Abstract
Cell mechanical properties on a whole cell basis have been widely studied, whereas local intracellular variations have been less well characterized and are poorly understood. To fill this gap, here we provide detailed intracellular maps of regional cytoskeleton (CSK) stiffness, loss tangent, and rate of structural rearrangements, as well as their relationships to the underlying regional F-actin density and the local cytoskeletal prestress. In the human airway smooth muscle cell, we used micropatterning to minimize geometric variation. We measured the local cell stiffness and loss tangent with optical magnetic twisting cytometry and the local rate of CSK remodeling with spontaneous displacements of a CSK-bound bead. We also measured traction distributions with traction microscopy and cell geometry with atomic force microscopy. On the basis of these experimental observations, we used finite element methods to map for the first time the regional distribution of intracellular prestress. Compared with the cell center or edges, cell corners were systematically stiffer and more fluidlike and supported higher traction forces, and at the same time had slower remodeling dynamics. Local remodeling dynamics had a close inverse relationship with local cell stiffness. The principal finding, however, is that systematic regional variations of CSK stiffness correlated only poorly with regional F-actin density but strongly and linearly with the regional prestress. Taken together, these findings in the intact cell comprise the most comprehensive characterization to date of regional variations of cytoskeletal mechanical properties and their determinants.
Keywords: cell mechanics, stiffness, remodeling, heterogeneity
evolutionary success of the eukaryotic cytoskeleton (CSK) is based on its abilities to perform tasks that demand contrasting material properties (31). For example, cell spreading (11), crawling (34), and invasion (72) require traction forces and mechanical strength on the one hand but adaptability and remodeling (8) on the other. During pattern formation (27) and gene expression (21) the CSK mediates transduction of mechanical cues into chemical signals. For most of these activities, the cell needs to tune CSK material properties not only globally at the whole cell level but also locally within the single cell (26). It is now known that corners of the micropatterned cell correspond to the region of greatest focal adhesion density (6), highest traction forces (3), and most active lamellar extension (45), but detailed mapping of CSK material properties within a single cell has yet to be defined.
Since Petersen et al.'s (48) measurements of intracellular stiffness, regional differences in cellular mechanical properties have been heavily emphasized (23, 28, 53, 68, 73). For example, Su et al. (61) have shown that in the migrating cell the leading edge is about two times stiffer than the trailing edge. Similarly, Shroff et al. (55) have shown that the local stiffness varies up to fivefold within a single cell and that the peripheral regions are the stiffest, while the cell center is the softest. Similar heterogeneities have been found in other cell types and with other methodologies (2, 29, 30, 39). To account for regional differences in cell stiffness it has been suggested that stress fibers are locally thicker or denser at stiffer locations (2, 29, 30, 40, 61, 62), and common to all these studies is the further assertion—based upon only limited data—that local variations in cell mechanical properties must be closely linked to variations in the local density of the F-actin network.
Nonetheless, both the tensegrity model and experiments from prestressed actin gels and stretched cells would suggest that the prestress should be the central factor determining cellular stiffness (22, 25, 49, 51, 52, 59, 60, 70, 71). However, as a potential contributor to the local variations of CSK stiffness, the local variation of the CSK prestress has not yet been evaluated experimentally in the living cell. To address this issue, we provide here comprehensive maps of local static and dynamic mechanical properties of the human airway smooth muscle (HASM) cell. We plated each cell on a micropatterned substrate with square extracellular matrix (ECM) islands. Micropatterning provided several experimental advantages. Across all cells that were studied, this approach created well-defined cell corners with no appreciable variation in cell spreading or cell shape. For that reason, responses could be averaged across an ensemble of cells with virtually identical geometries, and statistical power could be amplified further by taking advantage of the eightfold symmetry condition implied by square cell geometry and the associated rectilinear coordinate system onto which all data could be unambiguously mapped. We studied 462 positions on a total of 193 cells.
We measured three static properties locally: cell height, using atomic force microscopy (AFM) (54), F-actin density, using immunofluorescent imaging (56), and traction forces that the cell exerts on its elastic substrate, using Fourier-transform traction microscopy (FTTM) (10). In addition, we measured the rate of CSK structural rearrangements, using spontaneous (unforced) nanoscale displacements of a CSK-bound bead as an index (8, 9), and the complex elastic modulus, using forced bead displacements and optical magnetic twisting cytometry (OMTC) (16–18, 67). Finally, from the measured traction distribution and geometry we were able to compute for the first time the internal distribution of the local cytoskeletal prestress. Our principal finding is that systematic regional variations of CSK stiffness correlate quite poorly with regional F-actin density but correlate strongly and linearly with the regional prestress.
METHODS
Cell culture.
HASM cells were isolated from tracheal smooth muscle of human lung transplant donors (approved by the University of Pennsylvania Committee on Studies Involving Human Subjects) as described previously (44). Cells at passages 4–7 were used in all experiments. After cells reached confluence in plastic dishes, they were serum deprived for 24 h before being trypsinized and seeded on micropatterned substrates.
Acrylamide gel.
We adapted the technique of polyacrylamide substrate preparation described previously (47). The mixing ratio of acrylamide solution was adjusted to obtain a Young's modulus of 4 kPa (74). It is well established that the stiffness of the cell substrate influences both cell traction forces and cell stiffness (46, 58). However, in this report we restrict attention to the case of regional variations with a single value of substrate stiffness falling within the physiological range.
