Abstract
Access to pediatric HIV treatment in resource-limited settings has risen significantly. However, little is known about the quality of care that pediatric or adolescent patients receive. The objective of this study is to explore quality of HIV care and treatment in Nigeria and to determine the association between quality of care, loss-to-follow-up and mortality. A retrospective cohort study was conducted including patients ≤18 years of age who initiated ART between November 2002 and December 2011 at 23 sites across 10 states. 1,516 patients were included. A quality score comprised of 6 process indicators was calculated for each patient. More than half of patients (55.5%) were found to have a high quality score, using the median score as the cut-off. Most patients were screened for tuberculosis at entry into care (81.3%), had adherence measurement and counseling at their last visit (88.7% and 89.7% respectively), and were prescribed co-trimoxazole at some point during enrollment in care (98.8%). Thirty-seven percent received a CD4 count in the six months prior to chart review. Mortality within 90 days of ART initiation was 1.9%. A total of 4.2% of patients died during the period of follow-up (mean: 27 months) with 19.0% lost to follow-up. In multivariate regression analyses, weight for age z-score (Adjusted Hazard Ratio (AHR): 0.90; 95% CI: 0.85, 0.95) and high quality indicator score (compared a low score, AHR: 0.43; 95% CI: 0.26, 0.73) had a protective effect on mortality. Patients with a high quality score were less likely to be lost to follow-up (Adjusted Odds Ratio (AOR): 0.42; 95% CI: 0.32, 0.56), compared to those with low score. These findings indicate that providing high quality care to children and adolescents living with HIV is important to improve outcomes, including lowering loss to follow-up and decreasing mortality in this age group.
Introduction
Though numerous challenges have limited scale-up of pediatric antiretroviral therapy (ART), significant progress has been made. [1]–[3] Access to pediatric ART in resource limited settings has risen more than 7-fold from 75,000 children receiving ART in low and middle income countries in 2005 to 562,000 by the end of 2011. [4] As pediatric treatment becomes more widely available, determining standards for and measuring the quality of HIV care and treatment that children receive has become a high priority. In order to derive the full benefit of ART, children and adolescents must be provided with high quality care and treatment that addresses their multifaceted needs, including adherence counseling, disclosure support, laboratory monitoring, and opportunistic infection screening along with other critical services.
The Institute of Medicine defines health care quality as the extent to which health services provided to individuals and populations improve desired health outcomes and are consistent with current professional knowledge. [5] Performance indicators are measurement tools that may be used to assess health care quality. [6] These indicators fall into three categories: (1) structure (characteristics of the health care setting such as human resource availability); (2) process (aspects of the encounter with the patient such as which tests are ordered); or (3) outcome (the patient's subsequent health status). [7]–[10] Structure and process may influence outcome, indirectly or directly. [11] Understanding this relationship is essential for quality improvement efforts, particularly in settings where resources to institute structural or procedural change are limited. Numerous studies conducted in sub-Saharan Africa have assessed the impact of selected structural changes, such as task-shifting and decentralization of services, on HIV treatment outcomes. [12]–[17] Process indicators capturing key services, such as semi-annual CD4 count monitoring, routine opportunistic infection screening and regular adherence support, are frequently collected for programmatic monitoring and evaluation. However, the correlation between process indicators such as these and clinical outcomes of HIV care and treatment has not been well explored in resource limited settings. [18], [19]
Moreover, quality assessment of pediatric HIV care and treatment lags far behind that of adults internationally. Several national programs have identified process and outcomes indicators to guide improvement efforts. However, few reports of HIV care quality that focus on pediatric or adolescent services have been published. [20]–[22] Reports describing clinical outcomes in this age group have documented significant challenges, with numerous studies noting high rates of loss to follow-up and early mortality. [23]–[28] Many factors—including late presentation, malnutrition, lack of caregiver involvement, nondisclosure, and HIV-related stigma—contribute to these findings. [27], [29], [30] Whether the quality of care received by children and young adults is associated with loss to follow-up or mortality has not been assessed. As pediatric and adolescent ART becomes more widely available globally, identifying appropriate measures of quality and determining their association with outcomes is critical and should help guide future programmatic planning.
Nigeria is home to the second largest number of people living with HIV in the world after South Africa. In 2012, approximately 3,400,000 people in Nigeria were living with HIV, including 430,000 children. [31] Though coordinated efforts have been underway in Nigeria to increase access to ART for pediatric patients since 2005, a significant disparity exists between pediatric and adult ART coverage (7% versus 26%, respectively). [32] Limited data exist describing clinical outcomes or quality of care in pediatric or adolescent patients on ART in Nigeria. [33], [34] The objective of this study is to explore quality of care received by pediatric and adolescent patients receiving ART in Nigeria and to determine the association between quality of care and loss to follow-up and mortality.
Methods
Ethical Review
The study protocol and assessment tools were submitted to the National Health Research Ethics Committee of Nigeria (NHREC) and approved on April 9th, 2011. Individual signed, written informed consent from participants was waived by NHREC. Patient information was de-identified prior to analysis. Unique patient identifiers were assigned to each patient to protect patient confidentiality.
Study design
A retrospective cohort study was conducted including patients enrolled in care between November 2002 and December 2011. This chart review was a component of a larger assessment of access to pediatric and adolescent treatment services funded by the United States Government/PEPFAR Nigeria Program. [File S1] Purposive sampling was used to select 23 sites providing antiretroviral therapy from 10 states across the 6 geopolitical zones of Nigeria. At the time of this assessment, all pediatric HIV care and treatment in Nigeria was provided at hospitals that offered secondary and tertiary-level specialty services. The criteria guiding site selection included geographic location and setting (i.e., urban, peri-urban, or rural). Safety concerns limited inclusion of sites in certain states. All treatment sites are monitored by the Federal Ministry of Health and supported by a variety of US government funded implementing partners (IPs).
