Abstract
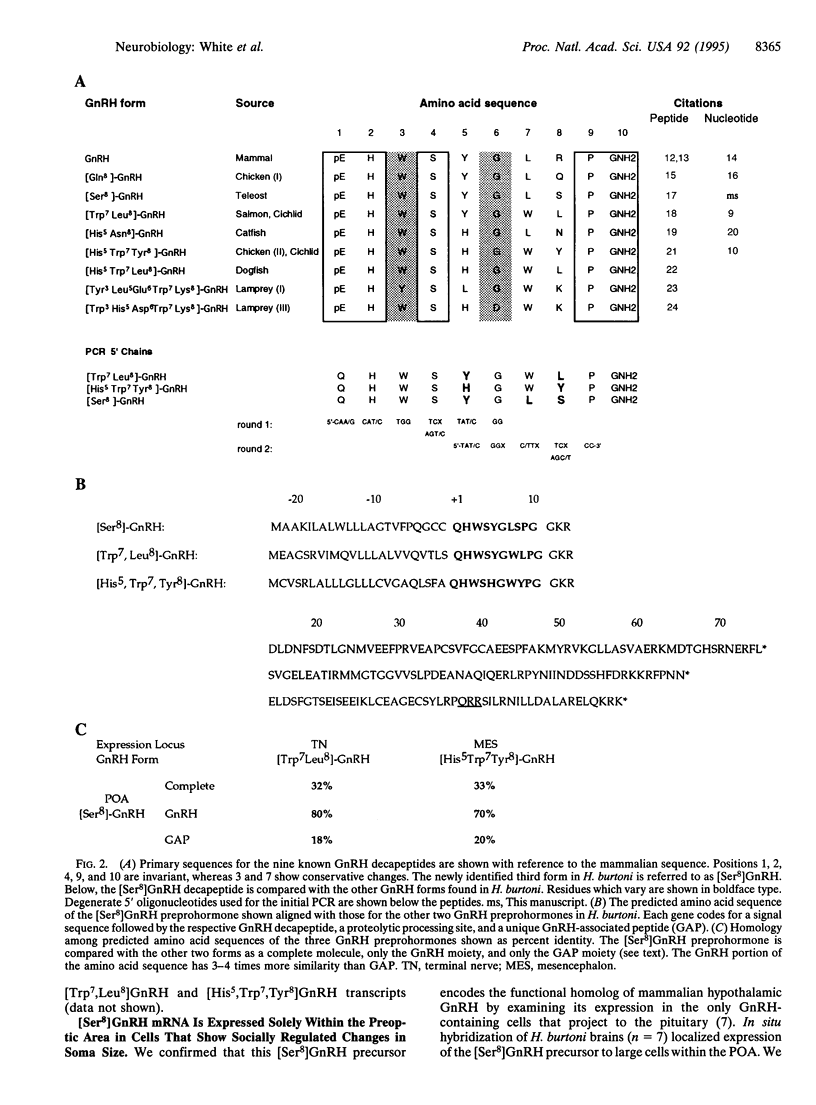
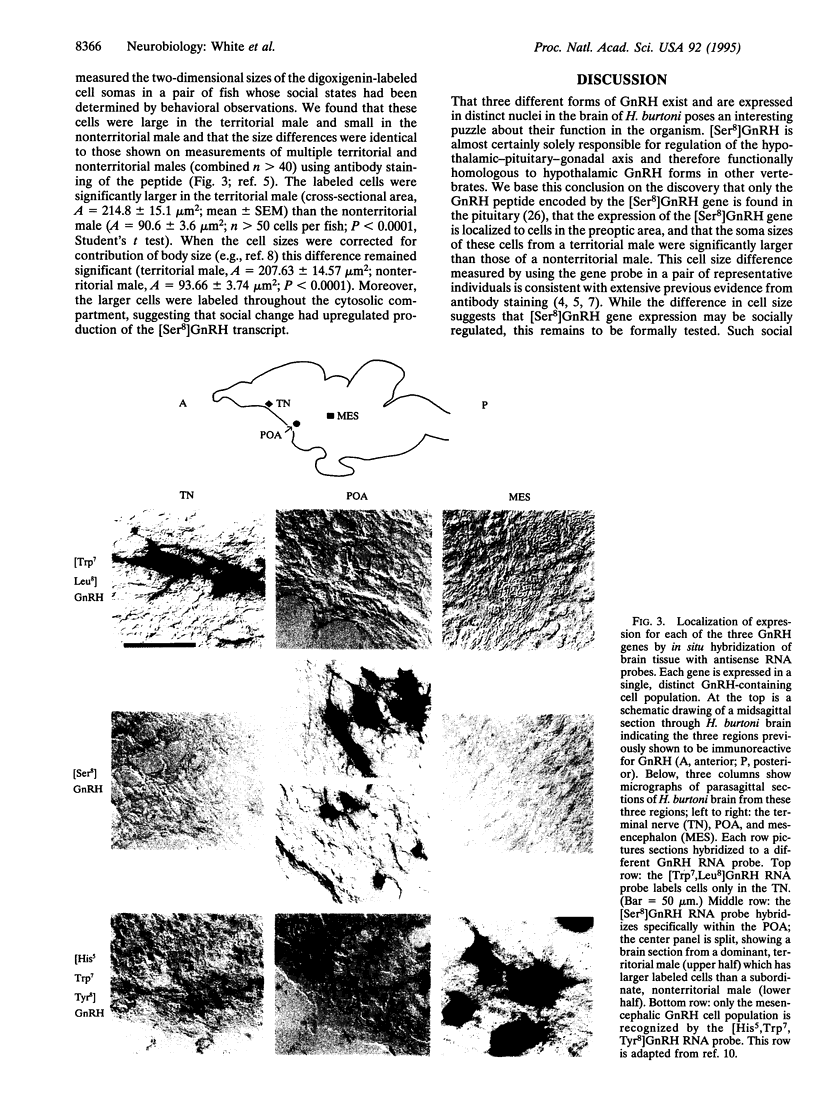
Gonadotropin-releasing hormone (GnRH) is known and named for its essential role in vertebrate reproduction. Release of this decapeptide from neurons in the hypothalamus controls pituitary gonadotropin levels which, in turn, regulate gonadal state. The importance of GnRH is underscored by its widespread expression and conservation across vertebrate taxa: five amino acids are invariant in all nine known forms, whereas two others show only conservative changes. In most eutherian mammals, only one form, expressed in the hypothalamus, is thought to exist, although in a recent report, antibody staining in developing primates suggests an additional form. In contrast, multiple GnRH forms and expression loci have been reported in many non-mammalian vertebrates. However, evidence based on immunological discrimination does not always agree with analysis of gene expression, since GnRH forms encoded by different genes may not be reliably distinguished by antibodies. Here we report the expression of three distinct GnRH genes in a teleost fish brain, including the sequence encoding a novel GnRH preprohormone. Using in situ hybridization, we show that this form is found only in neurons that project to the pituitary and exhibit changes in soma size depending on social and reproductive state. The other two GnRH genes are expressed in other, distinct cell populations. All three genes share the motif of encoding a polypeptide consisting of GnRH and a GnRH-associated peptide. Whereas the GnRH moiety is highly conserved, the GnRH-associated peptides are not, reflecting differential selective pressure on different parts of the gene. GnRH forms expressed in nonhypothalamic regions may serve to coordinate reproductive activities of the animal.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman J. P., Mason A. J., Hayflick J. S., Seeburg P. H. Isolation of the gene and hypothalamic cDNA for the common precursor of gonadotropin-releasing hormone and prolactin release-inhibiting factor in human and rat. Proc Natl Acad Sci U S A. 1986 Jan;83(1):179–183. doi: 10.1073/pnas.83.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoss M., Burgus R., Blackwell R., Vale W., Fellows R., Guillemin R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun. 1971 Jul 2;44(1):205–210. doi: 10.1016/s0006-291x(71)80179-1. [DOI] [PubMed] [Google Scholar]