Micropatterning.
We used the MEMPAT (membrane-based patterning) technique to make square cells (15, 43). In brief, polydimethylsiloxane (PDMS) membranes with 50 × 50-μm square holes were fabricated with conventional micromachining procedures and put onto the center of a glass-bottomed well or on top of the acrylamide gel surface. We then coated the open surfaces with bovine collagen type I (Cohesion). Collagen solutions (5 µg/ml and 100 µg/ml,) respectively) were used to coat glass and gel surfaces, respectively (1, 17). After incubation at 4°C overnight, we lifted off the membrane and blocked the uncoated area with 1% bovine serum albumin (Sigma).
The size of the 50-μm square was chosen based on the average area of isolated HASM cells (66). We plated serum-deprived cells (∼1,500 cells/well) sparsely, washed them two times with fresh medium after 1 h, and incubated them overnight before starting experiments. Single cells conforming to the micropatterned square shape were used for measurements.
Atomic force microscopy.
We measured the height profile of the micropatterned cell with AFM (Asylum Research); three-dimensional images were taken in contact mode with a pyramid tip [spring constant (k) ∼0.03 N/m, Novascan].
Optical magnetic twisting cytometry.
Detailed descriptions and validations of this technique have been given elsewhere (9, 17, 18, 50, 67). Briefly, to probe the rheology of the CSK, ferrimagnetic beads (4.1-μm diameter) were coated with a synthetic peptide containing the Arg-Gly-Asp (RGD) sequence (Integra Lifesciences) and allowed to adhere randomly to the apical cell surface. RGD binds mostly with β1-integrin, which has been shown to be distributed relatively homogeneously (41). The effect of RGD's binding with integrin in OMTC measurement has been discussed in depth elsewhere (50, 67). In brief, integrins are activated by binding with RGD, and their activation induces local remodeling, but these remodeled structures are no different from those that all adherent cells form at anchorage sites that connect cells to the ECM. Also, we showed previously (9) that cellular stiffness measured with beads coated with ligands that activate integrins is similar to the stiffness measured with poly-l-lysine-coated beads, which do not form focal adhesion. We then measured the complex modulus as a function of frequency by applying oscillatory magnetic fields and measuring the resultant bead motions. At a given frequency of oscillatory magnetic fields (f), the complex modulus is g̃(f) = T̃/D̃, where T is specific torque, D is the displacement, and the tilde denotes Fourier transform. The real part of the complex modulus is the storage modulus (g′), and the imaginary part is the loss modulus (g″). The ratio g″/g′ is the loss tangent.
We first recorded spontaneous motions of beads as a measure of the rate of structural rearrangements (described below). Beads were then magnetized with a short (<1 ms) magnetic field (>1,000 G) and twisted with a small magnetic field (10 G) in the OMTC protocol. To avoid time-dependent changes, we used a low specific torque (∼20 Pa) and finished measurements within 30 min (12).
Within the linear range, we established previously (16, 18) that CSK rheology corresponds to the power law response g′ ∝ fx−1 over a wide range of frequencies. In such systems, the power law exponent x describes a range of CSK states ranging from Hookean elastic solidlike behavior (x = 1) to Newtonian fluidlike behavior (x = 2) (16, 57).
For each bead, the structural damping model, Eq. 1, was fitted to the complex modulus (g̃) in the frequency domain with respect to the three parameters g, x, and μ,
| (1) |
where g is a prefactor that we used as the elastic modulus or stiffness, ω is angular velocity (2πf), Γ[] is the gamma function, μ is a parameter to account for the effect of Newtonian viscosity, and i is (16). The units of g are pascals per nanometer.
Structural rearrangements.
Bead positions were recorded at a frequency of 12 frames/s for maximum time (tmax) ∼160 s as previously described (8, 9). We defined mean square displacement (MSD) of an individual bead as
where r(t) is the bead position at time t, Δt is the time lag, and angle brackets indicate an average over t (8). MSD of most beads increased with time according to a power law relationship, MSD(Δt) = D*(Δt/Δt1)α, where Δt1 = 1 s, thus nondimensionalizing the time dependence. The coefficient D* has dimensions in square nanometers, and the exponent α of each bead was calculated from a least-square fit of a power law to the MSD data for Δt between 4 s and tmax/4 (9). All the beads used for MSD data were included in OMTC data.
Fourier-transform traction microscopy.
Bright field and fluorescent images of the micropatterned cell on a gel were recorded. After the cell was trypsinized, another fluorescent image was taken to calculate the displacement field generated by the cell. Using these images, we determined the displacement field of the gel (65). The constrained traction field within a 50-μm square was calculated from the displacement field implementing the solution described by Butler et al. (10).
Immunofluorescent imaging and quantification of F-actin amount.
We stained F-actin with rhodamine phalloidin (Molecular Probes) (56). The structure of F-actin was scanned with a confocal microscope (Leica Microsystems) with fixed step size (0.2 μm), the images were stacked vertically, and intensities were summed (64). The fluorescent intensities were used as a measure of F-actin amount based on the fact that the scanning conditions and staining conditions were same.
Bead position and symmetry conditions.