Charts representing 10% of the total number of patients 0–18 years of age receiving antiretroviral therapy (ART) in the 10 states chosen were selected using random sampling. Each site was asked to provide a list of all enrolled patients 0–18 years of age meeting the inclusion criteria by medical record number. Chart design and contents were standardized in 2008 to include the same variables across all sites throughout the country, and sites maintained either paper or electronic records.
Patients were eligible for inclusion in the study if they were 0–18 years of age and initiated ART during the study period. Follow-up was censored either at the time of the loss to follow-up (as defined below), death or the end of the study period.
Outcomes
The two main outcomes were lost to follow-up and death. Loss to follow-up was defined as no evidence of a visit to the clinic or drug pick up for 90 days following the last scheduled appointment as documented in the chart. Death was only counted if verified by the patient's family or if death occurred within the hospital.
Independent variables including quality of care indicators
The primary independent variable of interest was a quality score comprised of six process indicators: (1) screening for tuberculosis at entry into care, (2) adherence measurement at last visit, (3) adherence counseling at last visit, (4) prescription of co-trimoxazole at any time since enrollment, (5) at least one CD4 count in the last six months, and (6) documented weight at last visit. These indicators are recommended by the World Health Organization and should be standard components of clinical practice as outlined by the 2005 and 2010 National Guidelines on Paediatric HIV and AIDS Treatment and Care in Nigeria. [35], [36] Recommendations regarding these process indicators did not change over time. Similar indicators have been used to assess pediatric HIV care and treatment quality in other studies conducted in resource limited settings. [22] Screening for tuberculosis in pediatric and adolescent patients includes symptom assessment (poor weight gain, fever, and cough), determination of contact history with a known TB case, clinical examination and radiology followed by sputum induction, if warranted. The standard procedure for adherence assessment in HIV clinics in Nigeria is to ask the patient or the caregiver the number of ART doses missed within the last three days. Review of pharmacy records, returned syrup measurement and pill counting are not routinely conducted. Adherence counseling should be offered at every visit. More intensive counseling is provided if the patient or caregiver reports missed ART doses. CD4 count testing and co-trimoxazole are provided free of charge at all sites. A quality score was calculated with one point assigned for each service received and zero points assigned if the service was not received, for a total of six points. For ease of interpretation, the score was categorized into “high quality” versus “low quality” using the median score as a cutoff for bivariate and multivariate regression models.
Additional patient characteristics and clinical variables related to the outcomes of interest were also collected including: gender, age at ART initiation, CD4 count/percentage (baseline and most recent value), baseline weight/height (to determine age-for-weight z-score), current ART regimen, and facility type (rural, peri-urban and urban). Viral load measurement was not standard of care during the study period, and therefore was not included as an outcome measure.
Baseline immunosuppression at entry into care was calculated using patient age and either initial CD4 count or initial CD4 percentage. For patients less than two years of age, “severe immunosuppression” was defined as an initial CD4 count less than 750 cells/mm3 or a percentage less than 15%, while “moderate immunosuppression” was defined as CD4 count between 750 and 1500 cells/mm3 or a percentage of between 15% and 25%. “No immunosuppression” was defined as an initial CD4 count of 1500 cells/mm3 or more or a percentage of 25% or more. For patients between two and five years of age, “severe immunosuppression” was defined as an initial CD4 count less than 500 cells/mm3 or percentage less than 15%, “moderate immunosuppression” as an initial CD4 count of between 500 and 1000 cells/mm3 or percentage of between 15% and 25%, and “no immunosuppression” as an initial CD4 count of 1000 cells/mm3 or more or percentage of 25% or more. For patients five years of age or older, “severe immunosuppression” was defined as an initial CD4 count less than 200 cells/mm3 or percentage less than 15%, “moderate immunosuppression” as an initial CD4 count of between 200 and 500 cells/mm3 or percentage of between 15% and 25%, and “no immunosuppression” as an initial CD4 count of 500 cells/mm3 or more or percentage of 25% or more. [35]
Data Analysis
Means and standard deviations were computed for continuous variables and counts with percentages for categorical variables. Differences in mortality and loss to follow-up by age group and differences in quality indicators by year of initial visit were compared using chi-square statistics. Bivariate methods were used to examine the relationships of individual independent variables with the primary outcomes of survival and loss-to-follow up, including Kaplan-Meier estimation with log rank testing. One predictor Cox proportional hazards regression models were used for survival and one predictor logistic regression models for loss to follow-up.
In survival analyses, associations were estimated using hazard ratios (HR) with 95 percent confidence intervals (CI). In the analysis of loss to follow-up, associations were estimated using odds ratios (OR) with 95 percent CIs. The multivariate logistic regression models included independent variables that were significant in the bivariate models defined as p<0.05 and/or were potential confounders of the relationship between the quality score and the outcomes (e.g., weight for age (z-score) and age at ART initiation). The multivariate Cox regression model was limited to four predictors due to the number of deaths in the sample, using a guide of ten events or deaths required per predictor. [37]
The proportional hazards assumption was checked by graphical methods and by testing interaction terms that included the log of follow-up time. The discrimination ability of the logistic models was measured by c-statistics with calibration assessed using Hosmer-Lemeshow chi-square statistics and their associated p-values. Where data were sufficient, we tested for interactions among the independent variables in these models. Patient-level variability across sites was investigated using the intra-cluster correlation coefficient (ICC). The ICC was close to zero, indicating similarity between within-site variability and variability across sites. We employed an alpha of 0.05 in all statistical tests to determine statistical significance. All data management and statistical analyses were performed using SAS for Windows version 9.2.
