Abstract
Aggregation of α-synuclein plays a crucial role in the pathogenesis of synucleinopathies, a group of neurodegenerative diseases including Parkinson disease (PD), dementia with Lewy bodies (DLB), diffuse Lewy body disease (DLBD) and multiple system atrophy (MSA). The common feature of these diseases is a pathological deposition of protein aggregates, known as Lewy bodies (LBs) in the central nervous system. The major component of these aggregates is α-synuclein, a natively unfolded protein, which may undergo dramatic structural changes resulting in the formation of β-sheet rich assemblies. In vitro studies have shown that recombinant α-synuclein protein may polymerize into amyloidogenic fibrils resembling those found in LBs. These aggregates may be uptaken and propagated between cells in a prion-like manner. Here we present the mechanisms and kinetics of α-synuclein aggregation in vitro, as well as crucial factors affecting this process. We also describe how PD-linked α-synuclein mutations and some exogenous factors modulate in vitro aggregation. Furthermore, we present a current knowledge on the mechanisms by which extracellular aggregates may be internalized and propagated between cells, as well as the mechanisms of their toxicity.
Keywords: alpha-synuclein, aggregation, Parkinson disease, prion-like features
Introduction
Human α-synuclein is a natively unfolded 140-amino-acid residue protein widely expressed in neurons, found predominantly in presynaptic terminals.1-3 It was originally identified at the nuclear level, accounting for the designation “syn-nuclein”,4 but subsequently described as a predominantly cytoplasmic protein.5-7 While an ultimate definition of α-synuclein function has not been provided yet, different studies have shown its role in mediating dopamine synthesis, release and reuptake in dopaminergic neurons, as strongly suggested by the protein presynaptic location and its interaction with membranes.8-13 Additional studies reported α-synuclein upregulation in response to oxidative stress and excitotoxicity, suggesting a role for the protein in protecting neuronal cells against damage.14 In particular, α-synuclein may cooperate with the cysteine-string protein-α (CSPα)—a synaptic vesicle protein with co-chaperone activity—in preventing neurodegeneration.15
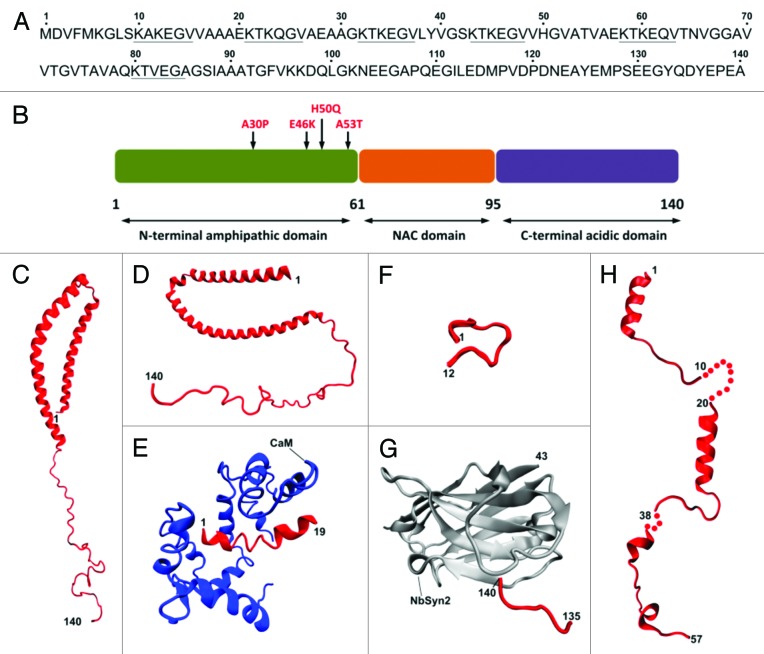
Structurally, α-synuclein features an N-terminal domain (residues 1–60), a central hydrophobic portion denoted as non-Aβ component of Alzheimer diseases amyloid (NAC, residues 61–95) and a C-terminal negatively charged region (residues 96–140). Both the N-terminal and NAC domains contain six highly conserved hexameric (KTKEGV) motifs (Fig. 1A and B).16 In its native monomeric state, α-synuclein behaves as an intrinsically unstructured protein, characterized by the lack of a stable tertiary structure.17,18 However, it may adopt a secondary structure upon binding to protein interactors or lipid membranes. Early studies reported that the N-terminal domain of α-synuclein displays α-helical folding in the presence of artificial membranes such as sodium dodecyl sulfate micelles.19-22 Solution state NMR studies on the structures of micelle-bound α-synuclein have shown that the protein contains two distinct α-helices in the region encompassing residues 1–92, while the C-terminal results predominantly unstructured (Fig. 1C and D).23,24 Other studies have attempted to obtain structural information about α-synuclein segments complexed with physiological partners such as calmodulin25 or synphilin-1,26 with a nanobody27 or the maltose binding protein28 (Fig. 1E–H). A recent work has reported that natively cell-extracted α-synuclein may be present as a tetramer formed by α-helical folded monomers with no binding to lipid membranes,29 but a further work has shown that α-synuclein in mammalian cells exists as unfolded monomer when dissociated from lipid membranes.30
Figure 1. Structural features of monomeric α-synuclein. Primary amino acidic sequence (UniProtKB: P37840) of human α-synuclein with the imperfect exapeptide repeats16 underlined (A) and schematic representation of the biochemically different domains including the currently identified PD-related mutations (B). Currently available NMR and X-ray crystal structures of α-synuclein segments (in red) bound to micelles (C and D) PDB: 1XQ8 and 2KKW, respectively, calmodulin, CaM (E) PDB: 2M55, synphylin-1 (F) PDB: 2JN5, nanobody, NbSyn2 (G) PDB: 2X6M and maltose binding protein (H) PDB: 3Q25, 2Q26, 3Q27.
Overall the structural studies on the α-synuclein monomer suggest a model where the lack of secondary structure motifs is the first event leading to its misfolding and aggregation in pathological and disease-related amyloid assemblies found in Parkinson disease (PD) and other synucleinopathies.
In the next sections, we present an overview of the strategies currently used to produce synthetic α-synuclein assemblies and their possible implications for the future of PD research.
In Vitro Aggregation Assays of α-Synuclein
Deposition of Lewy bodies (LBs) and Lewy neurites (LNs) in the brain is a major pathological hallmark of several neurodegenerative diseases, including PD, dementia with Lewy bodies (DLB), diffuse Lewy body disease (DLBD), and multiple system atrophy (MSA). LBs are spherical cytoplasmic inclusions, 5–25 µm in diameter, formed by aggregated proteins, with a dense eosinophilic core and a clearer surrounding halo. The major component of the proteinaceous filaments of LBs and LNs is α-synuclein.31
In vitro studies have revealed that recombinant α-synuclein can assemble into fibrils with morphologies and staining characteristics similar to those extracted from disease-affected brains. Wild-type (WT) α-synuclein fibrils formed in vitro are 8–10 nm in height as determined by atomic force microscopy (AFM) and about 10 nm in width (electron microscopy, EM), while fibrils of PD LBs observed in tissue slices are reported to be about 10 nm in width.32-34 Moreover, the presence of “twisted” fibrils as well as some small filaments of 5 nm width is a common feature of fibrils extracted from brains and some fibrils formed in vitro.31,32
Aggregation of α-synuclein plays a critical role in PD and related neurodegenerative disorders; hence in vitro aggregation assays are powerful molecular tools for studying the mechanisms of neurodegeneration. Understanding the mechanisms by which a soluble α-synuclein is transformed into insoluble β-rich fibrils, as well as describing the structural features of these fibrils are still questions to be addressed in future research. Additionally, α-synuclein aggregation assays may be feasible tools for the development of new drug compounds capable of interfering with α-synuclein fibril formation. Increasing evidence demonstrates that in vitro formed fibrils are internalized by cells and may be propagated in a prion-like manner leading to cytotoxicity. Thus, in vitro aggregation of α-synuclein may yield crucial components for studying molecular mechanisms of aggregate uptake, spreading and toxicity.