With bright field images, local positions of beads on a square cell were calculated relative to the lower left corner of the square with the same center of mass algorithm that we used for bead tracking (17). The symmetry of micropatterned square lattice allowed us to map all data onto a single triangle corresponding to one-eighth of the square, thus increasing the point density by eightfold (7). With the four symmetry lines of a square (horizontal, vertical, and 2 diagonals), all data were mapped onto a right-triangular octant. We also applied the same symmetry transformation on tractions, cell heights, and F-actin fluorescent intensities.
Statistical analysis.
Statistical analyses were performed with unpaired t-test assuming unequal variance. Statistical significance was determined at the level of P < 0.05.
Mapping schemes.
From pooled data over all cells studied, we created regional distribution maps with a grid spacing of 0.5 μm, smoothed over a 6-μm-radius circle. Maps presented here depict geometric mean (g and D*) or arithmetic mean (x) values over each circle, consistent with the distribution of these variables previously noted (9, 17). Arithmetic means of traction data, cell height, and F-actin density were taken over all cells.
Prestress calculation.
We define prestress as the maximum principal stress within our cell model, as described here. To calculate the prestress we used finite element methods (FEM). We constructed a three-dimensional finite element model of one-eighth of the cell (see Fig. 1A, inset) based on the AFM measurements. Displacements normal to the reflection-symmetry planes and the cell-substrate interface are constrained to be zero. Tractions obtained from FTTM were reversed in direction and applied to the bottom plane of the cell. This is a precise application of Newton's third law: the reciprocity of action and reaction. With these boundary conditions and the assumptions of an isotropic, linearly elastic material (Young's modulus = 10 kPa, Poisson ratio = 0.499), we calculated the stress distribution within the cell. It should be noted that as long as the strains are small, the recovered stresses given traction boundary conditions do not depend on the elastic modulus of the medium. We therefore chose a Young's modulus sufficiently high to ensure remaining in the linear regime. The two-dimensional contour map of prestress was generated by averaging the stress along the axis normal to the basal plane of the cell.
Fig. 1.
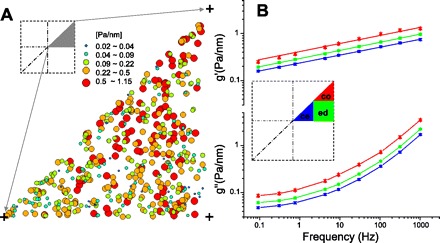
A: local elastic modulus g values (Pa/nm) measured by optical magnetic twisting cytometry (OMTC) were, by symmetry, folded onto an octant of the square cell (dark triangle, inset). B: raw data were segregated into 3 groups: center (ce), edge (ed), and corner (co) (inset). Average storage (g′) and loss (g″) modulus of each region show power law dynamics as a function of frequency. Data presented are as in Table 1; g′ and g″ in each region were significantly different from each other at all frequencies except for g′ at 1 kHz.
RESULTS
Random variability.
We obtained mechanical data from a total of 462 beads distributed among 193 different micropatterned cells. To minimize the effect of bead-bead interaction, all beads studied were apart from any other beads by at least 2 bead diameters (17). Data for all beads were mapped into one cell octant (Fig. 1A, inset). The distribution of bead positions showed no obvious spatial bias and spanned the space of interest uniformly. The local elastic modulus or stiffness, g, was coded by both the color and the size of each datum and showed that local stiffness is highly variable (Fig. 1A). The histogram of g conformed to a log-normal distribution, as has been reported previously by ourselves (17, 76) and confirmed widely by others (4, 14, 36, 69). Even though there was minimal variation in cell size or cell shape, the variation in g approached 2 orders of magnitude.
Systematic regional distribution.
As a crude first analysis, we segregated beads into three groups: center, edge, and corner (Fig. 1B, inset). The data in Fig. 1B show that the geometric mean of the elastic storage modulus (g′) increased as a weak power law function of frequency (16) in all three regions. The corner was approximately twice as stiff as the center, with the edge being intermediate; this result is compatible with previous reports (5, 55, 61). The loss modulus (g″) was also weakly dependent on frequency and showed regional differences comparable to the storage modulus. When we compared data in each region, the stiffness (g) was significantly higher in the corner than the other regions and the center was the softest region (Table 1). The Newtonian viscosity term μ in the corner followed a trend similar to that of g, but the effect was small. The power law exponent x was higher in the corner than the other regions; this difference was quantitatively small, but with the large size of the data set did reach statistical significance. There was no difference between the edge and the center. In summary, the corner region was stiffer, more viscous (the Newtonian μ), and more fluidlike than the other regions.
Table 1.
