Abstract
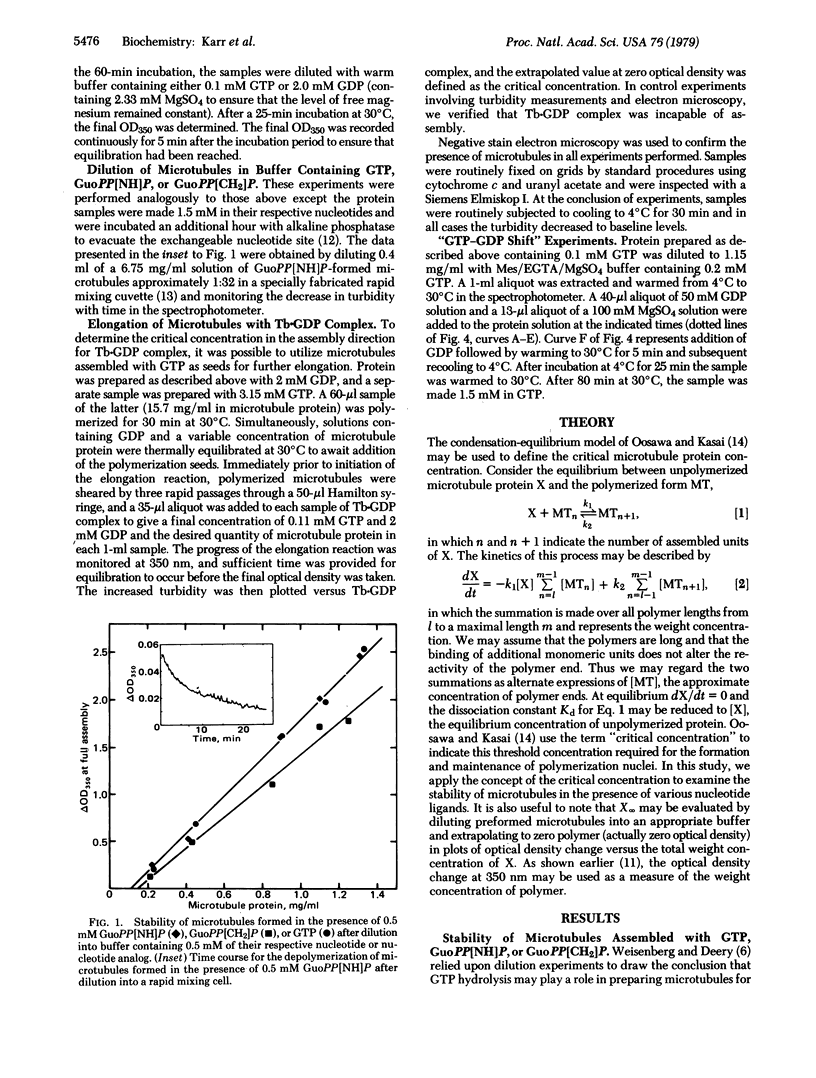
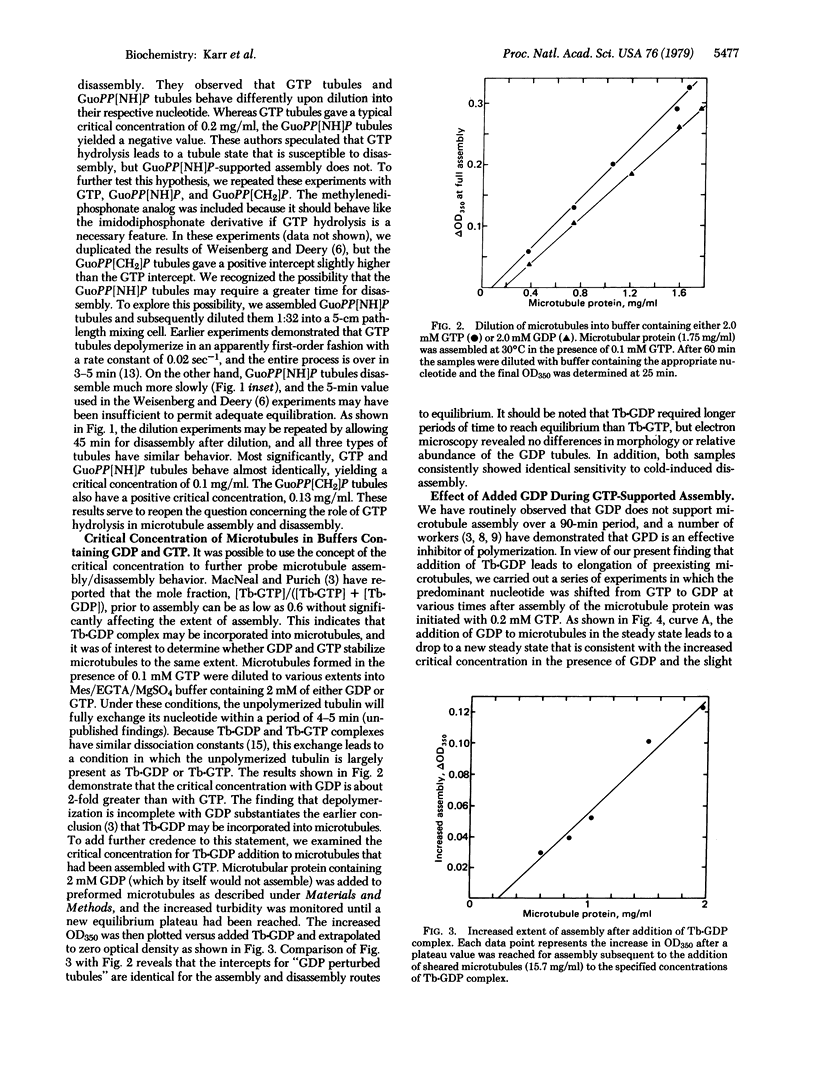
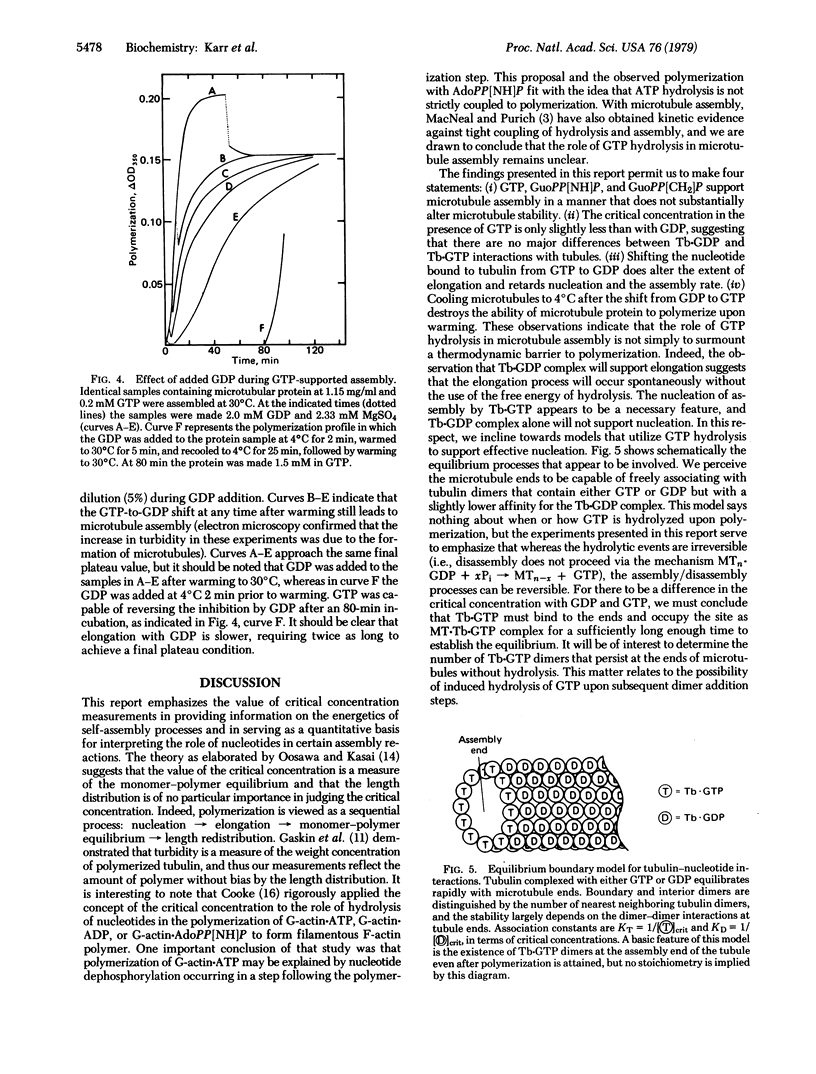
Critical concentrations for formation of microtubules from subunits with GTP and its beta, gamma-imido and beta, gamma-methylene analogs are similar when adequate time is given for equilibration. Dilution of microtubules into GTP and GDP yielded values of 0.1 and 0.19 mg/ml for the critical concentration, results similar to those reported by Carlier and Pantaloni [Carlier, M. & Pantaloni, D. (1978) Biochemistry 17, 1908-1915]. GDP is capable of supporting elongation of preformed microtubules, but it efficiently poisons the nucleation events. Reported experiments also demonstrate that the critical tubulin concentration of the tubulin-GDP complex can be accurately measured in both the assembly and disassembly directions. Evidence is presented that GTP is involved in early nucleation events but that microtubules are stabilized in the presence of either GTP or GDP. These results are discussed in terms of a condensation-equilibrium model in which tubulin subunits equilibrate rapidly with microtubule ends, and their affinity for the ends is governed by the nucleotide ligand at the exchangeable site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Kaziro Y. Effect of guanine nucleotides on the assembly of brain microtubles: ability of 5'-guanylyl imidodiphosphate to replace GTB in promoting the polymerization of microtubules in vitro. Biochem Biophys Res Commun. 1976 Mar 22;69(2):369–376. doi: 10.1016/0006-291x(76)90531-3. [DOI] [PubMed] [Google Scholar]