Strategies for the purification of recombinant α-synuclein
In vitro fibrillization assays require high amounts of highly pure α-synuclein protein. Recombinant α-synuclein is usually produced in E. coli cells and purified from either whole cell extract or periplasmic extract. Traditionally, the protein is purified from the whole cell extract by sonication or boiling, or ammonium sulfate precipitation followed by anion exchange and size exclusion chromatography purifications.35-37 Ren and collaborators have shown that E.coli-expressed α-synuclein without signal sequence mostly localizes in the bacterial periplasm.38 The protein translocation appeared to be mediated by the signal recognition particle (SRP)-dependent pathway facilitated by the α-synuclein C-terminal domain (residues 90–140) as a major part responsible for the translocation.38 Therefore, a new efficient and fast method has been developed for α-synuclein extraction and purification from periplasm.39 Briefly, the protein is first extracted from bacterial periplasm by osmotic shock, followed by anion-exchange chromatography purification. One L of E.coli LB medium culture yields about 80 mg of highly pure α-synuclein.39
Tools for monitoring α-synuclein fibril formation in vitro
In vitro fibrillization assays require powerful tools for detecting fibril formation and monitoring the fibrillization kinetics in real time. Two common histological dyes, Thioflavin T (ThT) and Congo red (CR), are used to this end. The mechanism of interaction between both dyes and amyloid fibrils remains poorly understood; both compounds seem nevertheless to interact with the cross-β structures, exhibiting changes in fluorescence. ThT is a benzothiazole dye that exhibits enhanced fluorescence upon binding to amyloid fibrils and constitutes the mostly used extrinsic probe for monitoring fibrillization kinetics in vitro. In the presence of fibrils, ThT gives rise to an excitation maximum of 450 nm and enhanced emission at 482 nm, whereas unbound ThT is not fluorescent at these wavelengths.40 ThT is a reliable and specific fibril-detecting tool, which does not interfere with the kinetics of α-synuclein fibrillization.41 However, despite its specificity toward fibrils, a slight increase in ThT fluorescence has been detected after binding to amorphous α-synuclein aggregates.42 The use of ThT for monitoring fibril formation should be optimized and negative controls are needed, as spectral properties of this dye may vary under different conditions. Additionally, being ThT a charged and hydrophobic molecule, it may non-specifically bind to some proteins by electrostatic and hydrophobic interactions.43
CR seems unsuitable for monitoring α-synuclein aggregation, since it exhibits high affinity binding to α-synuclein and has an inhibitory effect on the fibrillization assay.44 Some studies have indicated that CR may interfere with protein misfolding and aggregation by stabilizing native protein monomers or partially folded intermediates.45
Since the sensitivity and specificity of ThT and CR have been questioned, several efforts have been made to design extrinsic probes and spectroscopic techniques for monitoring the fibrillization process. Celej and coworkers reported that N-arylaminonaphthalenesulfonate (NAS) and its derivatives act as non-covalent, highly sensitive probes of α-synuclein fibril formation.46 Recently, it has been shown that protein aggregation reactions can be spectroscopically monitored without the need for extrinsic labels. Some studies have reported that amyloid-like structure is associated with an intrinsic fluorescent phenomenon that occurs in the visible range.47-49 The physical mechanism underlying the intrinsic fluorescence emission remains unknown. It has been suggested that the hydrogen-bonded water molecules within the cross-β structure might play a key role in the fluorescence emission of the fibrils. Direct excitation of the fibrils could induce electronic transitions in peptides because of a partial delocalization of peptide electrons elicited by the presence of hydrogen bonds.47 Pinotsi and collaborators have shown that this phenomenon can be used for monitoring the aggregation of proteins with no need for extrinsic labels, thus avoiding potential interference with the aggregation process.50 This method appears feasible and reproducible; it requires a confocal microscope and it has been successfully used for monitoring α-synuclein fibrillization in vitro.50
Mechanism and kinetics of fibril formation in vitro
During the fibrillization process, a soluble protein is converted into insoluble highly ordered aggregates exhibiting β-sheet enriched structures. Fibrillization of globular proteins requires the presence of partially unfolded conformations in order to adopt a structure competent for self-assembly into fibrils.51 Partial unfolding exposes the hydrophobic regions of the protein, thus facilitating the interactions between exposed amyloidogenic regions of two proteins and subsequent formation of higher order species. Several factors destabilizing the native fold of the protein, such as mutations, denaturants, changes in pH, or elevated temperature, have been shown to enhance fibril formation.51
Fibrillization of natively unfolded proteins such as α-synuclein presents intriguing issues, since it remains unclear how an intrinsically unstructured protein may form highly organized β-structured fibrils. Partial folding of the protein seems to represent a crucial step in the fibrillization pathway.51 Natively unfolded proteins are characterized by low hydrophobicity and a large net charge.52 It is supposed that any factors increasing protein hydrophobicity or decreasing protein net charge may lead to its partial folding and thus promote the fibrillogenic pathway. The overall hydrophobicity of a protein increases with higher temperature.53 It has been reported that either a decrease in pH or an increase in temperature leads α-synuclein to adopt a partially folded conformation, a change correlated with the enhanced formation of fibrils.41
The model for α-synuclein fibrillization has been proposed as follows:
| U ↔ I ↔ nucleus → F, |
where U corresponds to natively unfolded conformation, I: partially folded intermediate, nucleus: fibril nucleus, and F: fibril. This model describes the two key kinetic steps in the fibrillization process. The first step is the conformational transformation of α-synuclein into the aggregation-competent, partially folded intermediate; the second is the formation of the assembly-competent oligomers (nuclei), which is followed by oligomer growth and fibril formation. The model suggests that any factor that promotes intermediate formation (for example point mutations, non-polar molecules, cations, and others) will also promote α-synuclein fibrillization.41
The kinetics of α-synuclein fibrillization is commonly described as a sigmoidal curve, composed of an initial lag phase (nucleation) when the amount of fibrils is too low to be detected, a subsequent exponential phase (elongation) when fibril concentration increases rapidly, and a final plateau phase indicating the end of fibril formation. The length of lag phase and the fibril growth rates depend on several factors, including initial protein concentration, pH, temperature, agitation and other factors that will be discussed below.
Moreover, the fibrillization process can be catalyzed by adding preformed fibrils (seeds), which act as nuclei.54-56 This effect has been termed “‘nucleation-dependent’ fibrillization.” The added seeds may act as catalytic sites that induce conformational changes in α-synuclein and accelerate the reaction rates.54
To compare the effects of different reaction conditions on fibrillization kinetics, ThT fluorescence is commonly plotted as a function of time and fitted to a sigmoidal curve using the equation:
where Y is the fluorescence intensity, x is the time, and x0 is the time to 50% of maximal fluorescence. Thus, the apparent rate constant, kapp, for fibril growth is given by 1/τ and the lag time is given by x0–2τ.57
To test the effects of different factors on fibrillization kinetics, usually the lag phase, the apparent rate constant and/or the maximum intensity of ThT emission intensity at the end of fibril formation are calculated. These values obtained from tested samples are compared using commonly available statistic tests.58 The fibrillization kinetics has been shown to be fairly similar among different amyloidogenic proteins. However, the kinetic profiles do not display always the typical sigmoidal shape since multiple steps may be present, thus affecting the interpretation of the correct lag phase and the apparent rate constant. Optimizing the reproducibility of protein fibrillization is crucial to obtain reliable results.58
Various data confirmed that in vitro aggregation of α-synuclein may yield different final products, such as fibrils, soluble oligomers, or insoluble “amorphous aggregates.” Aggregation assays resulted in the formation of several oligomeric species with different morphologies, including spherical, chain-like, and annular oligomers.59 However, the mechanism of conversion among different oligomers is not fully understood.
The generation of different α-synuclein conformations has also been associated with the pathogenesis of PD and other synucleinopathies; fibrillar forms seem nevertheless to be the main component of LBs.31
Structural features of human α-synuclein fibrils
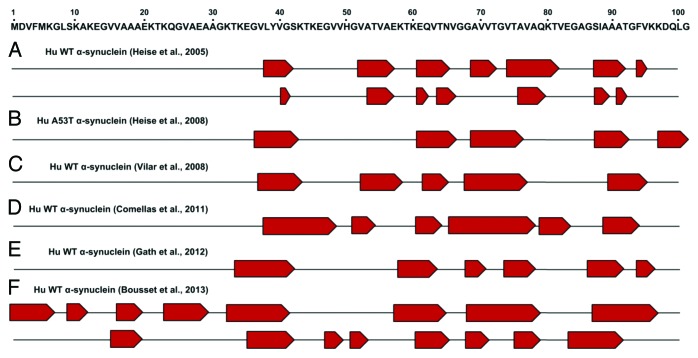
The assembly of physiologically expressed proteins into oligomeric and fibrillar aggregates with cross-β structure is an essential event of different human neurodegenerative diseases, such as PD, Alzheimer disease, and prion diseases. It is generally accepted that aggregation takes place from partially structured states of proteins. However, the role of the residual structures present in such conformational states is not well understood. α-synuclein fibrillar aggregates are pathological hallmarks of PD and other related synucleinopathies. The structural organization of α-synuclein amyloid fibrils has been researched extensively. Amyloid fibrils are composed of several protofilaments arranged into the classical cross-β conformation, with individual β-strands running perpendicular to the fiber axis.34,60,61 Findings from electron paramagnetic resonance (EPR)62,63 and magic-angle spinning solid-state NMR (ssNMR) have shown that the amyloidogenic β-sheet core is mostly located within the central NAC domain.64-71 Figure 2 illustrates an updated view of the β-sheet organization into the α-synuclein amyloid fibrils as determined by ssNMR. Although the fibril core spans approximately residues 38 to 100, structural variations of local β-strands have been reported inside this region. A recent report has expanded current knowledge of the α-synuclein segments involved in amyloid structural reorganization. In particular, Bousset and collaborators identified two novel α-synuclein assemblies characterized by ordered β-sheet organization starting from residue 1 to 38; this region appeared unstructured in all previous ssNMR studies.65 Interestingly, the same group has reported that the two α-synuclein polymorphs showed marked differences in their biological functions. Overall the EPR and ssNMR studies highlight the remarkable conformational plasticity of α-synuclein, which can adopt different monomeric, oligomeric, or fibrillar assemblies depending on the environment.
Figure 2. α-synuclein fibril core regions as determined by ssNMR studies. Upper panel: schematic representation of the α-synuclein segment from residue 1 to 100 involved in structural changes. Lower panel: proposed β-strand (indicated as red pentagons) organization of different human (Hu) α-synuclein fibrillar assemblies determined by different groups: (A) reference 68 (B) reference 67 (C) reference 69 (D) reference 70 (E) reference 71 (F) reference 65.
Factors affecting the kinetics of α-synuclein fibrillization in vitro
Oligomers and fibrils produced in vitro under diverse conditions may result in different structural properties and consequently exert different biological functions.72 As mentioned above, several factors including the initial concentration of monomeric protein, molecular crowding, agitation, pH, temperature, and ionic strength, have been shown to greatly affect the kinetics of α-synuclein fibrillization.32,41,73-75 Thus, developing a reliable and reproducible aggregation assay is necessary to screen the effects of exogenous factors on the fibrillization process, as well as to produce different aggregate species for structural studies and test their biological functions. Here, we characterize crucial factors that determine the kinetics of fibril formation in vitro.