Results
Study population
A total of 1,516 patients were sampled from 23 sites. Most patients (73.6%) received care at urban facilities (Table 1). The average human resource distribution for pediatric HIV care and treatment was similar across facilities with an average of 23 full-time clinical staff (including doctors, nurses, pharmacists and counselors) per site. Approximately one-quarter of patients were 24 months old or younger at ART initiation, while 40.0% were between two and six years old, 21.1% were six to nine years old, and 14.9% were 10 to 18 years old (Table 1). Most patients had an initial visit and initiated ART between 2008 and 2011. Approximately one-half of patients were male (52.8%). Almost half of patients were severely immunosuppressed at baseline (46.3%), 32.5% were moderately immunosuppressed, and 21.3% were not immunosuppressed. The mean weight for age (z-score) at baseline was −1.08 (±4.04).
Table 1. Characteristics of sampled pediatric and adolescent patients (age 0 to 18 years).
| All Patients (n = 1516) | ||
| N | Mean (SD) or % | |
| Demographics | ||
| Age at ART initiation | ||
| 0–24 months | 363 | 24.0% |
| 25–71 months | 605 | 40.0% |
| 6–9 years | 318 | 21.1% |
| 10–18 years | 225 | 14.9% |
| Gender (Male) | 799 | 52.8% |
| Clinical factors | ||
| Baseline immunosuppression1 | ||
| Severe | 666 | 46.3% |
| 0–24 months | 165 | 24.8% |
| 25–71 months | 256 | 38.4% |
| 6–9 years | 124 | 18.6% |
| 10–18 years | 121 | 18.2% |
| Moderate | 468 | 32.5% |
| 0–24 months | 106 | 22.7% |
| 25–71 months | 202 | 43.3% |
| 6–9 years | 92 | 19.7% |
| 10–18 years | 67 | 14.4% |
| No suppression | 306 | 21.3% |
| 0–24 months | 47 | 15.4% |
| 25–71 months | 126 | 41.3% |
| 6–9 years | 97 | 31.8% |
| 10–18 years | 35 | 11.5% |
| Weight for age (z-score) | 1382 | −1.08 (+4.04) |
| Most recent CD4 count among those alive | ||
| <350 cells/mm3 | 369 | 27.3% |
| ≥350 cells/mm3 | 983 | 72.7% |
| Treatment | ||
| Duration of follow-up in months | 1511 | 27.7 (+19.7) |
| Current ART regimens | ||
| AZT/3TC/NVP | 1236 | 81.5% |
| Regimens containing d4T | 81 | 5.3% |
| Other regimen | 196 | 13.0% |
| Patients enrolled by facility type | ||
| Rural | 199 | 13.1% |
| Peri-urban | 201 | 13.3% |
| Urban | 1116 | 73.6% |
| Quality indicators | ||
| Screened for tuberculosis at entry into care | 1115 | 81.3% |
| Adherence counseling documented at last visit | 1311 | 89.7% |
| Adherence measured at last visit | 1296 | 88.7% |
| Ever prescribed co-trimoxazole | 1482 | 98.8% |
| Alive and not lost to follow up with at least one CD4 count in last six months | 518 | 37.0% |
| Weight documented in chart at patient's last visit | 1049 | 72.2% |
| High quality indicator score2 | 842 | 55.5% |
For patients less than two years of age, severe immunosuppression was defined as an initial CD4 count less than 750 cells/mm3 or percentage less than 15%, moderate immunosuppression as an initial CD4 count of between 750 and 1500 cells/mm3 or percentage of between 15% and 25%, and no immunosuppression as an initial CD4 count of 1500 cells/mm3 or more, or percentage of 25% or more. For patients between two and five years of age, severe immunosuppression was defined as an initial CD4 count less than 500 cells/mm3 or percentage less than 15%, moderate immunosuppression as an initial CD4 count of between 500 and 1000 cells/mm3 or percentage of between 15% and 25%, and no immunosuppression as an initial CD4 count of 1000 cells/mm3 or more, or percentage of 25% or more. For patients between five years of age or older, severe immunosuppression was defined as an initial CD4 count less than 200 cells/mm3 or percentage less than 15%, moderate immunosuppression as an initial CD4 count of between 200 and 500 cells/mm3 or percentage of between 15% and 25%, and no immunosuppression as an initial CD4 count of 500 cells/mm3 or more, or percentage of 25% or more.
1 point assigned for each service received (screened for tuberculosis, adherence counseling at last visit, adherence measured by patient/caregiver self-report at last visit, ever prescribed co-trimoxazole, alive and not lost to follow-up with at least one CD4 count in the last six months, and weight documented in chart at patient's last visit, and 0 points assigned if the service was not received, for a total of 6 points. A high score was defined as having the median score or above (>4 points).
The mean duration of follow-up time was 27.7 months (±19.7). Most patients were on an ART regimen comprised of AZT/3TC/NVP (81.5%) at the time of chart review. For those on regimens containing d4T, the mean length of time on the regimen was 36.7 months (±18.5).