Average values of x, g, μ, D*, and α from each region and P values between regions
| No. of Beads | x | g, Pa/nm | μ, 10−4 Pa·s·nm−1 | No. of Beads | D*, nm2 | α | |
|---|---|---|---|---|---|---|---|
| Center | 149 | 1.176 ± 0.003 | 0.178 ± 0.013 | 2.51 ± 0.16 | 108 | 43.9 ± 3.7 | 1.58 ± 0.02 |
| Edge | 221 | 1.180 ± 0.003 | 0.222 ± 0.015 | 3.28 ± 0.20 | 178 | 31.1 ± 2.0 | 1.55 ± 0.02 |
| Corner | 92 | 1.201 ± 0.007 | 0.289 ± 0.027 | 5.12 ± 0.45 | 78 | 29.4 ± 2.9 | 1.62 ± 0.03 |
| P value | ce-ed | 0.48 | 0.026 | 0.0021 | N/A | 0.0014 | 0.26 |
| ce-co | 0.0017 | <0.0001 | <0.0001 | N/A | 0.0026 | 0.45 | |
| ed-co | 0.0053 | 0.025 | <0.0001 | N/A | 0.65 | 0.059 |
Data shown are means ± SE for power law exponent x, viscosity (μ), and exponent α and geometric means ± the log SE times the geometric mean for stiffness (g) and local mean square displacement prefactor D*. ce, Center; ed, edge; co, corner; N/A, not applicable.
Stiffness data were smoothed and mapped back onto the original square cell geometry (Fig. 2). Stiffness decreased gradually from the corners toward the center. The edge was relatively soft, and there was little change in stiffness along horizontal and vertical lines of symmetry (Fig. 2A). However, corners had slightly higher values of the power law exponent x (Fig. 2B) and were thus more fluidlike than other cell regions. The cell center, overlying the nucleus, was very slightly more fluidlike than surrounding regions, and a ring area around the nucleus was the most solidlike region. The distribution of x was comparable to that in a recent report (63).
Fig. 2.
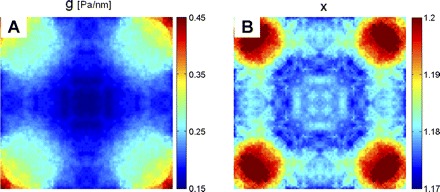
Distribution of local stiffness g (A) and power law exponent x (B).
Structural rearrangements.
Among all 364 beads we measured, spontaneous bead motions showed superdiffusive MSDs at lag times Δt > 1 s; this result is comparable to those in previous reports (8, 9). These data indicated, further, that for all time lags spontaneous motions were larger at the cell center than at edges or corners (data not shown). When the MSD was fitted to a power law at large time lags (Δt > 4 s), the prefactor D* was significantly higher in the center than the other regions, but there was no significant difference between the edge and the corner (Table 1). The exponent α showed no significant difference between groups (P > 0.05). When we mapped D* onto a square grid, D* was highest in the center and smallest in the corners (Fig. 3A). To compare directly the local cell remodeling with local stiffness, we divided the maps of D* and stiffness (g) into small squares (5 × 5 μm2) and cross-plotted the mean values of D* and g within each square (Fig. 3B). Consistent with our previous work in semiconfluent cells that are not micropatterned (8), there was a strong inverse association between regional distributions of D* and those of g.
Fig. 3.
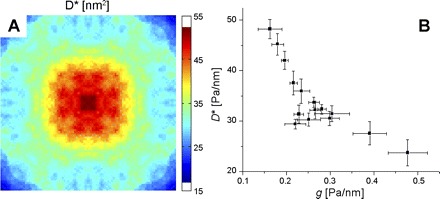
A: distribution of local mean square displacement (MSD) prefactor D*, evaluated from the MSD at lag time Δt = 1 s. B: cross plot of local MSD prefactor D* vs. local stiffness g. Plotted are mean and SD within small squares (5 × 5 μm2) into which we divided the maps of D* and stiffness g.
Height profile.
To examine the relationship, if any, between regional cell height (h) and local cell stiffness, h was measured with AFM (Fig. 4A). When we compared local cell height h and local stiffness g, the local stiffness increased as the local cell height decreased. However, for reasons addressed in the discussion, quantifying the direct influence that cell height has on the stiffness as measured by OMTC is not possible.
Fig. 4.
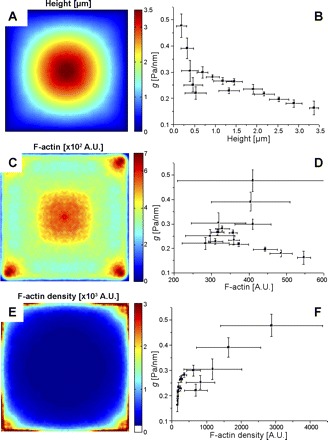
A: average height of cell measured with atomic force microscopy (AFM) (n = 5). B: cross plot of local stiffness g vs. local cell height. C: mean fluorescent intensity of F-actin in a square cell (n = 36). A.U., arbitrary units. D: cross plot of g vs. local F-actin amount. E: distribution of the local F-actin density calculated by dividing F-actin amount by cell height. F: cross plot of g vs. local F-actin density.
F-actin distribution.
To further investigate the role of F-actin in regional heterogeneity of cell mechanical properties, we stained F-actin with rhodamine phalloidin and measured the regional distribution of the amount of F-actin by confocal microscopy. We projected the fluorescent intensities within each slice onto the bottom plane, summing the intensities; this represents the amount of F-actin per unit area. F-actin was concentrated around the cell corners and cell center (Fig. 4C). There was no clear relationship between local cell stiffness and local F-actin amount (Fig. 4D). This failure is not surprising because stiffness would be expected to be related to F-actin concentration rather than amount, which in turn is proportional to local cell height. We therefore divided the local F-actin amount by the local cell height to find the local F-actin density (i.e., amount of F-actin per unit volume). F-actin density was almost constant in the cell center and perinuclear regions and high in the corner and edge regions (Fig. 4E). The relationship between the local stiffness and the local F-actin density was generally monotonic, with stiffness increasing with F-actin density, but the relationship was not simple (Fig. 4F). In particular, at higher densities a roughly linear relationship was found, whereas at lower densities the stiffness appeared to vary almost independently of F-actin content. As we show below, the complexity of this relationship arises in part because there is a hidden independent variable, the regional prestress.