α-synuclein concentration
Initial concentration of monomeric α-synuclein is a crucial factor determining the kinetics of fibril formation. Commonly, 0.5 to 1 mg/mL (35–70 µM) of α-synuclein is used during fibrillization assays.39,74,76,77 Other studies have used higher concentrations, namely 5 to 7 mg/mL.78,79 The minimal α-synuclein concentration, below which fibrils are not formed, has been estimated to be approximately 0.2 mg/mL.80 The kinetics of fibril formation is a concentration-dependent process; however, the concentration dependence almost disappears in the presence of higher protein concentrations. Above the supercritical concentration, nucleation is no longer rate-limiting and the fibril growth rate is almost independent of the protein concentration.81 In fact, increasing α-synuclein concentration has been reported to decrease the lag-phase of fibrillization.82 Supercritical concentration of α-synuclein has been estimated to be about 8.2 mg/ml (~ 574 µM); however this may vary according to the conditions used for the fibrillization reaction.42
Molecular crowding
Macromolecular crowding is another factor significantly affecting aggregation, as it may dramatically accelerate α-synuclein fibril formation.74,83 The effect of molecular crowding on protein aggregation in vitro has been examined by using concentrated solutions of a model “crowding agent” such as polyethylene glycols (PEG), polysaccharides (dextrans or Ficolls), or proteins (for example, lysozyme and bovine serum albumin). All these molecules accelerated α-synuclein fibrillization in vitro.83 The crowding agents had little effect on the conformation of α-synuclein, as shown by far-UV CD spectroscopy.74 It seems that the mechanism by which crowding agents affect fibrillization depends on their concentrations. It has been suggested that at lower crowding agent concentrations, the major effect of macromolecular crowding on protein aggregation is due to excluded volume effect, which favors self-association of α-synuclein following the increase of the effective protein concentration. At a higher concentration, viscosity effects of the polymer solutions may become dominant.74
Agitation
As reported by several studies, agitation is yet another crucial factor impacting the kinetics of the fibrillization process. Agitation of α-synuclein solutions leads to a dramatic increase in the rate of fibrillization, shortening the lag phase from weeks to hours.32,84 Uversky and collaborators have reported that vigorous agitation was the major determinant of the fibrillization rate. Very vigorous agitation attenuated the effects of ionic strength, protein concentration and various additives on the rate of fibril formation.85 The exact mechanism by which agitation induces fibrillization is unknown, however it could increase the air-water interface, thus leading to a partial folding of α-synuclein and the formation of the fibrillization intermediate.82 Moreover, agitation may increase the number of fibril ends through fragmentation or increase the collision of monomeric and oligomeric species interacting with fibril ends.86
Agitation is commonly performed either in a glass vial with a mini stir bar or in microplates on a fluorescence plate reader. With the former method, termed single time-point dilution ThT assay,39,41,75,87,88 aliquots are taken out at given time intervals for ThT fluorescence measurement, whereas in the latter approach (in situ real-time assay) ThT fluorescence is monitored in real time at regular intervals.58,73,74 Agitation speed in different assays ranges from 120 rpm89 to 1000 rpm.78 Agitation with glass beads has been shown to significantly decrease the lag time of the fibrillization and to increase the reproducibility of the assay.58
Reproducibility is an important issue in in vitro fibrillization assays, as it may vary significantly across sample replicates and among experiments.58 Giehm and Otzen have tested factors that influence the reproducibility of the fibrillization of α-synuclein in plate reader assays. The authors have determined that increased reproducibility is correlated with decreased lag time. Orbital agitation using a small glass bead in each well significantly increased lag time and was crucial to obtain good reproducibility.58
Temperature, pH and ionic strength
Several data have indicated that decreasing the pH or increasing the temperature substantially accelerated the kinetics of α-synuclein fibrillization, affecting both the lag time and the elongation rate. Elevated temperatures have been shown to strengthen hydrophobic interactions whereas lower pH decreased the net charge of the protein.41,53 Both factors induced some structural changes in α-synuclein, as shown by far-UV CD and FTIR spectroscopies, and consequently contributed to a reversible transformation of α-synuclein into a partially folded intermediate, whose formation is crucial for the fibrillization process.41,75
The rate of fibril formation has been shown to increase dramatically with increasing ionic strength, reaching the maximal effect by 300 mM NaCl. Anions have been suggested to induce partial folding of α-synuclein at neutral pH, thus increasing the population of the amyloidogenic intermediate. CD spectra confirmed that at 20 mM concentration, different salts induced partial folding of α-synuclein.73
Exogenous factors modulating α-synuclein fibrillization in vitro
Although the specific etiology of PD is unknown, several factors, both genetic and environmental, may increase the risk of developing the disease.90 In vitro aggregation assays are easy, fast, and powerful tools for screening potential factors that may affect α-synuclein aggregation and thus contribute to PD pathogenesis. Several studies have reported that α-synuclein aggregation may be triggered by environmental and other exogenous factors, such as pesticides, heavy metals, polycations, organic solvents, and some small chemical compounds.91,92 Here, we briefly characterize factors that have been shown either to accelerate or inhibit α-synuclein aggregation in vitro. Table 1 summarizes exogenous factors that have been involved in the modulation of α-synuclein aggregation.
Table 1. Exogenous factors affecting α-synuclein fibril formation in vitro.
| Factor | Description | References |
|---|---|---|
| Factors accelerating fibrillization | ||
| Agrochemicals | Pesticides (rotenone, dieldrin, paraquat) bound to the partially folded intermediate conformation of α-synuclein and induced its conformational changes to β-sheet enriched structures. | 85,89 |
| Polycations | α-synuclein bound to different polycations (spermine, polylysine, polyarginine, polyethyleneimine) forming stable complexes. Complex formation did not cause significant changes in α-synuclein secondary structures, as shown by far-UV CD spectroscopy. | 77 |
| Histones | Bovine histone H1 and bovine core histones (a mixture of H2a, H2b, H3 and H4) accelerated α-synuclein fibrillization in a dose-dependent manner. The formation of α-synuclein-histone complexes was not accompanied by significant changes in either secondary structure (CD) or globular structure (SAX). | 103 |
| Metal ions | Several divalent and trivalent ions (copper, iron, cobalt and manganese) accelerated α-synuclein fibrillization. Metal ions induced a conformational change of α-synuclein and led to the formation of a partially folded intermediate. | 76 |
| Glycosaminoglycans (GAGs) | Some glycosaminoglycans (heparin, heparin sulfate, agrin) and other highly sulfated polymers (dextran sulfate) significantly accelerated the formation of α-synuclein fibrils in vitro. Binding to α-synuclein induced conformational changes of the protein. | 87,157 |
| Sodium dodecyl sulfate | SDS stimulated α-synuclein fibrillization in a highly reproducible manner. SDS-induced fibrils did not exhibit the classical rod-like structure. Fibrillization in the presence of SDS seems to present an alternative fibrillization pathway. | 158 |
| Organic solvents | Organic solvents affected fibrillization kinetics in a concentration-dependent fashion. Low concentrations of alcohols accelerated the rate of fibrillization, whereas at high concentrations the effect was dependent on the type of alcohol—simple alcohols induced β-helix-rich conformation, fluorinated alcohols promoted α-helix-rich species. All solvents induced folding of α-synuclein, as shown by far-UV CD and FTIR spectra. | 73,111 |
| Factors inhibiting fibrillization | ||
|---|---|---|
| Small chemical compounds | Different chemical classes of compounds have been shown to inhibit α-synuclein fibrillization in vitro (polyphenols, benzothiazoles, phenothiazoles, steroids, Congo red and its derivatives, flavonoids). Some of them were able to disaggregate the preformed fibrils. | 44,104 |
| Heat shock proteins (Hsp) | Small Hsp proteins (αB-crystallin, HspB8, Hsp27, HspB2B3) inhibited α-synuclein fibrillization. The formation of aggregates suggested that Hsp proteins may redirect α-synuclein from a fibril-formation pathway toward an amorphous aggregation pathway. Hsp70 inhibited α-synuclein filament formation by binding to soluble prefibrillar intermediates. |
105-108 |
| PAMAM dendrimers | PAMAM dendrimers (generations G3, G4, and G5) inhibited fibrillization of α-synuclein and promoted the breakdown of pre-existing fibrils. They induced structural changes in α-synuclein and redirected the protein to an amorphous aggregation pathway. | 109 |
| β- and γ-synucleins | β- and γ-synucleins increased the lag time and dramatically reduced the elongation rate of α-synuclein. β- and γ-synucleins could be incorporated into oligomeric intermediates leading to their stabilization. | 159 |
| Catecholamines | Several catecholamines, including L-DOPA and dopamine, were able to inhibit α-synuclein fibrillization and to dissolve the preformed fibrils in vitro. | 160 |
| Phospholipids | Acidic phospholipids induced α-helical structure in α-synuclein, thus preventing the formation of fibrils. | 161 |
| Rifampicin | Rifampicin inhibited α-synuclein fibrillization and disaggregated existing fibrils by stabilizing monomeric and soluble oligomeric forms. | 162 |
| Trehalose | Trehalose, a disaccharide, inhibited α-synuclein fibrillization and stimulated the formation of large amorphous aggregates. | 163 |
| Oxidative modifications | Oxidation of α-synuclein methionines, N-terminal acetylation, and Tyr-nitration have been reported as protective factors against fibrillization. | 164-166 |
Pesticides and herbicides
Uversky and collaborators have reported that certain pesticides and herbicides (rotenone, dieldrin, and paraquat) dramatically accelerated the rate of fibril formation in vitro.85 It has been suggested that the relatively hydrophobic pesticides may bind to the partially folded α-synuclein intermediate, induce its conformational changes and consequently lead to fibril formation.85 In fact, epidemiological studies have shown increased risk of PD in populations exposed to pesticides.93,94
Polycations
Polycations, such as spermine, polylysine, polyarginine, and polyethylenimine, dramatically accelerated α-synuclein fibrillization in vitro. α-synuclein-polycation complex formation was not accompanied by significant structural changes in α-synuclein.77 Electrostatic interactions between polycations and α-synuclein have been suggested to lead to neutralization of the protein net negative charge and to the partially folded amyloidogenic intermediate that initiates fibril formation.77
Metal ions
Several works have described how some metal ions affected α-synuclein fibrillization in vitro. Di- and trivalent ions (e.g., copper, iron, cobalt, and manganese) accelerated the rate of fibril formation. Metal ions stimulated α-synuclein conformational change and led to the development of a partially folded intermediate, a possible critical precursor of fibrils.76,95 Metal exposure has been suggested as one of the factors that may increase the risk of PD. Several epidemiological studies have shown that exposure to some metals, including manganese, iron, aluminum, and copper, was associated with a higher risk of PD.96-99 Moreover, elevated levels of iron, zinc, and aluminum have been reported in the substantia nigra of PD patients, thus confirming their involvement in the pathogenesis.100-102
Histones
Some histones have been shown to form a tight complex with α-synuclein and accelerate its fibrillization in a dose-dependent manner.103 This effect may be physiologically important, as the translocation of α-synuclein into the nucleus and its binding to histones may play a large role in α-synuclein pathology.