- Bailhache T., Arazam A., Klungland H., Aleström P., Breton B., Jego P. Localization of salmon gonadotropin-releasing hormone mRNA and peptide in the brain of Atlantic salmon and rainbow trout. J Comp Neurol. 1994 Sep 15;347(3):444–454. doi: 10.1002/cne.903470310. [DOI] [PubMed] [Google Scholar]
- Bogerd J., Zandbergen T., Andersson E., Goos H. Isolation, characterization and expression of cDNAs encoding the catfish-type and chicken-II-type gonadotropin-releasing-hormone precursors in the African catfish. Eur J Biochem. 1994 Jun 1;222(2):541–549. doi: 10.1111/j.1432-1033.1994.tb18896.x. [DOI] [PubMed] [Google Scholar]
- Bond C. T., Francis R. C., Fernald R. D., Adelman J. P. Characterization of complementary DNA encoding the precursor for gonadotropin-releasing hormone and its associated peptide from a teleost fish. Mol Endocrinol. 1991 Jul;5(7):931–937. doi: 10.1210/mend-5-7-931. [DOI] [PubMed] [Google Scholar]
- Choi W. S., Kim M. O., Lee B. J., Kim J. H., Sun W., Seong J. Y., Kim K. Presence of gonadotropin-releasing hormone mRNA in the rat olfactory piriform cortex. Brain Res. 1994 Jun 13;648(1):148–151. doi: 10.1016/0006-8993(94)91914-3. [DOI] [PubMed] [Google Scholar]
- Davis M. R., Fernald R. D. Social control of neuronal soma size. J Neurobiol. 1990 Dec;21(8):1180–1188. doi: 10.1002/neu.480210804. [DOI] [PubMed] [Google Scholar]
- Dellovade T. L., King J. A., Millar R. P., Rissman E. F. Presence and differential distribution of distinct forms of immunoreactive gonadotropin-releasing hormone in the musk shrew brain. Neuroendocrinology. 1993 Aug;58(2):166–177. doi: 10.1159/000126529. [DOI] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Dunn I. C., Chen Y., Hook C., Sharp P. J., Sang H. M. Characterization of the chicken preprogonadotrophin-releasing hormone-I gene. J Mol Endocrinol. 1993 Aug;11(1):19–29. doi: 10.1677/jme.0.0110019. [DOI] [PubMed] [Google Scholar]
- Francis R. C., Soma K., Fernald R. D. Social regulation of the brain-pituitary-gonadal axis. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7794–7798. doi: 10.1073/pnas.90.16.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haygood M. G. Spreadsheet macros for coloring sequence alignments. Biotechniques. 1993 Dec;15(6):1084–1089. [PubMed] [Google Scholar]
- Jones S. W. Chicken II luteinizing hormone-releasing hormone inhibits the M-current of bullfrog sympathetic neurons. Neurosci Lett. 1987 Sep 23;80(2):180–184. doi: 10.1016/0304-3940(87)90650-1. [DOI] [PubMed] [Google Scholar]
- King J. A., Millar R. P. Structure of chicken hypothalamic luteinizing hormone-releasing hormone. I. Structural determination on partially purified material. J Biol Chem. 1982 Sep 25;257(18):10722–10728. [PubMed] [Google Scholar]