Traction forces.
For a square micropatterned cell on an elastic gel, cell-induced displacements of the elastic substrate were biggest at the cell corners, and, as others have found (3, 6, 45, 65), the traction forces were concentrated in these corners (Fig. 5A, inset). There was minimal traction in the cell center, and the directions of traction vectors were mostly centripetal (data not shown).
Fig. 5.
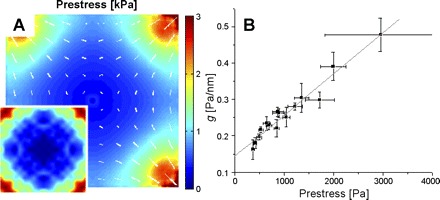
A: distribution of prestress within a square cell calculated with finite element methods (FEM). Direction and length of lines are the direction and magnitude of the prestress. These were determined from the cell height and tractions at the cell-substrate interface, the latter shown in inset (n = 13), with the magnitude of the tractions color coded. B: cross plot of local stiffness g vs. local prestress. Dotted line is a linear fit.
Prestress.
We computed the full tensor for intracellular stress as a function of x,y,z, using finite element analysis of a continuum model for a cell subjected to the tractions obtained from FTTM. At each x,y location, we computed the average over z of the maximum principal stress and defined this scalar as the local prestress. Similarly, the orientation of the prestress was taken as the average over z of the orientation of the maximum principal stress. We confirmed that the average prestress over an imaginary plane transecting the cell was equivalent, by a balance of forces, to the summation of tractions at the cell-gel interface (59). The first finding was that the prestress within a single cell was not homogeneous. The prestress was largest in the corners and decreased along the diagonal line toward cell center, where it was smallest but not zero (Fig. 5A). The direction of prestress along the diagonal was centripetal and parallel to the direction of traction. The prestress along the edge of a square cell, however, was parallel to the cell boundary and perpendicular to the direction of traction. When we compared the regional stiffness to the regional prestress, a simple and close positive relationship was revealed (Fig. 5B).
DISCUSSION
Here we report three major findings. First, the adherent HASM cell has regionally heterogeneous distributions of CSK mechanical properties; corners are more fluidlike and stiffer and have slower remodeling dynamics. Second, the regional remodeling rate is inversely correlated with the regional stiffness. Third, regional mechanical properties correlate only poorly with regional actin density but correlate strongly and linearly with the regional prestress. In this discussion we first address the experimental limitations and cytoskeletal dynamics, and then go on to address the relationship of our findings to data already in the literature. We conclude with new interpretations of these findings.
Limitations surrounding OMTC have been described in detail elsewhere (9, 18, 67). Of particular importance to our study of systematic regional variations of cytoskeletal mechanical properties, regional variations in cell height complicate the picture because bead rotation or displacement depends on both the degree of bead embedding into the cell and the proximity of the cell-glass interface (37, 42). To infer cytoskeletal elastic moduli from raw mechanical data, all experimental probes of cell mechanics require some model of cytoskeletal deformation. In general, regardless of the experimental probe, variations of model validity across different cell regions, and the biases they might engender, are unknown. In particular, as the cell height decreases (such as in the corners) any model's relationship between the elastic modulus and the bead displacement diverges, and, in consequence, it is in precisely these regions that any predictions from the model are exquisitely sensitive to small changes in cell height and bead embedding. Both of these are difficult to measure, especially the latter, and for these reasons we feel it most appropriate to focus on the interpretation of our primary measurements rather than using a model analysis of questionable utility. Nevertheless, it is true that these factors do contribute to the regional stiffness heterogeneity that we report here; their specific quantitative role remains an open question.
In contrast to prior investigations of the regional distributions of cell material properties, this report includes characterization of dissipation (friction) by two different measurements. First, from the complex elastic modulus measured with OMTC, we computed the loss tangent, given by the ratio of loss modulus to storage modulus [this is traditionally quantified by x = 1 + (2/π)tan−1 (loss tangent) (18)]. Second, we measured the rate of CSK remodeling through the MSD of spontaneous motion of the beads. In previous studies, we have found that the loss modulus is proportional to the storage modulus, implying near constancy of the loss tangent or hysteresivity, over a wide frequency range in lung parenchyma (19), smooth muscle strips (20), and even whole cells (16). Here we found that within a single cell the loss tangent is nearly constant, leading to a small, albeit systematic, variation in x (Fig. 2B). It should be noted that this variation is quite small compared with the stiffness variations (Fig. 2A). Hence, the local loss modulus is mostly determined by the local storage modulus, and where stiffness is higher, such as in the cell corners, the loss modulus is correspondingly higher. Although the fluctuation-dissipation theorem and the generalized Stokes-Einstein relationship break down in the living CSK (8, 13, 33, 67, 75), in regions where the loss modulus is the greatest there is nonetheless a general slowing down of remodeling dynamics (Fig. 3B).