Small chemical compounds
Many therapeutic strategies for PD aim at developing chemical compounds and small molecules capable of inhibiting fibril formation. Masuda and collaborators have tested 79 small compounds belonging to different chemical classes (including polyphenols, benzothiazoles, phenothiazoles, steroids, Congo red and its derivatives) for their ability to inhibit the assembly of α-synuclein into filaments in vitro.44 Several of these compounds inhibited α-synuclein assembly; among them, polyphenols proved to be an especially effective class of anti-aggregation compounds. The mechanism underlying the polyphenol-induced inhibition is unclear, but these compounds seem to stabilize soluble, prefibrillar intermediates.44 Meng and coworkers have tested 48 compounds representative of different classes of flavonoids for their ability to inhibit α-synuclein fibril formation in vitro. A variety of flavonoids inhibited α-synuclein in vitro fibrillization to varying extents: some of them (myricetin, tricetin, 6-HP and baicalein) completely inhibited fibril formation and many were able to disaggregate preformed fibrils.104
Heat shock proteins
Heat shock proteins (Hsp) have been reported to interact with α-synuclein and inhibit its aggregation in vitro.105-108 The small Hsp αB-crystallin, a molecular chaperone, inhibited α-synuclein fibrillization. The interaction of α-synuclein with αB-crystallin resulted in large irregular aggregates, suggesting that the chaperone protein redirected α-synuclein from a fibril-formation pathway toward an amorphous aggregation pathway.105 Hsp70 protein inhibited α-synuclein filament formation by binding to soluble prefibrillar intermediates.106,107 Moreover, different Hsp proteins (HspB8, Hsp27, HspB2B3, and αB-crystallin) inhibited the α-synuclein aggregation process, as shown by ThT assay and AFM imaging.108
PAMAM dendrimers
Another class of compounds exerting anti-aggregation effects includes polyamidoamine (PAMAM) dendrimers—highly branched polymers possessing a well-defined spherical geometry.109 PAMAM dendrimers (generations G3, G4, and G5) inhibit fibrillization of α-synuclein and promote the breakdown of pre-existing fibrils. They have been shown to induce structural changes in α-synuclein and redirect the protein to an amorphous aggregation pathway.
Organic solvents
Some factors affecting α-synuclein fibril formation have been reported to inhibit or accelerate the fibrillization depending on their concentration or reaction conditions. Munishkina and collaborators have tested the effect of different organic solvents on α-synuclein fibrillization kinetics.73 Far-UV CD, FTIR and MS spectra confirmed that they induced folding of α-synuclein.73,95 The concentration and composition of different alcohols (ethanol, methanol, and propanol) differently affected the fibrillization rate of α-synuclein.73 Notably, recent studies have established that overexpression of α-synuclein and the consequent higher risk for developing PD is modulated by chronic long-term alcohol exposure.110,111
Effect of PD-related mutations on α-synuclein fibrillization in vitro
Three autosomal dominant missense point mutations in the SNCA gene have been identified in early-onset PD: A53T, A30P, and E46K.112-114 Recently, two independent groups have discovered the novel pathogenic mutation H50Q.115,116 Several works have shown that PD-linked mutations A53T, A46K, and H40Q promoted α-synuclein aggregation in vitro, yet neither of them affected the overall structure of the α-synuclein monomer.32,75,115-120
Mutations A53T and E46K have been shown to significantly accelerate fibrillization in vitro when compared with WT protein.117 Although the A53T and E46K mutated α-synuclein fibrils exhibited distinct morphologies, the rate of amyloid formation of both mutants was similar.121,122 A53T fibrils were about 5–14 nm in width and appeared twisted, with a crossover spacing of about 100 nm; E46K fibrils had often a twisted appearance too, with width varying between about 5 and 14 nm, but with shorter crossover spacing of about 43 nm.121 Recently, Lemkau and collaborators have found by ssNMR that both mutated fibrils possessed site-specific perturbations in their secondary structures, which could alter the overall structural arrangement of the fibril.123
Similarly to WT α-synuclein protein, both mutants generated also prefibrillar intermediates with pore-like activity.60,118,124
The effect of the A30P mutation on fibrillization is not clear, as some data reported that it formed fibrils slightly faster than WT protein,37 whereas others showed that this mutation decreased fibril formation.60,125 Conway and collaborators have observed that A30P had a slower propensity to form fibrils than WT, and it preferentially adopted a soluble, protofibrillar conformation, whereas the WT protein readily progressed to mature fibrils.60 In spite of that, some data have shown that A30P fibril morphologies were similar to those formed from WT protein.34,121 Both WT and A30P fibrils had a filament width of 6–9 nm, which varied slightly along the length of the filaments.121
The recently identified H50Q mutant has been shown to aggregate more readily than WT protein, but more slowly than A53T and E46K.120 AFM studies have shown that, similarly to WT protein, the H50Q protein oligomerized through small oligomers, from protofibrils to mature fibrils.120
Current Knowledge on Internalization and Propagation of α-Synuclein Aggregates
Increasing evidence argues that α-synuclein may transfer from cell to cell, suggesting that prion-like propagation may mediate the pathological progression of PD. Braak and colleagues have proposed that α-synuclein pathology begins in the lower brainstem and olfactory bulb and spreads into the limbic system and eventually to the cortex.126 Two independent studies have revealed host-to-graft propagation of α-synuclein-positive Lewy-like pathology, providing further evidence for prion-like spreading of the pathology.127,128 Embryonic mesencephalic neurons grafted into the neostriatum of PD patients have been shown to accumulate LB pathology. Moreover, α-synuclein was transmitted via endocytosis to neighboring neurons, forming Lewy-like inclusions. α-synuclein was also transmitted from the affected neurons to engrafted neuronal precursor cells in a transgenic (Tg) model of PD-like pathology.129
Several recent studies investigated whether α-synuclein aggregates formed in vitro from recombinant protein may be internalized by cells and eventually recruit endogenous α-synuclein to form LB-like pathology. Increasing evidence suggests that misfolded α-synuclein aggregates, both fibrils and oligomers, may be uptaken and propagated among cells in a prion-like manner and induce pathology in healthy cells.78,79,129,130
Luk and collaborators have shown that addition of in vitro preformed α-synuclein fibrils into culture medium induces intracellular α-synuclein aggregation in different cell lines overexpressing this protein.78 The fibrils were shown to rapidly recruit endogenous soluble protein converting this into detergent-insoluble inclusions, while monomeric and oligomeric α-synuclein showed only a negligible effect. Moreover, α-synuclein inclusions undergo several modifications characteristic for LBs, such as hyperphosphorylation, ubiquitination and accumulation of cytoplasmic vesicles around the periphery of the inclusions.78 Morphological and biochemical similarities between intracellular inclusions and LBs suggest that fibrillar seeds may have a central role in the initial formation of LBs and other disease-associated inclusions. Luk et al. also tested whether exogenous aggregates could induce aggregation of endogenous α-synuclein expressed by primary cells not overexpressing the protein.131 In fact, preformed fibrils entered primary hippocampal neurons and promoted recruitment of soluble α-synuclein into insoluble LB-like aggregates. Consistent with previous results, endogenous aggregates were hyperphosphorylated and ubiquitinated, confirming their LB-like properties. Moreover, formation of endogenous aggregates resulted in the decrease of some synaptic proteins, impairments in neuronal excitability and connectivity and, eventually, neuron loss.131
Another study has shown that intracerebral inoculations of symptomatic mouse brain lysates or preformed α-synuclein fibrils into Tg asymptomatic mice overexpressing the human A53T mutant triggered PD-like pathology and dramatically reduced the survival time of animals.132 PD-like pathology was shown to spread to distal CNS regions. The most severe α-synuclein pathology was detected in brain regions that project to, or receive input from the inoculation sites, suggesting that the propagation of pathology occurs between associated neuronal populations.132 Furthermore, intrastriatal inoculation of synthetic α-synuclein fibrils into wild-type non-Tg mice also led to cell-to-cell transmission of pathologic α-synuclein and LB-like pathology. The α-synuclein pathology caused progressive loss of dopamine neurons in the substantia nigra pars compacta and impairment in motor coordination.130
Injections of in vitro preformed human or mouse α-synuclein fibrils in the substantia nigra of non-Tg mice have also induced LB-like pathology, while mice injected with soluble α-synuclein did not exhibit pathological symptoms.133 Exogenously injected fibrils could be detected for less than one week, but induction of α-synuclein pathology was observed after three months. The pathology induced by exogenous fibrils was similar to that triggered by intracerebral injection of sarcosyl-insoluble α-synuclein from brains of patients with DLB.79
Sacino and collaborators have obtained similar results.134 They have observed that neonatal cerebral injections of exogenous α-synuclein fibrils induced pathology after prolonged incubation times. The injected α-synuclein was rapidly cleared within a few days and inclusion pathology, which arose from endogenous protein, was developed after months. Tg mice overexpressing α-synuclein were more prone to develop pathology. Moreover, the authors have shown that injections of the non-amyloidogenic form of α-synuclein (Δ71–82) induced similar pathology, suggesting that the pathogenesis might be induced not solely by conformation-dependent templating events.134
Altogether these data strongly confirm that exogenous α-synuclein amyloid fibrils produced from recombinant protein have prion-like properties, as they are sufficient to seed and induce pathology in vivo.