- Le Douarin N. M. Cell line segregation during peripheral nervous system ontogeny. Science. 1986 Mar 28;231(4745):1515–1522. doi: 10.1126/science.3952494. [DOI] [PubMed] [Google Scholar]
- Lovejoy D. A., Fischer W. H., Ngamvongchon S., Craig A. G., Nahorniak C. S., Peter R. E., Rivier J. E., Sherwood N. M. Distinct sequence of gonadotropin-releasing hormone (GnRH) in dogfish brain provides insight into GnRH evolution. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6373–6377. doi: 10.1073/pnas.89.14.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H., Baba Y., Nair R. M., Arimura A., Schally A. V. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1334–1339. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- Miller K. E., Kriebel R. M. Peptidergic innervation of caudal neurosecretory neurons. Gen Comp Endocrinol. 1986 Dec;64(3):396–400. doi: 10.1016/0016-6480(86)90074-2. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hasegawa Y., Nomura M., Igarashi M., Kangawa K., Matsuo H. Identification of the second gonadotropin-releasing hormone in chicken hypothalamus: evidence that gonadotropin secretion is probably controlled by two distinct gonadotropin-releasing hormones in avian species. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3874–3878. doi: 10.1073/pnas.81.12.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muske L. E. Evolution of gonadotropin-releasing hormone (GnRH) neuronal systems. Brain Behav Evol. 1993;42(4-5):215–230. doi: 10.1159/000114156. [DOI] [PubMed] [Google Scholar]
- Ngamvongchon S., Rivier J. E., Sherwood N. M. Structure-function studies of five natural, including catfish and dogfish, gonadotropin-releasing hormones and eight analogs on reproduction in Thai catfish (Clarias macrocephalus). Regul Pept. 1992 Nov 20;42(1-2):63–73. doi: 10.1016/0167-0115(92)90024-o. [DOI] [PubMed] [Google Scholar]
- Oka Y., Matsushima T. Gonadotropin-releasing hormone (GnRH)-immunoreactive terminal nerve cells have intrinsic rhythmicity and project widely in the brain. J Neurosci. 1993 May;13(5):2161–2176. doi: 10.1523/JNEUROSCI.13-05-02161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. F., Fischer W. H., Park M., Craig A. G., Rivier J. E., White S. A., Francis R. C., Fernald R. D., Licht P., Warby C. Primary structure of solitary form of gonadotropin-releasing hormone (GnRH) in cichlid pituitary; three forms of GnRH in brain of cichlid and pumpkinseed fish. Regul Pept. 1995 May 4;57(1):43–53. doi: 10.1016/0167-0115(95)00014-3. [DOI] [PubMed] [Google Scholar]