Quality of care
Most patients were screened for tuberculosis at entry into care (81.3%) and had adherence counseling at their last visit (89.7%) (Table 1). Similarly, the majority of patients had their adherence measured at their last visit (88.7%). Almost all patients had been prescribed co-trimoxazole at some point during their enrollment in care (98.8%). Weight for age was documented at the last visit in 72.2% of charts evaluated. However, less than half of patients alive and in care at the time of the chart review had obtained a CD4 count in the six months prior to chart review (37.0%). A higher percentage of patients who were >10 years old were screened for TB compared to those who were <10 years (87.4% vs. 80.3%, p = 0.0148). Over half of patients had a quality score of the median or higher (greater than four points out of six) (55.5%). No significant difference was noted in overall quality score between adolescents (10–18) and younger patients (p = 0.325).
Two quality indicators improved over time. The percentage of patients with a CD4 count in the last six months (p<0.0001) and weight for age documented at the last visit (p = 0.0034) was higher for those with an initial visit in more recent years (2008 to 2011) than for those who enrolled in care prior to 2008.
Outcomes
Documented mortality within 90 days of ART initiation was 1.9% (Table 2). A total of 4.2% of patients died during the period of follow-up. Mortality was highest for those 24 months old or less at ART initiation (35.9%) (p = NS). Loss to follow-up was 19.0% during the follow-up period, with 3.6% of patients lost within six months of ART initiation and 6.9% lost within the first twelve months. Loss to follow-up was highest for those 25 to 71 months old at ART initiation (35.9%), compared to the other age groups (p = 0.0130). No significant difference was noted in mortality or loss to follow-up between adolescents (age 10–18) and younger patients (p = 0.583 and p = 0.565, respectively).
Table 2. Mortality and loss to follow-up by age at ART initiation.
| Mortality | n | % | p value 1 |
| Within 90 days of ART Initiation | 30 | 1.9% | |
| During period of follow-up | 64 | 4.2% | |
| By age at ART initiation | |||
| 0–24 months | 23 | 35.9% | NS |
| 25–71 months | 20 | 31.3% | |
| 6–9 years | 13 | 20.3% | |
| 10–18 years | 8 | 12.5% | |
| Loss to follow-up | |||
| Within 6 months of ART Initiation | 52 | 3.6% | |
| Within 12 months of ART Initiation | 100 | 6.9% | |
| During period of follow-up | 276 | 19.0% | |
| By age at ART initiation | |||
| 0–24 months | 83 | 30.4% | p = 0.0130 |
| 25–71 months | 98 | 35.9% | |
| 6–9 years | 48 | 17.6% | |
| 10–18 years | 44 | 16.1% |
Differences between age groups significant at p<0.05.
NS, not significant.
Bivariate results
Patients with a high quality score were more likely to survive over time (p = 0.0011) and less likely to be loss to follow-up (p<0.0001) than patients with a low quality indicator score (Figures 1 and 2). Gender was not statistically significant in bivariate results for mortality or loss to follow-up. A one-point increase in weight for age z-score had a protective effect on mortality (HR: 0.90; 95% CI: 0.85, 0.95) (Table 3). Patients 24 months or younger at ART initiation had a greater likelihood of death (HR: 1.76; 95% CI: 1.06, 2.94) and loss to follow-up (OR: 1.56; 95% CI: 1.16, 2.09), compared to those older than 24 months at ART initiation. Patients with severe immunosuppression were more likely to die (HR: 6.11; 95% CI: 1.89, 19.76) and be lost to follow-up (OR: 1.48; 95% CI: 1.02, 2.14) than patients with no suppression. Overall, a high quality score had a protective effect on mortality (HR: 0.43; 95% CI: 0.26, 0.73) and loss to follow-up (OR: 0.40; 95% CI: 0.31, 0.53) compared to those with a low score.
Figure 1. Kaplan-Meier survival by quality indicator score (high vs. low).
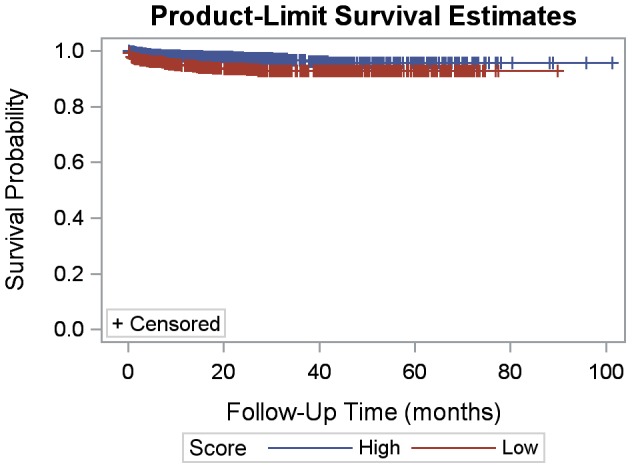
Figure 2. Kaplan-Meier loss to follow-up by quality indicator score (high vs. low).