A typical hallmark of prion diseases is the existence of different “strains,” arising from the same proteins but having different incubation periods, PrPSc distribution and lesion profiles in the brain, as well as different protease cleavage patterns.135 The existence of different strains has not been identified so far for other neurodegenerative diseases. Recently, Bousset and collaborators have generated in vitro two different high-molecule assemblies of α-synuclein, prepared in buffers with different salt concentrations.65 These assemblies, designated fibrils and ribbons, adopted different conformations, as confirmed by transmission electron microscopy (TEM), FTIR, X-ray diffraction, and ssNMR. Moreover, proteinase-K digestion revealed different cleavage profiles. Fibrils and ribbons exhibited marked differences in their propensity to bind and penetrate cells, toxicity and seeded aggregation of endogenous α-synuclein in vivo. Moreover, they imprinted their intrinsic structure to endogenous α-synuclein, suggesting that different strain-like assemblies may account for the development and progression of different synucleinopathies.65
The mechanism by which exogenous aggregates may be internalized by cells is not clear. Previous studies on fibrils comprised of polyglutamine repeats and tau proteins have shown that exogenous fibrils can be internalized by cells by simple addition into culture medium.136,137 Some studies have reported that a similar internalization process occurs also for α-synuclein,72,129,138 while others have shown that fibrils were efficiently introduced into cells only in the presence of a transduction reagent.56,78 Transduction was performed using cationic-liposomal protein transduction reagent or lipofectamine reagent.56,139
Thus the ability of α-synuclein aggregates to enter into cells and induce aggregation of endogenous protein may depend on the precise structure of the aggregated protein. Danzer and collaborators have observed that, depending on in vitro aggregation conditions, α-synuclein oligomers formed distinct populations that differed in their biophysical properties and cellular effects.72,140 Only some types of oligomers were internalized by primary neuronal cells and neuronal cell lines, and induced endogenous α-synuclein aggregation, while others induced cell death via disruption of cellular ion homeostasis.72,140
Another study has confirmed that the mechanism of internalization and propagation may depend on the assembly state of the protein: oligomers and fibrils were uptaken into cells via endocytosis, while the monomeric protein passively crossed the plasma membrane.138 Internalization of fibrillar α-synuclein required physiological temperatures and dynamin-1 activity, which were necessary for endocytosis. Moreover, it has been suggested that extracellular fibrils were internalized by receptor-mediated endocytosis. Internalized aggregates moved through the endosomal pathway and were degraded by lysosomes.138 Internalization of α-synuclein preformed fibrils was increased by the presence of wheat germ agglutinin, which bound N-acetyl-glucosamine and sialic acids at the cell surface and induced adsorptive-mediated endocytosis, suggesting that some glycoproteins might be important players in fibril internalization.131
Once α-synuclein aggregates reach acceptor cells, they could seed aggregation of endogenous protein in a prion-like fashion and eventually the newly formed aggregates may be released into the extracellular space. Vesicle-mediated exocytosis could be responsible for release of α-synuclein aggregates. Multiple forms of α-synuclein have been detected in the cerebrospinal fluid, blood plasma and saliva, suggesting an underlying secretory process.141 It has been shown that cultured neuronal cells secreted α-synuclein monomers and aggregates by non-classical vesicle-mediated exocytosis. Moreover, the secretion was elevated in response to proteasomal and mitochondrial dysfunctions associated with PD pathogenesis.142
Recently, it has been reported that fibrillar α-synuclein was transferred along axons through anterograde axonal transport, released, and subsequently uptaken by additional neurons. The axonal transfer of fibrils may explain the spread of LB between anatomically connected brain areas.143
Moreover, tunneling nanotubes (TNTs) have been suggested to play a major role in the propagation of α-synuclein pathology. TNTs are tubular membrane bridges interconnecting cells over long distances, thus enabling the exchange of molecules and cytoplasmic content between cells.144 TNTs could be involved in the intracellular propagation of PrPSc.145 Till now, TNTs have not been implicated in PD but their involvement in the spread of α-synuclein pathology cannot be ruled out.
Toxicity of α-Synuclein Aggregates
An increasing number of studies, both in vitro and in vivo, have suggested that prefibrillar oligomers and protofibrils, rather than mature fibrils, may feature toxic species of α-synuclein and may cause neuronal injury and cell death.146-149 The process of fibril formation, rather than the fibrils themselves, has been suggested as a key element in α-synuclein toxicity. The presence of some α-synuclein prefibrillar species may indicate an attempt by neurons to convert toxic oligomers to fibrils, which are more stable, less dynamic structures and exhibit reduced toxicity.147
Membrane permeabilization by oligomeric species represents a potential mechanism of α-synuclein mediated cytotoxicity. Monomeric α-synuclein was found to bind negatively charged phospholipid membranes and undergo the structural transition into α-helix.150,151 It has been shown that the protofibrillar α-synuclein species binds negatively charged vesicles more strongly than the monomer. Moreover, protofibrillar species, but not monomeric protein or fibrils, have permeabilized negatively charged vesicles to calcium.150 Two PD-linked variants, A53T and A30P, show greater permeabilizing activity than WT protein, while this activity is reduced for E46K.118,124
The mechanism of membrane permeabilization is not clear, but protofibrillar oligomers have been suggested to permeabilize membranes in a pore-like fashion.124 They may integrate into the membrane, creating pores or channel-like structures causing uncontrolled membrane permeability. Alternatively, oligomers or protofibrils could increase the conductivity of the membrane without the formation of pores.152
Membrane permeabilization may initiate several pathological events leading to changed ion homeostasis, induction of oxidative stress, altered signaling pathways, dysfunction of mitochondria, inflammation and eventually cell death.153 In fact, several works have found that exogenous oligomers led to an increase in intracellular Ca2+ level, diminished levels of some synaptic proteins, production of reactive oxygen species and cell death.131,149,150,154 Moreover, in mice intracerebro-ventricular injections of α-synuclein oligomers increased the activity of calcineurin, a key negative regulator of synaptic plasticity and memory function, thus resulting in a decrease of synaptic plasticity and memory deficits.149
It seems that toxicity of α-synuclein oligomers may depend on their structure. Danzer and collaborators developed novel methods for generating different α-synuclein oligomeric species, which have been tested for their biological effects on SH-SY5Y cells.72 Only spherical oligomers, 2 to 6 nm in height, increased intracellular calcium level and led to caspase-3 activation, suggesting that some structural properties of oligomeric α-synuclein may be important to induce toxicity.72 Moreover, Bousset and collaborators have tested the toxicity of two α-synuclein assemblies (fibrils and ribbons) with different structural properties:65 fibrils resulted more toxic than ribbons. In fact, fibrils induced activation of caspase-3, production of ROS and permeabilized lipid vesicles to a higher extent than ribbons.65
Although large sets of data suggest that prefibrillar assemblies represent toxic species of α-synuclein, it has been shown that a homogenous population of fibrils, rather than their precursor on-assembly pathway oligomers, is highly toxic to cells.155 Fibrils have been shown to permeabilize membrane vesicles and to alter calcium homeostasis. Moreover, cells exposure to increasing concentrations of fibrils resulted in the activation of caspase-3 in a concentration-dependent manner and cell death, whereas oligomeric α-synuclein did not affect cell viability and did not activate caspase-3. The authors argued that the discrepancy with previous results on oligomer toxicity originates from the comparison of protein particles at different concentrations. In previous reports, the concentration of different aggregates was usually expressed as a function of the initial soluble protein concentration and not as a real concentration of particles in solution.155
Conclusions
Increasing evidence indicates that aggregation of α-synuclein is a critical factor in the etiology of PD and other synucleinopathies. Several studies have focused on explaining the structural features of α-synuclein and the mechanism leading to its aggregation and fibrillization. These investigations may explain molecular mechanisms occurring in living cells and leading to disease development. In vitro aggregation assays are powerful tools for studying the mechanisms responsible for the formation of different aggregate species of α-synuclein, their structural properties and biological functions. Moreover, aggregation assays are crucial to develop new drug compounds or novel therapy strategies to prevent disease development and progression. Recent studies clearly indicate that misfolded α-synuclein aggregates may be internalized by cells and propagated in a prion-like manner, leading to toxicity. In fact, Watts and collaborators have shown that α-synuclein aggregates formed in the brains of MSA patients are transmissible in a prion-like fashion.156
Prion-like transmission and propagation of misfolded proteins seems to be a process common to several neurodegenerative disorders, not only PD, but also Alzheimer`s disease, Huntington`s disease, amyloid lateral sclerosis, and others.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the European Union’s Seventh Framework Programme (FP7/2007–2013) under grant agreement n° 222887—the PRIORITY project.
