Abstract
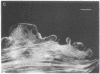
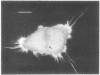
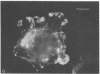
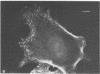
A fluorescent derivative of phalloidin has been synthesized possessing high affinity to filamentous actin. This compound was used for visualization of actin-containing structures in eukaryotic nonmuscle cells. Due to its low molecular weight (1250), fixation for formaldehyde was sufficient to render the membrane permeable for the labeled peptide. Bundles of microfilaments are the predominant pattern in the flat rat kangaroo PtK1 cells, whereas a net of concentric fibers characterizes the more spherical bovine kidney MDBK cells. Specificity of staining was confirmed by competition experiments with unlabeled phalloidin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cande W. Z., Lazarides E., McIntosh J. R. A comparison of the distribution of actin and tubulin in the mammalian mitotic spindle as seen by indirect immunofluorescence. J Cell Biol. 1977 Mar;72(3):552–567. doi: 10.1083/jcb.72.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulstich H., Schäfer A. J., Weckauf M. The dissociation of the phalloidin-actin complex. Hoppe Seylers Z Physiol Chem. 1977 Feb;358(2):181–184. doi: 10.1515/bchm2.1977.358.1.181. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Montesano R., Tuchweber B., Salas M., Orci L. Phalloidin-induced hyperplasia of actin filaments in rat hepatocytes. Lab Invest. 1975 Nov;33(5):562–569. [PubMed] [Google Scholar]
- Goldman R. D., Lazarides E., Pollack R., Weber K. The distribution of actin in non-muscle cells. The use of actin antibody in the localization of actin within the microfilament bundles of mouse 3T3 cells. Exp Cell Res. 1975 Feb;90(2):333–344. doi: 10.1016/0014-4827(75)90323-7. [DOI] [PubMed] [Google Scholar]
- Govindan V. M., Faulstich H., Wieland T., Agostini B., Hasselbach W. In-vitro effect of phalloidin on plasma membrane preparation from rat liver. Naturwissenschaften. 1972 Nov;59(11):521–522. doi: 10.1007/BF00609837. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Wang K., Singer S. J. Intracellular distributions of mechanochemical proteins in cultured fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3883–3887. doi: 10.1073/pnas.74.9.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman I. M., Pollard T. D. Actin localization in fixed dividing cells stained with fluorescent heavy meromyosin. Exp Cell Res. 1978 Jun;114(1):15–25. doi: 10.1016/0014-4827(78)90030-7. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. Relationships between fibronectin (LETS protein) and actin. Cell. 1978 Nov;15(3):875–886. doi: 10.1016/0092-8674(78)90272-6. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Actin, alpha-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976 Feb;68(2):202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Immunofluorescence studies on the structure of actin filaments in tissue culture cells. J Histochem Cytochem. 1975 Jul;23(7):507–528. doi: 10.1177/23.7.1095651. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz F., Glossmann H., Frimmer M. Binding of 3 H-desmethylphalloin to isolated plasma membranes from rat liver. Naunyn Schmiedebergs Arch Pharmacol. 1972;273(4):341–351. doi: 10.1007/BF00499668. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Sanger J. W. Changing patterns of actin localization during cell division. Proc Natl Acad Sci U S A. 1975 May;72(5):1913–1916. doi: 10.1073/pnas.72.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W. Presence of actin during chromosomal movement. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2451–2455. doi: 10.1073/pnas.72.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V., Celis J. E. Filament arrangements in negatively stained cultured cells: the organization of actin. Cytobiologie. 1978 Feb;16(2):308–325. [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Binding of deoxyribonuclease I to actin: a new way to visualize microfilament bundles in nonmuscle cells. J Histochem Cytochem. 1978 Sep;26(9):745–749. doi: 10.1177/26.9.361884. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Osborn M., Weber K. Visualization of the same PtK2 cytoskeletons by both immunofluorescence and low power electron microscopy. Exp Cell Res. 1978 Nov;117(1):47–61. doi: 10.1016/0014-4827(78)90426-3. [DOI] [PubMed] [Google Scholar]
- Wehland J., Osborn M., Weber K. Phalloidin-induced actin polymerization in the cytoplasm of cultured cells interferes with cell locomotion and growth. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5613–5617. doi: 10.1073/pnas.74.12.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T., Faulstich H. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous Amanita mushrooms. CRC Crit Rev Biochem. 1978 Dec;5(3):185–260. doi: 10.3109/10409237809149870. [DOI] [PubMed] [Google Scholar]
- Wieland T. Modification of actins by phallotoxins. Naturwissenschaften. 1977 Jun;64(6):303–309. doi: 10.1007/BF00446784. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Yamada K. M., Yamada S. S., Pouysségur J., Pastan I. Microfilament bundles and cell shape are related to adhesiveness to substratum and are dissociable from growth control in cultured fibroblasts. Cell. 1977 Mar;10(3):375–380. doi: 10.1016/0092-8674(77)90024-1. [DOI] [PubMed] [Google Scholar]