- Arai T., Kaziro Y. Role of GTP in the assembly of microtubules. J Biochem. 1977 Oct;82(4):1063–1071. doi: 10.1093/oxfordjournals.jbchem.a131777. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Kinetic analysis of cooperativity in tubulin polymerization in the presence of guanosine di- or triphosphate nucleotides. Biochemistry. 1978 May 16;17(10):1908–1915. doi: 10.1021/bi00603a017. [DOI] [PubMed] [Google Scholar]
- Cooke R. The role of the bound nucleotide in the polymerization of actin. Biochemistry. 1975 Jul 15;14(14):3250–3256. doi: 10.1021/bi00685a035. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Caplow M. Microtubular protein reaction with nucleotides. Biochem Biophys Res Commun. 1976 Jan 12;68(1):127–135. doi: 10.1016/0006-291x(76)90019-x. [DOI] [PubMed] [Google Scholar]
- Karr T. L., Purich D. L. Examination of tubulin-nucleotide interactions by protein fluorescence quenching measurements. Biochem Biophys Res Commun. 1978 Oct 30;84(4):957–961. doi: 10.1016/0006-291x(78)91676-5. [DOI] [PubMed] [Google Scholar]
- Karr T. L., White H. D., Purich D. L. Characterization of brain microtubule proteins prepared by selective removal of mitochondrial and synaptosomal components. J Biol Chem. 1979 Jul 10;254(13):6107–6111. [PubMed] [Google Scholar]
- Kobayashi T. Dephosphorylation of tubulin-bound guanosine triphosphate during microtubule assembly. J Biochem. 1975 Jun;77(6):1193–1197. [PubMed] [Google Scholar]