References
- 1.Zhang L, Zhang C, Zhu Y, Cai Q, Chan P, Uéda K, Yu S, Yang H. Semi-quantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons: an immunogold electron microscopic study using a C-terminal specific monoclonal antibody. Brain Res. 2008;1244:40–52. doi: 10.1016/j.brainres.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 2.Maroteaux L, Scheller RH. The rat brain synucleins; family of proteins transiently associated with neuronal membrane. Brain Res Mol Brain Res. 1991;11:335–43. doi: 10.1016/0169-328X(91)90043-W. [DOI] [PubMed] [Google Scholar]
- 3.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–75. doi: 10.1016/0896-6273(95)90302-X. [DOI] [PubMed] [Google Scholar]
- 4.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–15. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- 6.Papp MI, Komoly S. Filamentous Glial Cytoplasmic Inclusions in the Cns of Patients with Various Combinations of Striatonigral Degeneration (Snd), Olivopontocerebellar Atrophy (Opca) and Shy-Drager Syndrome (Sds) Clin Neuropathol. 1988;7:195. [Google Scholar]
- 7.Lee HJ, Lee SJ. Characterization of cytoplasmic alpha-synuclein aggregates. Fibril formation is tightly linked to the inclusion-forming process in cells. J Biol Chem. 2002;277:48976–83. doi: 10.1074/jbc.M208192200. [DOI] [PubMed] [Google Scholar]
- 8.Baptista MJ, O’Farrell C, Daya S, Ahmad R, Miller DW, Hardy J, Farrer MJ, Cookson MR. Co-ordinate transcriptional regulation of dopamine synthesis genes by alpha-synuclein in human neuroblastoma cell lines. J Neurochem. 2003;85:957–68. doi: 10.1046/j.1471-4159.2003.01742.x. [DOI] [PubMed] [Google Scholar]
- 9.Yavich L, Tanila H, Vepsäläinen S, Jäkälä P. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–70. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fountaine TM, Wade-Martins R. RNA interference-mediated knockdown of alpha-synuclein protects human dopaminergic neuroblastoma cells from MPP(+) toxicity and reduces dopamine transport. J Neurosci Res. 2007;85:351–63. doi: 10.1002/jnr.21125. [DOI] [PubMed] [Google Scholar]
- 11.Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15:916–26. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- 12.Wersinger C, Prou D, Vernier P, Sidhu A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003;17:2151–3. doi: 10.1096/fj.03-0152fje. [DOI] [PubMed] [Google Scholar]
- 13.Wersinger C, Sidhu A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci Lett. 2003;340:189–92. doi: 10.1016/S0304-3940(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 14.Sidhu A, Wersinger C, Moussa CE, Vernier P. The role of alpha-synuclein in both neuroprotection and neurodegeneration. Ann N Y Acad Sci. 2004;1035:250–70. doi: 10.1196/annals.1332.016. [DOI] [PubMed] [Google Scholar]
- 15.Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–96. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Deleersnijder A, Gerard M, Debyser Z, Baekelandt V. The remarkable conformational plasticity of alpha-synuclein: blessing or curse? Trends Mol Med. 2013;19:368–77. doi: 10.1016/j.molmed.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Tompa P. Intrinsically unstructured proteins evolve by repeat expansion. Bioessays. 2003;25:847–55. doi: 10.1002/bies.10324. [DOI] [PubMed] [Google Scholar]
- 18.Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27:527–33. doi: 10.1016/S0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- 19.Bisaglia M, Tessari I, Pinato L, Bellanda M, Giraudo S, Fasano M, Bergantino E, Bubacco L, Mammi S. A topological model of the interaction between alpha-synuclein and sodium dodecyl sulfate micelles. Biochemistry. 2005;44:329–39. doi: 10.1021/bi048448q. [DOI] [PubMed] [Google Scholar]
- 20.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 21.Eliezer D, Kutluay E, Bussell R, Jr., Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–73. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 22.Chandra S, Chen X, Rizo J, Jahn R, Südhof TC. A broken alpha -helix in folded alpha -Synuclein. J Biol Chem. 2003;278:15313–8. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- 23.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem. 2005;280:9595–603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 24.Rao JN, Jao CC, Hegde BG, Langen R, Ulmer TS. A combinatorial NMR and EPR approach for evaluating the structural ensemble of partially folded proteins. J Am Chem Soc. 2010;132:8657–68. doi: 10.1021/ja100646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruschus JM, Yap TL, Pistolesi S, Maltsev AS, Lee JC. NMR structure of calmodulin complexed to an N-terminally acetylated α-synuclein peptide. Biochemistry. 2013;52:3436–45. doi: 10.1021/bi400199p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie YY, Zhou CJ, Zhou ZR, Hong J, Che MX, Fu QS, Song AX, Lin DH, Hu HY. Interaction with synphilin-1 promotes inclusion formation of alpha-synuclein: mechanistic insights and pathological implication. FASEB J. 2010;24:196–205. doi: 10.1096/fj.09-133082. [DOI] [PubMed] [Google Scholar]
- 27.De Genst EJ, Guilliams T, Wellens J, O’Day EM, Waudby CA, Meehan S, Dumoulin M, Hsu ST, Cremades N, Verschueren KH, et al. Structure and properties of a complex of α-synuclein and a single-domain camelid antibody. J Mol Biol. 2010;402:326–43. doi: 10.1016/j.jmb.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhao M, Cascio D, Sawaya MR, Eisenberg D. Structures of segments of α-synuclein fused to maltose-binding protein suggest intermediate states during amyloid formation. Protein Sci. 2011;20:996–1004. doi: 10.1002/pro.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–10. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fauvet B, Mbefo MK, Fares MB, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, et al. α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287:15345–64. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–73. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–20. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 33.Forno LS, Langston JW. Lewy Bodies and Aging. J Neuropathol Exp Neurol. 1990;49:278. doi: 10.1097/00005072-199005000-00058. [DOI] [Google Scholar]
- 34.Serpell LC, Berriman J, Jakes R, Goedert M, Crowther RA. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid-like cross-beta conformation. Proc Natl Acad Sci U S A. 2000;97:4897–902. doi: 10.1073/pnas.97.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–15. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 36.Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–22. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 37.Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, et al. Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J Biol Chem. 1999;274:9843–6. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 38.Ren G, Wang X, Hao S, Hu H, Wang CC. Translocation of alpha-synuclein expressed in Escherichia coli. J Bacteriol. 2007;189:2777–86. doi: 10.1128/JB.01406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang C, Ren G, Zhou H, Wang CC. A new method for purification of recombinant human alpha-synuclein in Escherichia coli. Protein Expr Purif. 2005;42:173–7. doi: 10.1016/j.pep.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson MR. Techniques to study amyloid fibril formation in vitro. Methods. 2004;34:151–60. doi: 10.1016/j.ymeth.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem. 2001;276:10737–44. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 42.Giehm L, Lorenzen N, Otzen DE. Assays for α-synuclein aggregation. Methods. 2011;53:295–305. doi: 10.1016/j.ymeth.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Eisert R, Felau L, Brown LR. Methods for enhancing the accuracy and reproducibility of Congo red and thioflavin T assays. Anal Biochem. 2006;353:144–6. doi: 10.1016/j.ab.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Masuda M, Suzuki N, Taniguchi S, Oikawa T, Nonaka T, Iwatsubo T, Hisanaga S, Goedert M, Hasegawa M. Small molecule inhibitors of alpha-synuclein filament assembly. Biochemistry. 2006;45:6085–94. doi: 10.1021/bi0600749. [DOI] [PubMed] [Google Scholar]
- 45.Frid P, Anisimov SV, Popovic N. Congo red and protein aggregation in neurodegenerative diseases. Brain Res Rev. 2007;53:135–60. doi: 10.1016/j.brainresrev.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Celej MS, Jares-Erijman EA, Jovin TM. Fluorescent N-arylaminonaphthalene sulfonate probes for amyloid aggregation of alpha-synuclein. Biophys J. 2008;94:4867–79. doi: 10.1529/biophysj.107.125211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Mercato LL, Pompa PP, Maruccio G, Della Torre A, Sabella S, Tamburro AM, Cingolani R, Rinaldi R. Charge transport and intrinsic fluorescence in amyloid-like fibrils. Proc Natl Acad Sci U S A. 2007;104:18019–24. doi: 10.1073/pnas.0702843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan FTS, Kaminski Schierle GS, Kumita JR, Bertoncini CW, Dobson CM, Kaminski CF. Protein amyloids develop an intrinsic fluorescence signature during aggregation. Analyst. 2013;138:2156–62. doi: 10.1039/c3an36798c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharpe S, Simonetti K, Yau J, Walsh P. Solid-State NMR characterization of autofluorescent fibrils formed by the elastin-derived peptide GVGVAGVG. Biomacromolecules. 2011;12:1546–55. doi: 10.1021/bm101486s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinotsi D, Buell AK, Dobson CM, Kaminski Schierle GS, Kaminski CF. A label-free, quantitative assay of amyloid fibril growth based on intrinsic fluorescence. Chembiochem. 2013;14:846–50. doi: 10.1002/cbic.201300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rochet JC, Lansbury PT., Jr. Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol. 2000;10:60–8. doi: 10.1016/S0959-440X(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 52.Uversky VN, Gillespie JR, Fink AL. Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins. 2000;41:415–27. doi: 10.1002/1097-0134(20001115)41:3<415::AID-PROT130>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 53.Baldwin RL. Temperature dependence of the hydrophobic interaction in protein folding. Proc Natl Acad Sci U S A. 1986;83:8069–72. doi: 10.1073/pnas.83.21.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL. alpha-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson’s disease. J Biol Chem. 1999;274:19509–12. doi: 10.1074/jbc.274.28.19509. [DOI] [PubMed] [Google Scholar]