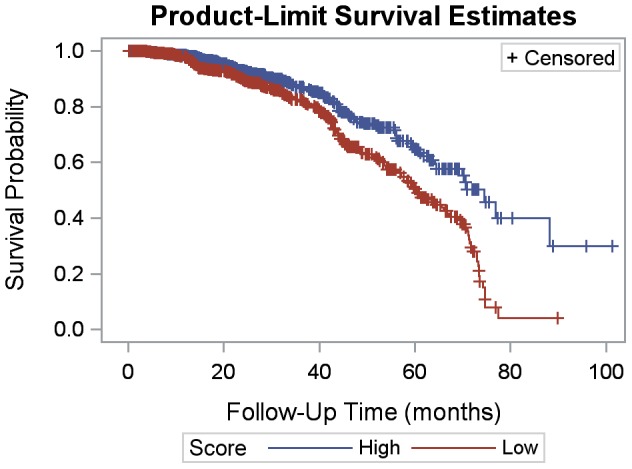
Table 3. Factors associated with mortality and loss to follow-up.
| Mortality | Mortality (n = 1315) | Loss to Follow-up | Loss to Follow-up (n = 1386) | |||||||
| Bivariate | Multivariate | Bivariate | Multivariate | |||||||
| N | HR (95% CI) | p-value | AHR (95% CI) | p-value | N | OR (95% CI) | p-value | AOR (95% CI) | p-value | |
| Male (Ref: Female) | 1509 | 1.50 (0.90, 2.49) | NS | — | — | 1450 | 1.00 (0.77, 1.30) | NS | — | — |
| Weight for age (z-score) | 1379 | 0.90 (0.85, 0.95) | 0.0003 | 0.92 (0.87, 0.98) | 0.0121 | 1328 | 0.99 (0.96, 1.03) | NS | — | — |
| Age at ART initiation ≤24 months (Ref: >24) | 1506 | 1.76 (1.06, 2.94) | 0.0297 | 1.00 (0.51, 1.99) | NS | 1447 | 1.56 (1.16, 2.09) | 0.0029 | 1.36 (0.99, 1.87) | NS |
| Baseline immunosuppression1 | 1435 | 1388 | ||||||||
| Severe | 6.11 (1.89, 19.76) | 0.0025 | 7.21 (1.72, 30.21) | 0.0068 | 1.48 (1.02, 2.14) | 0.0374 | 1.45 (0.99, 2.11) | NS | ||
| Moderate | 1.95 (0.53, 7.19) | NS | 2.88 (0.62, 13.39) | NS | 1.14 (0.77, 1.70) | NS | 1.13 (0.75, 1.70) | NS | ||
| No suppression | Ref | Ref | Ref | Ref | ||||||
| High quality indicator score2 (Ref: Low) | 1511 | 0.43 (0.26, 0.73) | 0.0015 | 0.47 (0.26, 0.87) | 0.0165 | 1452 | 0.40 (0.31, 0.53) | <0.0001 | 0.42 (0.32, 0.56) | <0.0001 |
HR, hazard ratio; AHR, adjusted hazard ratio; OR, odds ratio; AOR, adjusted odds ratio; CI, confidence interval; NS, not significant
For patients less than two years of age, severe immunosuppression was defined as an initial CD4 count less than 750 cells/mm3 or percentage less than 15%, moderate immunosuppression as an initial CD4 count of between 750 and 1500 cells/mm3 or percentage of between 15% and 25%, and no immunosuppression as an initial CD4 count of 1500 cells/mm3 or more, or percentage of 25% or more. For patients between two and five years of age, severe immunosuppression was defined as an initial CD4 count less than 500 cells/mm3 or percentage less than 15%, moderate immunosuppression as an initial CD4 count of between 500 and 1000 cells/mm3 or percentage of between 15% and 25%, and no immunosuppression as an initial CD4 count of 1000 cells/mm3 or more, or percentage of 25% or more. For patients between five years of age or older, severe immunosuppression was defined as an initial CD4 count less than 200 cells/mm3 or percentage less than 15%, moderate immunosuppression as an initial CD4 count of between 200 and 500 cells/mm3 or percentage of between 15% and 25%, and no immunosuppression as an initial CD4 count of 500 cells/mm3 or more, or percentage of 25% or more.
1 point assigned for each service received (screened for tuberculosis, adherence counseling at last visit, adherence measured by patient/caregiver self-report at last visit, ever prescribed co-trimoxazole, alive and not lost to follow-up with at least one CD4 count in the last six months, and weight documented in chart at patient's last visit and 0 points assigned if the service was not received, for a total of 6 points. A high score was defined as having the median score or above (>4 points).
Multivariate results
In multivariate Cox regression analyses, adjusting for other factors, weight for age z-score (AHR: 0.92; 95% CI: 0.87, 0.98) and high quality score (compared a low score, AHR: 0.47; 95% CI: 0.26, 0.87) had a protective effect on mortality. Patients with severe baseline immunosuppression were more likely to die than those with no immunosuppression (AHR: 7.21; 95% CI: 1.72, 30.21) (Table 3). Adjusting for other factors in multiple logistic regression analysis, patients with a high quality score were less likely to be lost to follow-up (AOR: 0.42; 95% CI: 0.32, 0.56), compared to those with low score.
Discussion
To our knowledge, this is the first report of a pediatric and adolescent HIV care and treatment quality assessment in Nigeria. Our findings suggest that providing high quality care to children and adolescents living with HIV is associated with lower loss to follow-up and mortality, providing compelling evidence that investing in quality has the potential to retain this age group in care and save lives.