- Powell J. F., Zohar Y., Elizur A., Park M., Fischer W. H., Craig A. G., Rivier J. E., Lovejoy D. A., Sherwood N. M. Three forms of gonadotropin-releasing hormone characterized from brains of one species. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12081–12085. doi: 10.1073/pnas.91.25.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M., Pfaff D. W. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989 Mar 9;338(6211):161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Adelman J. P. Characterization of cDNA for precursor of human luteinizing hormone releasing hormone. Nature. 1984 Oct 18;311(5987):666–668. doi: 10.1038/311666a0. [DOI] [PubMed] [Google Scholar]
- Sherwood N. M., Sower S. A., Marshak D. R., Fraser B. A., Brownstein M. J. Primary structure of gonadotropin-releasing hormone from lamprey brain. J Biol Chem. 1986 Apr 15;261(11):4812–4819. [PubMed] [Google Scholar]
- Sherwood N., Eiden L., Brownstein M., Spiess J., Rivier J., Vale W. Characterization of a teleost gonadotropin-releasing hormone. Proc Natl Acad Sci U S A. 1983 May;80(9):2794–2798. doi: 10.1073/pnas.80.9.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sower S. A., Chiang Y. C., Lovas S., Conlon J. M. Primary structure and biological activity of a third gonadotropin-releasing hormone from lamprey brain. Endocrinology. 1993 Mar;132(3):1125–1131. doi: 10.1210/endo.132.3.8440174. [DOI] [PubMed] [Google Scholar]
- White S. A., Bond C. T., Francis R. C., Kasten T. L., Fernald R. D., Adelman J. P. A second gene for gonadotropin-releasing hormone: cDNA and expression pattern in the brain. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1423–1427. doi: 10.1073/pnas.91.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. A., Fernald R. D. Gonadotropin-releasing hormone-containing neurons change size with reproductive state in female Haplochromis burtoni. J Neurosci. 1993 Feb;13(2):434–441. doi: 10.1523/JNEUROSCI.13-02-00434.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S., Nieburgs A., Elkabes S. Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the prenatal mouse: evidence for an embryonic origin in the olfactory placode. Brain Res Dev Brain Res. 1989 Apr 1;46(2):309–318. doi: 10.1016/0165-3806(89)90295-2. [DOI] [PubMed] [Google Scholar]
- van Weerd J. H., Sukkel M., Bongers A. B., van der Does H. M., Steynis E., Richter C. J. Stimulation of gonadal development by sexual interaction of pubertal African catfish, Clarias gariepinus. Physiol Behav. 1991 Feb;49(2):217–223. doi: 10.1016/0031-9384(91)90035-m. [DOI] [PubMed] [Google Scholar]