- MacNeal R. K., Purich D. L. Stoichiometry and role of GTP hydrolysis in bovine neurotubule assembly. J Biol Chem. 1978 Jul 10;253(13):4683–4687. [PubMed] [Google Scholar]
- Margolis R. L., Wilson L., Keifer B. I. Mitotic mechanism based on intrinsic microtubule behaviour. Nature. 1978 Mar 30;272(5652):450–452. doi: 10.1038/272450a0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- OOSAWA F., KASAI M. A theory of linear and helical aggregations of macromolecules. J Mol Biol. 1962 Jan;4:10–21. doi: 10.1016/s0022-2836(62)80112-0. [DOI] [PubMed] [Google Scholar]
- Penningroth S. M., Kirschner M. W. Nucleotide binding and phosphorylation in microtubule assembly in vitro. J Mol Biol. 1977 Oct 5;115(4):643–673. doi: 10.1016/0022-2836(77)90108-5. [DOI] [PubMed] [Google Scholar]
- Purich D. L., MacNeal R. K. Properties of tubulin treated with alkaline phosphatase to remove guanine nucleotides from the exchangeable binding site. FEBS Lett. 1978 Dec 1;96(1):83–86. doi: 10.1016/0014-5793(78)81067-9. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J., Dickinson P. J. Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules. Biochemistry. 1976 Sep 21;15(19):4248–4254. doi: 10.1021/bi00664a018. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J. Role of nucleotide hydrolysis in microtubule assembly. Nature. 1976 Oct 28;263(5580):792–793. doi: 10.1038/263792a0. [DOI] [PubMed] [Google Scholar]
- Zeeberg B., Hassid A., Caplow M. Microtubular protein catalytic interactions with nucleotides. J Biol Chem. 1977 Mar 25;252(6):2101–2105. [PubMed] [Google Scholar]