In this study, six process indicators were selected to measure quality. Overall, receipt of services was high. More than 80% of patients were screened for TB at entry into care. However, a higher percentage of adolescents were screened for TB compared to pediatric patients. Though the World Health Organization recommends screening for TB in pediatric patients as described, under-diagnosis and diagnostic delays are common. [38] Intensive training and improved strategies for early TB diagnosis in this age group are needed. Adherence was measured, and adherence counseling was provided to nearly 90% of patients at their last visit. Almost all patients were started on co-trimoxazole at some point since enrollment. Weight for age (z-score) was documented in approximately 70% of charts. The percentage of patients with documented weight for age (z-score) increased in 2008–2011 compared to previous years. Increasing site level experience, improved availability of resources (scales) and training may have contributed to this difference. Low quality scores were largely due to deficits in receipt of CD4 count testing within 6 months of the chart review. The low rates of CD4 count testing may have been due to human resource deficits, dysfunctional equipment, reagent stock-outs, or limited ability to transport samples to a central lab facility, all common challenges identified in a larger assessment of these sites. [File S1]
The quality score devised for this study was significantly correlated with both loss to follow-up and mortality, with a higher score associated with decreased loss to follow-up and increased survival. This finding correlates with limited data from studies in the US demonstrating the association between quality of HIV care and clinical outcomes. [39], [40] In this study, survival may have been lower in patients who did not receive selected process indicators because sub-optimal adherence and treatment failure were missed. Many of the patients who were lost to follow-up may have been too ill to return to the clinic. Higher weight for age z-score had a protective effect on mortality. Numerous studies have also noted this finding. [41]–[44]
Very limited clinical outcomes data are currently available from the Nigerian pediatric and adolescent HIV treatment program. Though the focus of this study was not solely on clinical outcomes, this study provides a reasonable estimate of loss to follow-up and mortality within the Nigerian national program. Early mortality was 1.9% across all age groups, while mortality during the period of follow-up was noted to be 4.2%. Compared to data from similar pediatric and adolescent cohorts in sub-Saharan Africa, mortality was lower in the Nigerian program. [44], [45] Similar to other studies, loss to follow-up was high at 19.0%. [46], [47]
This study has several limitations. Sites were not randomly selected because we wanted to include a geographically diverse sample. Furthermore, we were limited to certain states due to safety concerns. We did not measure all site characteristics that may have impacted the process indicators included in the quality score in this study. This was because our goal was to focus on process, not structure. Furthermore, we knew from a larger assessment that all the sites had similar resource availability. [File S1] Though we provided an estimate of pediatric program staffing, we were unable to account for individual provider characteristics such as years of experience treating pediatric or adolescent patients living with HIV or specialty training which may have impacted performance measures. In addition, as death was only documented in the chart if confirmation was obtained from the patient's family or caregiver or if death occurred in the hospital, it is not possible to know how many patients who were lost to follow up were actually dead. Staff members were trained to contact caregivers when patients were loss to follow-up, but standards regarding the timeliness of this contact were not well defined, and deaths may have been missed. Lastly, accurate measurement of quality is largely dependent upon the accuracy of documentation which is challenging to confirm in a retrospective study.
Pediatric and adolescent access to antiretroviral therapy in Nigeria is expanding. Since 2013, the number of facilities across the country that provide ART to children and adolescents has increased significantly. In order to expedite access to treatment, decentralization or down-referral of pediatric care to primary health clinics has been initiated. Efforts are also underway to standardize services across facilities and to ensure that quality improvement is incorporated into regular in service training. [Federal Ministry of Health of Nigeria, Personal Communication, January 15, 2013] As pediatric and adolescent ART access continues to expand in Nigeria and in similar resource limited settings, ensuring the quality of care that patients receive will be essential to reducing mortality and loss to follow-up. The next logical step toward achieving this goal in the Nigerian treatment program would be to use these data to inform a quality improvement intervention. Quality improvement studies are infrequently published in the medical literature. However, a few studies have demonstrated the efficacy of quality improvement in HIV care and treatment in resource limited settings. [48]–[50] More broadly, quality improvement methods strengthen health systems and help program planners utilize scarce resources more efficiently. [51] Considering the enormous investment that has been made in expanding access to pediatric and adolescent HIV treatment, assessments of quality followed by the development of appropriate interventions are critically important to maximize the benefits of ART.
Supporting Information
Greeson D, Ojikutu B, Kolapo U, Higgins Biddle M, Cabral H, et al. (2012) Rapid assessment of pediatric HIV treatment in Nigeria. Arlington, VA: USAID's AIDS Support and Technical Assistance Resources, AIDSTAR-One, Task Order 1. Available at: http://www.aidstar-one.com/focus_areas/treatment/resources/report/pediatric_tx_nigeria. Accessed 2013 Dec 14.
(PDF)
Funding Statement
This research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Agency for International Development under the terms of contract no. GHH-I-00–07–00059–00. Bisola Ojikutu and Molly-Higgins Biddle are employed by John Snow Inc. Usman Kolapo is employed by Indepth Precision. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kline MW (2006) Perspectives on the pediatric HIV/AIDS pandemic: catalyzing access of children to care and treatment. Pediatrics 117: 1388–93. [DOI] [PubMed] [Google Scholar]
- 2. Abrams EJ, Simonds RJ, Modi S, Rivadeneira E, Vaz P, et al. (2012) PEPFAR scale-up of pediatric HIV services: innovations, achievements, and challenges. J Acquir Immune Defic Syndr 60 Suppl 3 S105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meyers T, Moultrie H, Naidoo K, Cotton M, Eley B, et al. (2007) Challenges to pediatric HIV care and treatment in South Africa. J Infect Dis 196 Suppl 3 S474–81. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Treatment of HIV in Children (2013) Available at: http://www.who.int/hiv/topics/paediatric/en/index.html. Accessed 2014 Jun 1.
- 5.The Institute of Medicine. Crossing the Quality Chasm: The IOM Health Care Quality Initiative (1998) Available at: http://www.iom.edu/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed 2014 Jun 1.