- 55.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–6. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M. Seeded aggregation and toxicity of alpha-synuclein and tau: cellular models of neurodegenerative diseases. J Biol Chem. 2010;285:34885–98. doi: 10.1074/jbc.M110.148460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nielsen L, Khurana R, Coats A, Frokjaer S, Brange J, Vyas S, Uversky VN, Fink AL. Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry. 2001;40:6036–46. doi: 10.1021/bi002555c. [DOI] [PubMed] [Google Scholar]
- 58.Giehm L, Otzen DE. Strategies to increase the reproducibility of protein fibrillization in plate reader assays. Anal Biochem. 2010;400:270–81. doi: 10.1016/j.ab.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Horvath I, Weise CF, Andersson EK, Chorell E, Sellstedt M, Bengtsson C, Olofsson A, Hultgren SJ, Chapman M, Wolf-Watz M, et al. Mechanisms of protein oligomerization: inhibitor of functional amyloids templates α-synuclein fibrillation. J Am Chem Soc. 2012;134:3439–44. doi: 10.1021/ja209829m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conway KA, Harper JD, Lansbury PT., Jr. Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39:2552–63. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 61.Tycko R. Solid-state NMR studies of amyloid fibril structure. Annu Rev Phys Chem. 2011;62:279–99. doi: 10.1146/annurev-physchem-032210-103539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen M, Margittai M, Chen J, Langen R. Investigation of alpha-synuclein fibril structure by site-directed spin labeling. J Biol Chem. 2007;282:24970–9. doi: 10.1074/jbc.M700368200. [DOI] [PubMed] [Google Scholar]
- 63.Pornsuwan S, Giller K, Riedel D, Becker S, Griesinger C, Bennati M. Long-range distances in amyloid fibrils of α-synuclein from PELDOR spectroscopy. Angew Chem Int Ed Engl. 2013;52:10290–4. doi: 10.1002/anie.201304747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lv G, Kumar A, Giller K, Orcellet ML, Riedel D, Fernández CO, Becker S, Lange A. Structural comparison of mouse and human α-synuclein amyloid fibrils by solid-state NMR. J Mol Biol. 2012;420:99–111. doi: 10.1016/j.jmb.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, Madiona K, Olieric V, Böckmann A, Meier BH, et al. Structural and functional characterization of two alpha-synuclein strains. Nat Commun. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kloepper KD, Woods WS, Winter KA, George JM, Rienstra CM. Preparation of alpha-synuclein fibrils for solid-state NMR: expression, purification, and incubation of wild-type and mutant forms. Protein Expr Purif. 2006;48:112–7. doi: 10.1016/j.pep.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Heise H, Celej MS, Becker S, Riedel D, Pelah A, Kumar A, Jovin TM, Baldus M. Solid-state NMR reveals structural differences between fibrils of wild-type and disease-related A53T mutant alpha-synuclein. J Mol Biol. 2008;380:444–50. doi: 10.1016/j.jmb.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 68.Heise H, Hoyer W, Becker S, Andronesi OC, Riedel D, Baldus M. Molecular-level secondary structure, polymorphism, and dynamics of full-length alpha-synuclein fibrils studied by solid-state NMR. Proc Natl Acad Sci U S A. 2005;102:15871–6. doi: 10.1073/pnas.0506109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vilar M, Chou HT, Lührs T, Maji SK, Riek-Loher D, Verel R, Manning G, Stahlberg H, Riek R. The fold of alpha-synuclein fibrils. Proc Natl Acad Sci U S A. 2008;105:8637–42. doi: 10.1073/pnas.0712179105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Comellas G, Lemkau LR, Nieuwkoop AJ, Kloepper KD, Ladror DT, Ebisu R, Woods WS, Lipton AS, George JM, Rienstra CM. Structured regions of α-synuclein fibrils include the early-onset Parkinson’s disease mutation sites. J Mol Biol. 2011;411:881–95. doi: 10.1016/j.jmb.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gath J, Habenstein B, Bousset L, Melki R, Meier BH, Böckmann A. Solid-state NMR sequential assignments of α-synuclein. Biomol NMR Assign. 2012;6:51–5. doi: 10.1007/s12104-011-9324-3. [DOI] [PubMed] [Google Scholar]
- 72.Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–32. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munishkina LA, Phelan C, Uversky VN, Fink AL. Conformational behavior and aggregation of alpha-synuclein in organic solvents: modeling the effects of membranes. Biochemistry. 2003;42:2720–30. doi: 10.1021/bi027166s. [DOI] [PubMed] [Google Scholar]
- 74.Munishkina LA, Cooper EM, Uversky VN, Fink AL. The effect of macromolecular crowding on protein aggregation and amyloid fibril formation. J Mol Recognit. 2004;17:456–64. doi: 10.1002/jmr.699. [DOI] [PubMed] [Google Scholar]
- 75.Li J, Uversky VN, Fink AL. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry. 2001;40:11604–13. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- 76.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J Biol Chem. 2001;276:44284–96. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 77.Goers J, Uversky VN, Fink AL. Polycation-induced oligomerization and accelerated fibrillation of human alpha-synuclein in vitro. Protein Sci. 2003;12:702–7. doi: 10.1110/ps.0230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106:20051–6. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–38. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Raaij ME, van Gestel J, Segers-Nolten IMJ, de Leeuw SW, Subramaniam V. Concentration dependence of alpha-synuclein fibril length assessed by quantitative atomic force microscopy and statistical-mechanical theory. Biophys J. 2008;95:4871–8. doi: 10.1529/biophysj.107.127464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Powers ET, Powers DL. The kinetics of nucleated polymerizations at high concentrations: amyloid fibril formation near and above the “supercritical concentration”. Biophys J. 2006;91:122–32. doi: 10.1529/biophysj.105.073767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fink AL. Factors Affecting the Fibrillation of alpha-synuclein, a natively unfolded protein. In Misbehaving Proteins: Protein (Mis)Folding, Aggregation, and Stability. Springer, 2006. [Google Scholar]
- 83.Shtilerman MD, Ding TT, Lansbury PT., Jr. Molecular crowding accelerates fibrillization of alpha-synuclein: could an increase in the cytoplasmic protein concentration induce Parkinson’s disease? Biochemistry. 2002;41:3855–60. doi: 10.1021/bi0120906. [DOI] [PubMed] [Google Scholar]
- 84.Fink AL. Protein aggregation: folding aggregates, inclusion bodies and amyloid. Fold Des. 1998;3:R9–23. doi: 10.1016/S1359-0278(98)00002-9. [DOI] [PubMed] [Google Scholar]
- 85.Uversky VN, Li J, Fink AL. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett. 2001;500:105–8. doi: 10.1016/S0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- 86.Serio TR, Cashikar AG, Kowal AS, Sawicki GJ, Moslehi JJ, Serpell L, Arnsdorf MF, Lindquist SL. Nucleated conformational conversion and the replication of conformational information by a prion determinant. Science. 2000;289:1317–21. doi: 10.1126/science.289.5483.1317. [DOI] [PubMed] [Google Scholar]
- 87.Cohlberg JA, Li J, Uversky VN, Fink AL. Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from alpha-synuclein in vitro. Biochemistry. 2002;41:1502–11. doi: 10.1021/bi011711s. [DOI] [PubMed] [Google Scholar]
- 88.Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279:26846–57. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
- 89.Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–4. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 90.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–44. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 91.Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 92.Uversky VN, Eliezer D. Biophysics of Parkinson’s disease: structure and aggregation of alpha-synuclein. Curr Protein Pept Sci. 2009;10:483–99. doi: 10.2174/138920309789351921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–6. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 94.Giasson BI, Lee VM. A new link between pesticides and Parkinson’s disease. Nat Neurosci. 2000;3:1227–8. doi: 10.1038/81737. [DOI] [PubMed] [Google Scholar]
- 95.Natalello A, Benetti F, Doglia SM, Legname G, Grandori R. Compact conformations of α-synuclein induced by alcohols and copper. Proteins. 2011;79:611–21. doi: 10.1002/prot.22909. [DOI] [PubMed] [Google Scholar]
- 96.Zayed J, Ducic S, Campanella G, Panisset JC, André P, Masson H, Roy M. [Environmental factors in the etiology of Parkinson’s disease] Can J Neurol Sci. 1990;17:286–91. [PubMed] [Google Scholar]
- 97.Rybicki BA, Johnson CC, Uman J, Gorell JM. Parkinson’s disease mortality and the industrial use of heavy metals in Michigan. Mov Disord. 1993;8:87–92. doi: 10.1002/mds.870080116. [DOI] [PubMed] [Google Scholar]
- 98.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology. 1997;48:650–8. doi: 10.1212/WNL.48.3.650. [DOI] [PubMed] [Google Scholar]
- 99.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology. 1999;20:239–47. [PubMed] [Google Scholar]
- 100.Hirsch EC, Brandel JP, Galle P, Javoy-Agid F, Agid Y. Iron and aluminum increase in the substantia nigra of patients with Parkinson’s disease: an X-ray microanalysis. J Neurochem. 1991;56:446–51. doi: 10.1111/j.1471-4159.1991.tb08170.x. [DOI] [PubMed] [Google Scholar]
- 101.Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–75. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- 102.Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem. 1989;52:1830–6. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 103.Goers J, Manning-Bog AB, McCormack AL, Millett IS, Doniach S, Di Monte DA, Uversky VN, Fink AL. Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry. 2003;42:8465–71. doi: 10.1021/bi0341152. [DOI] [PubMed] [Google Scholar]
- 104.Meng X, Munishkina LA, Fink AL, Uversky VN. Effects of Various Flavonoids on the α-Synuclein Fibrillation Process. Parkinsons Dis. 2010;2010:650794. doi: 10.4061/2010/650794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rekas A, Adda CG, Andrew Aquilina J, Barnham KJ, Sunde M, Galatis D, Williamson NA, Masters CL, Anders RF, Robinson CV, et al. Interaction of the molecular chaperone alphaB-crystallin with alpha-synuclein: effects on amyloid fibril formation and chaperone activity. J Mol Biol. 2004;340:1167–83. doi: 10.1016/j.jmb.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 106.Dedmon MM, Christodoulou J, Wilson MR, Dobson CM. Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J Biol Chem. 2005;280:14733–40. doi: 10.1074/jbc.M413024200. [DOI] [PubMed] [Google Scholar]