Stress fibers are concentrated in the corner regions where the stiffness is highest, but also a large amount of fibers is distributed in the cell center where the stiffness is lowest (Fig. 4C). In consequence, the F-actin amount was found to have at best a poor correlation with the local stiffness (Fig. 4D). The nucleus itself is known to be quite stiff (32) and is well connected with the cytoskeletal network (35), but its role in determining local stiffness seems to be limited. Also, cytosolic actin filaments may exist in the center of the cell that are not part of adhesive structures but serve other functions and therefore do not contribute to cell stiffness. As noted in results, the more appropriate comparison would be between the local F-actin density (amount divided by height) and the local stiffness (Fig. 4F). In particular, the general trend is monotonic, and it even suggests the possibility of two distinct regimes: 1) in the center and perinuclear regions, the local stiffness varies significantly without appreciable change in F-actin density, and 2) in the corners and edges, the local stiffness changes roughly linearly with F-actin density (Fig. 4, E and F). These two different regimes confirm a role for F-actin density in setting the local cell stiffness that is complex and less than straightforward.
By contrast, for explaining cell-to-cell variations of stiffness it is well established that the single most important factor is the cytoskeletal prestress; this relationship is found to be one of simple direct proportionality (70) and holds true over a wide variety of pharmacological interventions (71) as well as in stressed reconstituted actin gels (22). We reasoned, therefore, that a similar relationship might govern interregional variations of the local prestress and the local stiffness, but interregional variations of the prestress had never before been measured. Here we were able to accomplish this by quantifying both local intracellular prestress, with cell height and traction data, and local stiffness. Our findings confirmed that the local stiffness is strongly and linearly related to the local prestress as quantified by maximum principal stress (Fig. 5B). Although regional cell stiffness increased with regional F-actin density, the regional stiffness was related more closely and more simply to the regional prestress. In that connection, myosins are distributed preferentially along cell periphery (38), but it remains unclear whether the distribution of regional prestress might be explained by local acto-myosin activity because cytoskeletal tension is transmitted over great distances (24) and is balanced through the interconnected F-actin network. While a close relationship between average cell stiffness and average cell prestress has been established previously across a population of cells (71), this result is the first to show that this relationship extends to intracellular variations.
In conclusion, our results support three major findings. First, cell corners are stiffer than the cell center. The corner of a micropatterned cell mimics the periphery of an unmicropatterned adherent cell where it builds focal adhesions, connected in turn to stress fibers bearing tensile stress and contributing to prestress. Second, remodeling dynamics are slower in the corner regions. This is consistent with the loss modulus increasing proportionately to the increasing stiffness, resulting in slower remodeling dynamics. Finally, systematic regional variations of CSK stiffness correlated only poorly with regional F-actin density but strongly and linearly with regional prestress. These findings quantify both the random and the systematic variations of intracellular mechanical properties that underlie localized cellular functions.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. G. Whitesides and B. Mayers of Harvard University for the help with micropatterning and the Center for Nanoscale Systems of Harvard University for the support of micromachining and AFM measurement. We also appreciate valuable comments and help from J. J. Fredberg’s lab members.
REFERENCES
- 1. An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? Am J Respir Cell Mol Biol 35: 55–64, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Azeloglu EU, Bhattacharya J, Costa KD. Atomic force microscope elastography reveals phenotypic differences in alveolar cell stiffness. J Appl Physiol 105: 652–661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol 3: 466–472, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Balland M, Desprat N, Icard D, Fereol S, Asnacios A, Browaeys J, Henon S, Gallet F. Power laws in microrheology experiments on living cells: comparative analysis and modeling. Phys Rev E Stat Nonlin Soft Matter Phys 74: 021911, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bausch AR, Moller W, Sackmann E. Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys J 76: 573–579, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 19: 677–695, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Brock A, Chang E, Ho CC, LeDuc P, Jiang X, Whitesides GM, Ingber DE. Geometric determinants of directional cell motility revealed using microcontact printing. Langmuir 19: 1611–1617, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Bursac P, Lenormand G, Fabry B, Oliver M, Weitz DA, Viasnoff V, Butler JP, Fredberg JJ. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater 4: 557–561, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Bursac P, Fabry B, Trepat X, Lenormand G, Butler JP, Wang N, Fredberg JJ, An SS. Cytoskeleton dynamics: fluctuations within the network. Biochem Biophys Res Commun 355: 324–330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282: C595–C605, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science 276: 1425–1428, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Deng L, Fairbank NJ, Fabry B, Smith PG, Maksym GN. Localized mechanical stress induces time-dependent actin cytoskeletal remodeling and stiffening in cultured airway smooth muscle cells. Am J Physiol Cell Physiol 287: C440–C448, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Deng L, Trepat X, Butler JP, Millet E, Morgan KG, Weitz DA, Fredberg JJ. Fast and slow dynamics of the cytoskeleton. Nat Mater 5: 636–640, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Desprat N, Richert A, Simeon J, Asnacios A. Creep function of a single living cell. Biophys J 88: 2224–2233, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duffy DC, Jackman RJ, Vaeth KM, Jensen F, Whitesides GM. Patterning electroluminescent materials with feature sizes as small as 5 um using elastomeric membranes as masks for dry lift-off. Adv Mater 11: 546–552, 1999 [Google Scholar]