- 6. Mainz J (2003) Defining and classifying clinical indicators for quality improvement. Int J Qual Health Care 15: 523–30. [DOI] [PubMed] [Google Scholar]
- 7. Brook RH, McGlynn EA, Cleary PD (1996) Quality of health care. Part 2: measuring quality of care. N Engl J Med 335: 966–70. [DOI] [PubMed] [Google Scholar]
- 8. Donabedian A (1988) The quality of care. How can it be assessed? JAMA 260: 1743–8. [DOI] [PubMed] [Google Scholar]
- 9. Donabedian A (1988) Quality assessment and assurance: unity of purpose, diversity of means. Inquiry 25: 173–92. [PubMed] [Google Scholar]
- 10.Brook RH, Davies Avery A, Greenfield S, Harris LJ, Lelah T, et al. (1977) Assessing the quality of medical care using outcome measures: an overview of the method. Med Care. 15 :suppl 1–165. [PubMed] [Google Scholar]
- 11. Campbell SM, Roland MO, Buetow SA (2000) Defining quality of care. Soc Sci Med 51: 1611–25. [DOI] [PubMed] [Google Scholar]
- 12. Emdin CA, Chong NJ, Millson PE (2003) Non-physician clinician provided HIV treatment results in equivalent outcomes as physician-provided care: a meta-analysis. J Int AIDS Soc 16: 18445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moon TD, Burlison JR, Blevins M, Shepherd BE, Baptista A, et al. (2011) Enrollment and programmatic trends and predictors of antiretroviral therapy initiation from president's emergency plan for AIDS Relief (PEPFAR)-supported public HIV care and treatment sites in rural Mozambique. Int J STD AIDS 22: 621–7. [DOI] [PubMed] [Google Scholar]
- 14. Morris MB, Chapula BT, Chi BH, Mwango A, Chi HF, et al. (2009) Use of task-shifting to rapidly scale-up HIV treatment services: experiences from Lusaka, Zambia. BMC Health Serv Res. 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brennan AT, Long L, Maskew M, Sanne I, Jaffray I, et al. (2011) Outcomes of stable HIV-positive patients down-referred from a doctor-managed antiretroviral therapy clinic to a nurse-managed primary health clinic for monitoring and treatment. AIDS 25: 2027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutcliffe CG, Bolton Moore C, van Dijk JH, Cotham M, Tambatamba B, et al. (2010) Secular trends in pediatric antiretroviral treatment programs in rural and urban Zambia: a retrospective cohort study. BMC Pediatr 29: 849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fayorsey RN, Saito S, Carter RJ, Gusmao E, Frederix K, et al. (2013) Decentralization of pediatric HIV care and treatment in five sub-Saharan African countries. J Acquir Immune Defic Syndr 62: e124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alemayehu YK, Bushen OY, Muluneh AT (2009) Evaluation of HIV/AIDS clinical care quality: the case of a referral hospital in North West Ethiopia. Int J Qual Health Care 21: 356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thanprasertsuk S, Supawitkul S, Lolekha R, Ningsanond P, Agins BD, et al. (2012) HIVQUAL-T: monitoring and improving HIV clinical care in Thailand, 2002–08. Int J Qual Health Care 24: 338–47. [DOI] [PubMed] [Google Scholar]
- 20. Ciampa PJ, Tique JA, Jumá N, Sidat M, Moon TD, et al. (2012) Addressing poor retention of infants exposed to HIV: a quality improvement study in rural Mozambique. J Acquir Immune Defic Syndr 60: e46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Were MC, Nyandiko WM, Huang KT, Slaven JE, Shen C, et al. (2013) Computer-generated reminders and quality of pediatric HIV care in a resource-limited setting. Pediatrics 131: e789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lolekha R, Chunwimaleung S, Hansudewechakul R, Leawsrisook P, Prasitsuebsai W, et al. (2010) Pediatric HIVQUAL-T: measuring and improving the quality of pediatric HIV care in Thailand, 2005–2007. Jt Comm J Qual Patient Saf 36: 541–51. [DOI] [PubMed] [Google Scholar]
- 23. Leroy V, Malateste K, Rabie H, Lumbiganon P, Ayaya S, et al. (2013) Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multiregional collaboration. J Acquir Immune Defic Syndr 62: 208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lamb MR, Fayorsey R, Nuwagaba Birbonwoha H, Viola V, Mutabazi V, et al. (2013) High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS 28: 559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. George E, Noël F, Bois G, Cassagnol R, Estavien L, et al. (2007) Antiretroviral therapy for HIV-1-infected children in Haiti. J Infect Dis 195: 1411–8. [DOI] [PubMed] [Google Scholar]
- 26. Nyandiko W, Vreeman R, Liu H, Shangani S, Sang E, et al. (2013) Nonadherence to clinic appointments among HIV-infected children in an ambulatory care program in western Kenya. J Acquir Immune Defic Syndr 63: e49–55. [DOI] [PubMed] [Google Scholar]
- 27. Walker AS, Prendergast AJ, Mugyenyi P, Munderi P, Hakim J, et al. (2012) Mortality in the year following antiretroviral therapy initiation in HIV-infected adults and children in Uganda and Zimbabwe. Clin Infect Dis 55: 1707–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weigel R, Estill J, Egger M, Harries AD, Makombe S, et al. (2012) Mortality and loss to follow-up in the first year of ART: Malawi national ART programme. AIDS 26: 365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marazzi MC, De Luca S, Palombi L, Scarcella P, Ciccacci F, et al. (2013) Predictors of adverse outcomes in HIV-1 infected children receiving combination antiretroviral treatment: results from a DREAM Cohort in Sub-Saharan Africa. Pediatr Infect Dis J. 2014 Mar 33(3): 295–300. [DOI] [PubMed] [Google Scholar]
- 30. Braitstein P, Songok J, Vreeman RC, Wools Kaloustian KK, Koskei P, et al. (2011) "Wamepotea" (they have become lost): outcomes of HIV-positive and HIV-exposed children lost to follow-up from a large HIV treatment program in western Kenya. J Acquir Immune Defic Syndr 57: e40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UNAIDS. Country profile: Nigeria. Available: http://www.unaids.org/en/regionscountries/countries/nigeria/. Accessed 2014 Jun 1.
