeradbiomed.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362(4):329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Bose SK, Ahmed I, Turkheimer FE, Pavese N, Brooks DJ. Adenosine A2 receptor availability in dyskinetic and nondyskinetic patients with Parkinson's disease. Neurology. 2011;76(21):1811–6. doi: 10.1212/WNL.0b013e31821ccce4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece TB, Davis JD, Okonkwo DO, Maxey TS, Ellman PI, Li X, et al. Adenosine A2A analogue reduces long-term neurologic injury after blunt spinal trauma. J Surg Res. 2004;121:130–4. doi: 10.1016/j.jss.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Rodnitzky RL. Upcoming treatments in Parkinson's disease, including gene therapy. Parkinsonism Relat Disord. 2012;18(Suppl. 1):S37–40. doi: 10.1016/S1353-8020(11)70014-1. [DOI] [PubMed] [Google Scholar]
- Rosim FE, Persike DS, Nehlig A, Amorim RP, de Oliveira DM, Fernandes MJ. Differential neuroprotection by A(1) receptor activation and A(2A) receptor inhibition following pilocarpine-induced status epilepticus. Epilepsy Behav. 2011;22(2):207–13. doi: 10.1016/j.yebeh.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Kim SF, Mollace V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications. Am J Physiol Regul Integr Comp Physiol. 2013;304(7):R473–87. doi: 10.1152/ajpregu.00355.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J, Reid AR. Caffeine inhibits antinociception by acetaminophen in the formalin test by inhibiting spinal adenosine A1 receptors. Eur J Pharmacol. 2011;674(2–3):248–54. doi: 10.1016/j.ejphar.2011.10.036. [DOI] [PubMed] [Google Scholar]
- Schenone S, Brullo C, Musumeci F, Bruno O, Botta M. A1 receptors ligands: past, present and future trends. Curr Top Med Chem. 2010;10(9):878–901. doi: 10.2174/156802610791268729. Review. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson's disease. Trends Neurosci. 2006;29(11):647–54. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Ribeiro JA. Adenosine receptors and the central nervous system. Handb Exp Pharmacol. 2009;193:471–534. doi: 10.1007/978-3-540-89615-9_16. [DOI] [PubMed] [Google Scholar]
- Snel J, Lorist MM. Effects of caffeine on sleep and cognition. Prog Brain Res. 2011;190:105–17. doi: 10.1016/B978-0-444-53817-8.00006-2. Review. [DOI] [PubMed] [Google Scholar]
- Song L, Kong M, Ma Y, Ba M, Liu Z. Inhibitory effect of 8-(3-chlorostryryl) caffeine on levodopa-induced motor fluctuation is associated with intracellular signaling pathway in 6-OHDA-lesioned rats. Brain Res. 2009;1276:171–9. doi: 10.1016/j.brainres.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Wong LY, Harris SL, Richardson JR, Khobahy I, Li W, et al. Delayed caffeine treatment prevents nigral dopamine neuron loss in a progressive rat model of Parkinson's disease. Exp Neurol. 2012;234(2):482–7. doi: 10.1016/j.expneurol.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW, Ceruti S, Abbracchio MP. Adenosine receptors and neurological disease: neuroprotection and neurodegeneration. Handb Exp Pharmacol. 2009;193:535–87. doi: 10.1007/978-3-540-89615-9_17. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Blennow K, Wallin A, Tarkowski A. Intracerebral production of tumor necrosis factor-α, a local neuroprotective agent, in Alzheimer disease and vascular dementia. J Clin Immunol. 1999;19(4):223–30. doi: 10.1023/a:1020568013953. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Liljeroth AM, Nilsson a, Minthon L, Blennow K. Decreased levels of intrathecal interleukin 1 receptor antagonist in Alzheimer's disease. Dement Geriatr Cogn Disord. 2001;12(5):314–7. doi: 10.1159/000051276. [DOI] [PubMed] [Google Scholar]
- Tomić MA, Vucković SM, Stepanović-Petrović RM, Ugresić N, Prostran MS, Bosković B. Peripheral anti-hyperalgesia by oxcarbazepine: involvement of adenosine A1 receptors. Pharmazie. 2006;61(6):566–8. [PubMed] [Google Scholar]
- Tsutsui S, Schnermann J, Noorbakhsh F, Henry S, Yong VW, Winston BW, et al. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci. 2004;24(6):1521–9. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lubitz DK, Lin RC, Jacobson KA. Cerebral ischemia in gerbils: effects of acute and chronic treatment with adenosine A2A receptor agonist and antagonist. Eur J Pharmacol. 1995;287:295–302. doi: 10.1016/0014-2999(95)00498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CJ, Li W, Chen JF. Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim Biophys Acta. 2011;1808(5):1358–79. doi: 10.1016/j.bbamem.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12(9):1005–15. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Xiao D, Cassin JJ, Healy B, Burdett TC, Chen JF, Fredholm BB, et al. Deletion of adenosine A or A(2a) receptors reduces l-3,4-dihydroxyphenylalanine-induced dyskinesia in a model of Parkinson's disease. Brain Res. 2011;1367:310–8. doi: 10.1016/j.brainres.2010.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ramkumar V, Toth LA. Adenosine and dopamine receptor interactions in striatum and caffeine-induced behavioral activation. Comp Med. 2007;57(6):538–45. Review. [PubMed] [Google Scholar]
- Xuesong C, Othman G, Jonathan DG. Caffeine protects against disruptions of the BBB in animal models of Alzheimer's and Parkinson's disease. J Alzheimers Dis. 2010;20:127–46. doi: 10.3233/JAD-2010-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaday S, Gupta SP, Srivastava G, Srivastava PK, Singh MP. Role of secondary mediators in caffeine-mediated neuroprotection in maneb- and paraquat-induced Parkinson's disease phenotype in the mouse. Neurochem Res. 2012;37(4):875–84. doi: 10.1007/s11064-011-0682-0. [DOI] [PubMed] [Google Scholar]
- Zeitlin R, Patel S, Burgess S, Arendash GW, Echeverria V. Caffeine induces beneficial changes in PKA signaling and JNK and ERK activities in the striatum and cortex of Alzheimer's transgenic mice. Brain Res. 2011;12(1417):127–36. doi: 10.1016/j.brainres.2011.08.036. [DOI] [PubMed] [Google Scholar]