- 16. Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett 87: 148102, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Fabry B, Maksym GN, Shore SA, Moore PE, Panettieri RA, Jr, Butler JP, Fredberg JJ. Selected Contribution: time course and heterogeneity of contractile responses in cultured human airway smooth muscle cells. J Appl Physiol 91: 986–994, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Taback NA, Millet EJ, Fredberg JJ. Time scale and other invariants of integrative mechanical behavior in living cells. Phys Rev E Stat Nonlin Soft Matter Phys 68: 041914, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol 67: 2408–2419, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Fredberg JJ, Jones KA, Nathan M, Raboudi S, Prakash YS, Shore SA, Butler JP, Sieck GC. Friction in airway smooth muscle: mechanism, latch, and implications in asthma. J Appl Physiol 81: 2703–2712, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Fredberg JJ, Kamm RD. Stress transmission in the lung: pathways from organ to molecule. Annu Rev Physiol 68: 507–541, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Gardel ML, Nakamura F, Hartwig JH, Crocker JC, Stossel TP, Weitz DA. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci USA 103: 1762–1767, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heidemann SR, Wirtz D. Towards a regional approach to cell mechanics. Trends Cell Biol 14: 160–166, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Hu S, Chen J, Fabry B, Numaguchi Y, Gouldstone A, Ingber DE, Fredberg JJ, Butler JP, Wang N. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am J Physiol Cell Physiol 285: C1082–C1090, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci 104: 613–627, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Ingber DE. Mechanosensation through integrins: cells act locally but think globally. Proc Natl Acad Sci USA 100: 1472–1474, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ingber DE. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol 50: 255–266, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Ji L, Loerke D, Gardel M, Danuser G. Probing intracellular force distributions by high-resolution live cell imaging and inverse dynamics. Methods Cell Biol 83: 199–235, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Kidoaki S, Matsuda T, Yoshikawa K. Relationship between apical membrane elasticity and stress fiber organization in fibroblasts analyzed by fluorescence and atomic force microscopy. Biomech Model Mechanobiol 5: 263–272, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Kidoaki S, Matsuda T. Shape-engineered vascular endothelial cells: nitric oxide production, cell elasticity, and actin cytoskeletal features. J Biomed Mater Res A 81: 728–735, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Krishnan R, Park CY, Lin Y, Mead J, Jaspers RT, Trepat X, Lenormand G, Tambe D, Smolensky AV, Knoll AH, Butler JP, Fredberg JJ. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS One 4: e5486, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuo KH, Seow CY. Contractile filament architecture and force transmission in swine airway smooth muscle. J Cell Sci 117: 1503–1511, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Lenormand G, Bursac P, Butler JP, Fredberg JJ. Out-of-equilibrium dynamics in the cytoskeleton of the living cell. Phys Rev E Stat Nonlin Soft Matter Phys 76: 041901, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Li S, Guan JL, Chien S. Biochemistry and biomechanics of cell motility. Annu Rev Biomed Eng 7: 105–150, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA 94: 849–854, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Massiera G, Van Citters KM, Biancaniello PL, Crocker JC. Mechanics of single cells: rheology, time dependence, and fluctuations. Biophys J 93: 3703–3713, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mijailovich SM, Kojic M, Zivkovic M, Fabry B, Fredberg JJ. A finite element model of cell deformation during magnetic bead twisting. J Appl Physiol 93: 1429–1436, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Murata K, Hirano K, Villa-Moruzzi E, Hartshorne DJ, Brautigan DL. Differential localization of myosin and myosin phosphatase subunits in smooth muscle cells and migrating fibroblasts. Mol Biol Cell 8: 663–673, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagayama M, Haga H, Kawabata K. Drastic change of local stiffness distribution correlating to cell migration in living fibroblasts. Cell Motil Cytoskeleton 50: 173–179, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Nagayama M, Haga H, Takahashi M, Saitoh T, Kawabata K. Contribution of cellular contractility to spatial and temporal variations in cellular stiffness. Exp Cell Res 300: 396–405, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Nishizaka T, Shi Q, Sheetz MP. Position-dependent linkages of fibronectin-integrin-cytoskeleton. Proc Natl Acad Sci USA 97: 692–697, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohayon J, Tracqui P, Fodil R, Fereol S, Laurent VM, Planus E, Isabey D. Analysis of nonlinear responses of adherent epithelial cells probed by magnetic bead twisting: a finite element model based on a homogenization approach. J Biomech Eng 126: 685–698, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Ostuni E, Kane R, Chen CS, Ingber DE, Whitesides GM. Patterning mammalian cells using elastomeric membranes. Langmuir 16: 7811–7819, 2000 [Google Scholar]
- 44. Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol Cell Physiol 256: C329–C335, 1989 [DOI] [PubMed] [Google Scholar]