- 32.UNAIDS (2011) Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. Geneva: WHO. Available at: http://www.who.int/hiv/pub/progress_report2011/en/ (Joint publication of WHO, Joint United Nations Programme on HIV/AIDS (UNAIDS), and United Nations Children's Fund (UNICEF). Accessed 2014 Jun 1.
- 33. Mukhtar Yola M, Adeleke S, Gwarzo D, Ladan ZF (2006) Preliminary investigation of adherence to antiretroviral therapy among children in Aminu Kano Teaching Hospital, Nigeria. Afr J AIDS Res 5: 141–144. [DOI] [PubMed] [Google Scholar]
- 34.Anigilaje EA, Olutola A (2013) Prevalence and clinical and immunoviralogical profile of human immunodeficiency virus-Hepatitis B co-infection among children in an antiretroviral therapy programme in Benue State, Nigeria. ISRN Pediatr. 932697. [DOI] [PMC free article] [PubMed]
- 35.World Health Organization (2010) Antiretroviral therapy for HIV infection in infants and children: towards universal access. Available at: http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. Accessed 2013 Dec 14. [PubMed]
- 36.Federal Ministry of Health of Nigeria (2010) National guidelines on pediatric HIV and AIDS treatment and care. Available at: http://www.aidstar-one.com/sites/default/files/treatment/national_treatment_guidelines/Nigeria_peds_2010_tagged.pdf. Accessed 2013 Dec 14.
- 37. Peduzzi P, Concato J, Feinstein AR, Holford TR (1995) Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol 48: 1503–10. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization (2011) Guidelines for intensified case finding for people living with HIV in resource constrained settings. Available at: http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf?ua=1. Accessed 2014 May 18.
- 39. Virga PH, Jin B, Thomas J, Virodov S (2012) Electronic health information technology as a tool for improving quality of care and health outcomes for HIV/AIDS patients. Int J Med Inform 81: e39–45. [DOI] [PubMed] [Google Scholar]
- 40. Horberg M, Hurley L, Towner W, Gambatese R, Klein D, et al. (2011) HIV quality performance measures in a large integrated health care system. AIDS Patient Care STDS 25: 21–8. [DOI] [PubMed] [Google Scholar]
- 41.Davies MA, May M, Bolton Moore C, Chimbetete C, Eley B, et al. (2013) Prognosis of Children with HIV-1 Infection Starting Antiretroviral Therapy in Southern Africa: A Collaborative Analysis of Treatment Programs. Pediatr Infect Dis J. [DOI] [PMC free article] [PubMed]
- 42. Zhao Y, Li C, Sun X, Mu W, McGoogan JM, et al. (2013) Mortality and treatment outcomes of China's National Pediatric antiretroviral therapy program. Clin Infect Dis 56: 735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zanoni BC, Phungula T, Zanoni HM, France H, Feeney ME (2011) Risk factors associated with increased mortality among HIV infected children initiating antiretroviral therapy (ART) in South Africa. PLoS One 6: e22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ekouevi DK, Azondekon A, Dicko F, Malateste K, Touré P, et al. (2011) 12-month mortality and loss-to-program in antiretroviral-treated children: the IeDEA pediatric West African database to evaluate AIDS (pWADA), 2000–2008. BMC Public Health 11: 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. KIDS-ART-LINC Collaboration (2008) Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr 49: 523–31. [DOI] [PubMed] [Google Scholar]
- 46. Bygrave H, Mtangirwa J, Ncube K, Ford N, Kranzer K, et al. (2012) Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PLoS One 7: e52856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Evans D, Menezes C, Mahomed K, Macdonald P, Untiedt S, et al. (2013) Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retroviruses 29: 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ciampa PJ, Tique JA, Jumá N, Sidat M, Moon TD, et al. (2012) Addressing poor retention of infants exposed to HIV: a quality improvement study in rural Mozambique. J Acquir Immune Defic Syndr 60: e46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sripipatana T, Spensley A, Miller A, McIntyre J, Sangiwa G, et al. (2007) Site-specific interventions to improve prevention of mother-to-child transmission of human immunodeficiency virus programs in less developed settings. Am J Obstet Gynecol 197: S107–S112. [DOI] [PubMed] [Google Scholar]
- 50. Doherty T, Chopra M, Nsibande D, Mngoma D (2009) Improving the coverage of the PMTCT programme through a participatory quality improvement intervention in South Africa. BMC Public Health 9: 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leatherman S, Ferris TG, Berwick D, Omaswa F, Crisp N (2010) The role of quality improvement in strengthening health systems in developing countries. Int J Qual Health Care 22: 237–243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Greeson D, Ojikutu B, Kolapo U, Higgins Biddle M, Cabral H, et al. (2012) Rapid assessment of pediatric HIV treatment in Nigeria. Arlington, VA: USAID's AIDS Support and Technical Assistance Resources, AIDSTAR-One, Task Order 1. Available at: http://www.aidstar-one.com/focus_areas/treatment/resources/report/pediatric_tx_nigeria. Accessed 2013 Dec 14.
(PDF)