- 107.Huang C, Cheng H, Hao S, Zhou H, Zhang X, Gao J, Sun QH, Hu H, Wang CC. Heat shock protein 70 inhibits alpha-synuclein fibril formation via interactions with diverse intermediates. J Mol Biol. 2006;364:323–36. doi: 10.1016/j.jmb.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 108.Bruinsma IB, Bruggink KA, Kinast K, Versleijen AAM, Segers-Nolten IMJ, Subramaniam V, Kuiperij HB, Boelens W, de Waal RM, Verbeek MM. Inhibition of α-synuclein aggregation by small heat shock proteins. Proteins. 2011;79:2956–67. doi: 10.1002/prot.23152. [DOI] [PubMed] [Google Scholar]
- 109.Rekas A, Lo V, Gadd GE, Cappai R, Yun SI. PAMAM dendrimers as potential agents against fibrillation of alpha-synuclein, a Parkinson’s disease-related protein. Macromol Biosci. 2009;9:230–8. doi: 10.1002/mabi.200800242. [DOI] [PubMed] [Google Scholar]
- 110.Janeczek P, Mackay RK, Lea RA, Dodd PR, Lewohl JM. Reduced expression of α-synuclein in alcoholic brain: influence of SNCA-Rep1 genotype. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00495.x. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Janeczek P, Lewohl JM. The role of α-synuclein in the pathophysiology of alcoholism. Neurochem Int. 2013;63:154–62. doi: 10.1016/j.neuint.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 112.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 113.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–8. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 114.Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–73. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 115.Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, Weir D, Thompson C, Szu-Tu C, Trinh J, et al. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov Disord. 2013;28:811–3. doi: 10.1002/mds.25421. [DOI] [PubMed] [Google Scholar]
- 116.Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, Houlden H, Schapira AH. A novel α-synuclein missense mutation in Parkinson disease. Neurology. 2013;80:1062–4. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280:7800–7. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 118.Fredenburg RA, Rospigliosi C, Meray RK, Kessler JC, Lashuel HA, Eliezer D, Lansbury PT., Jr. The impact of the E46K mutation on the properties of alpha-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46:7107–18. doi: 10.1021/bi7000246. [DOI] [PubMed] [Google Scholar]
- 119.Lemkau LR, Comellas G, Kloepper KD, Woods WS, George JM, Rienstra CM. Mutant protein A30P α-synuclein adopts wild-type fibril structure, despite slower fibrillation kinetics. J Biol Chem. 2012;287:11526–32. doi: 10.1074/jbc.M111.306902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ghosh D, Mondal M, Mohite GM, Singh PK, Ranjan P, Anoop A, Ghosh S, Jha NN, Kumar A, Maji SK. The Parkinson’s disease-associated H50Q mutation accelerates α-Synuclein aggregation in vitro. Biochemistry. 2013;52:6925–7. doi: 10.1021/bi400999d. [DOI] [PubMed] [Google Scholar]
- 121.Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M. Mutation E46K increases phospholipid binding and assembly into filaments of human alpha-synuclein. FEBS Lett. 2004;576:363–8. doi: 10.1016/j.febslet.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 122.Sacino AN, Thomas MA, Ceballos-Diaz C, Cruz PE, Rosario AM, Lewis J, Giasson BI, Golde TE. Conformational templating of α-synuclein aggregates in neuronal-glial cultures. Mol Neurodegener. 2013;8:17. doi: 10.1186/1750-1326-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lemkau LR, Comellas G, Lee SW, Rikardsen LK, Woods WS, George JM, Rienstra CM. Site-specific perturbations of alpha-synuclein fibril structure by the Parkinson’s disease associated mutations A53T and E46K. PLoS One. 2013;8:e49750. doi: 10.1371/journal.pone.0049750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Volles MJ, Lansbury PT., Jr. Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 125.Yonetani M, Nonaka T, Masuda M, Inukai Y, Oikawa T, Hisanaga S, Hasegawa M. Conversion of wild-type alpha-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. J Biol Chem. 2009;284:7940–50. doi: 10.1074/jbc.M807482200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 127.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–6. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 128.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–3. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 129.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–5. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–53. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012;209:975–86. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–38. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sacino AN, Brooks M, McGarvey NH, McKinney AB, Thomas MA, Levites Y, Ran Y, Golde TE, Giasson BI. Induction of CNS α-synuclein pathology by fibrillar and non-amyloidogenic recombinant α-synuclein. Acta Neuropathol Commun. 2013;1:38. doi: 10.1186/2051-5960-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8:552–61. doi: 10.1038/nrm2204. [DOI] [PubMed] [Google Scholar]
- 136.Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–25. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frost B, Ollesch J, Wille H, Diamond MI. Conformational diversity of wild-type Tau fibrils specified by templated conformation change. J Biol Chem. 2009;284:3546–51. doi: 10.1074/jbc.M805627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40:1835–49. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 139.Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106:20051–6. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Danzer KM, Krebs SK, Wolff M, Birk G, Hengerer B. Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J Neurochem. 2009;111:192–203. doi: 10.1111/j.1471-4159.2009.06324.x. [DOI] [PubMed] [Google Scholar]
- 141.Steiner JA, Angot E, Brundin P. A deadly spread: cellular mechanisms of α-synuclein transfer. Cell Death Differ. 2011;18:1425–33. doi: 10.1038/cdd.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–24. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Freundt EC, Maynard N, Clancy EK, Roy S, Bousset L, Sourigues Y, Covert M, Melki R, Kirkegaard K, Brahic M. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann Neurol. 2012;72:517–24. doi: 10.1002/ana.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol. 2008;18:414–20. doi: 10.1016/j.tcb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–36. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 146.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–9. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 147.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011;108:4194–9. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Martin ZS, Neugebauer V, Dineley KT, Kayed R, Zhang W, Reese LC, Taglialatela G. α-Synuclein oligomers oppose long-term potentiation and impair memory through a calcineurin-dependent mechanism: relevance to human synucleopathic diseases. J Neurochem. 2012;120:440–52. doi: 10.1111/j.1471-4159.2011.07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, Lansbury PT., Jr. Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40:7812–9. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 151.Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-Synuclein and Parkinson’s disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–8. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 152.Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem. 2004;279:46363–6. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 153.Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006;27:570–5. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 154.Cremades N, Cohen SIA, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, et al. Direct observation of the interconversion of normal and toxic forms of α-synuclein. Cell. 2012;149:1048–59. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pieri L, Madiona K, Bousset L, Melki R. Fibrillar α-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys J. 2012;102:2894–905. doi: 10.1016/j.bpj.2012.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, DeArmond SJ, Prusiner SB. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci U S A. 2013;110:19555–60. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu IH, Uversky VN, Munishkina LA, Fink AL, Halfter W, Cole GJ. Agrin binds alpha-synuclein and modulates alpha-synuclein fibrillation. Glycobiology. 2005;15:1320–31. doi: 10.1093/glycob/cwj014. [DOI] [PubMed] [Google Scholar]
- 158.Giehm L, Oliveira CLP, Christiansen G, Pedersen JS, Otzen DE. SDS-induced fibrillation of alpha-synuclein: an alternative fibrillation pathway. J Mol Biol. 2010;401:115–33. doi: 10.1016/j.jmb.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 159.Uversky VN, Li J, Souillac P, Millett IS, Doniach S, Jakes R, Goedert M, Fink AL. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. J Biol Chem. 2002;277:11970–8. doi: 10.1074/jbc.M109541200. [DOI] [PubMed] [Google Scholar]
- 160.Li J, Zhu M, Manning-Bog AB, Di Monte DA, Fink AL. Dopamine and L-dopa disaggregate amyloid fibrils: implications for Parkinson’s and Alzheimer’s disease. FASEB J. 2004;18:962–4. doi: 10.1096/fj.03-0770fje. [DOI] [PubMed] [Google Scholar]
- 161.Zhu M, Fink AL. Lipid binding inhibits alpha-synuclein fibril formation. J Biol Chem. 2003;278:16873–7. doi: 10.1074/jbc.M210136200. [DOI] [PubMed] [Google Scholar]
- 162.Li J, Zhu M, Rajamani S, Uversky VN, Fink AL. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem Biol. 2004;11:1513–21. doi: 10.1016/j.chembiol.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 163.Jiang T, Yu WB, Yao T, Zhi XL, Pan LF, Wang J, et al. Trehalose inhibits wild-type alpha-synuclein fibrillation and overexpression and protects against the protein neurotoxicity in transduced PC12 cells. Rsc Advances. 2013;3:9500–8. doi: 10.1039/c3ra40600h. [DOI] [Google Scholar]
- 164.Uversky V. α-Synuclein Aggregation and Parkinson’s Disease. In: Uversky V, Fink A, eds. Protein Misfolding, Aggregation, and Conformational Diseases: Springer US, 2007:61-110. [Google Scholar]
- 165.Chavarría C, Souza JM. Oxidation and nitration of α-synuclein and their implications in neurodegenerative diseases. Arch Biochem Biophys. 2013;533:25–32. doi: 10.1016/j.abb.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 166.Moriarty GM, Janowska MK, Kang L, Baum J. Exploring the accessible conformations of N-terminal acetylated α-synuclein. FEBS Lett. 2013;587:1128–38. doi: 10.1016/j.febslet.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]