- 45. Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J 16: 1195–1204, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94: 13661–13665, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Petersen NO, McConnaughey WB, Elson EL. Dependence of locally measured cellular deformability on position on the cell, temperature, and cytochalasin B. Proc Natl Acad Sci USA 79: 5327–5331, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pourati J, Maniotis A, Spiegel D, Schaffer JL, Butler JP, Fredberg JJ, Ingber DE, Stamenovic D, Wang N. Is cytoskeletal tension a major determinant of cell deformability in adherent endothelial cells? Am J Physiol Cell Physiol 274: C1283–C1289, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Puig-de-Morales M, Millet E, Fabry B, Navajas D, Wang N, Butler JP, Fredberg JJ. Cytoskeletal mechanics in adherent human airway smooth muscle cells: probe specificity and scaling of protein-protein dynamics. Am J Physiol Cell Physiol 287: C643–C654, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Rosenblatt N, Hu S, Chen J, Wang N, Stamenovic D. Distending stress of the cytoskeleton is a key determinant of cell rheological behavior. Biochem Biophys Res Commun 321: 617–622, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Rosenblatt N, Hu S, Suki B, Wang N, Stamenovic D. Contributions of the active and passive components of the cytoskeletal prestress to stiffening of airway smooth muscle cells. Ann Biomed Eng 35: 224–234, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Rotsch C, Jacobson K, Radmacher M. Dimensional and mechanical dynamics of active and stable edges in motile fibroblasts investigated by using atomic force microscopy. Proc Natl Acad Sci USA 96: 921–926, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sato M, Suzuki K, Ueki Y, Ohashi T. Microelastic mapping of living endothelial cells exposed to shear stress in relation to three-dimensional distribution of actin filaments. Acta Biomater 3: 311–319, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Shroff SG, Saner DR, Lal R. Dynamic micromechanical properties of cultured rat atrial myocytes measured by atomic force microscopy. Am J Physiol Cell Physiol 269: C286–C292, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Small J, Rottner K, Hahne P, Anderson KI. Visualising the actin cytoskeleton. Microsc Res Tech 47: 3–17, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Sollich P, Lequeux F, Hebraud P, Cates ME. Rheology of soft glassy materials. Phys Rev Lett 78: 2020–2023, 1997 [Google Scholar]
- 58. Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J 93: 4453–4461, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stamenovic D, Coughlin MF. The role of prestress and architecture of the cytoskeleton and deformability of cytoskeletal filaments in mechanics of adherent cells: a quantitative analysis. J Theor Biol 201: 63–74, 1999 [DOI] [PubMed] [Google Scholar]
- 60. Stamenovic D, Suki B, Fabry B, Wang N, Fredberg JJ. Rheology of airway smooth muscle cells is associated with cytoskeletal contractile stress. J Appl Physiol 96: 1600–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Su J, Brau RR, Jiang X, Whitesides GM, Lange MJ, So PT. Geometric confinement influences cellular mechanical properties II—intracellular variances in polarized cells. Mol Cell Biomech 4: 105–118, 2007 [PubMed] [Google Scholar]
- 62. Su J, Jiang X, Welsch R, Whitesides GM, So PT. Geometric confinement influences cellular mechanical properties I—adhesion area dependence. Mol Cell Biomech 4: 87–104, 2007 [PubMed] [Google Scholar]
- 63. Sunyer R, Trepat X, Fredberg JJ, Farre R, Navajas D. The temperature dependence of cell mechanics measured by atomic force microscopy. Phys Biol 6: 25009, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thery M, Pepin A, Dressaire E, Chen Y, Bornens M. Cell distribution of stress fibres in response to the geometry of the adhesive environment. Cell Motil Cytoskeleton 63: 341–355, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Tolic-Norrelykke IM, Butler JP, Chen J, Wang N. Spatial and temporal traction response in human airway smooth muscle cells. Am J Physiol Cell Physiol 283: C1254–C1266, 2002 [DOI] [PubMed] [Google Scholar]
- 66. Tolic-Norrelykke IM, Wang N. Traction in smooth muscle cells varies with cell spreading. J Biomech 38: 1405–1412, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal physical responses to stretch in the living cell. Nature 447: 592–595, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tseng Y, Kole TP, Wirtz D. Micromechanical mapping of live cells by multiple-particle-tracking microrheology. Biophys J 83: 3162–3176, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Van Citters KM, Hoffman BD, Massiera G, Crocker JC. The role of F-actin and myosin in epithelial cell rheology. Biophys J 91: 3946–3956, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang N, Naruse K, Stamenovic D, Fredberg JJ, Mijailovich SM, Tolic-Norrelykke IM, Polte T, Mannix R, Ingber DE. Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci USA 98: 7765–7770, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol 282: C606–C616, 2002 [DOI] [PubMed] [Google Scholar]
- 72. Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci 96: 379–386, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yanai M, Butler JP, Suzuki T, Sasaki H, Higuchi H. Regional rheological differences in locomoting neutrophils. Am J Physiol Cell Physiol 287: C603–C611, 2004 [DOI] [PubMed] [Google Scholar]
- 74. Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60: 24–34, 2005 [DOI] [PubMed] [Google Scholar]
- 75. Zhou EH, Trepat X, Park CY, Lenormand G, Oliver MN, Mijailovich SM, Hardin C, Weitz DA, Butler JP, Fredberg JJ. Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition. Proc Natl Acad Sci USA 106: 10632–10637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou EH, Lim CT, Quek ST. Power-law rheology analysis of cells undergoing micropipette aspiration. Biomech Model Mechanobiol (February 2010). doi:10.1007/s10237-010-0197-7 [DOI] [PubMed] [Google Scholar]


