Abstract
Predicting the response to immunosuppressive therapy could provide useful information to help the clinician define treatment strategies for patients with aplastic anemia. In our current study, we evaluated the relationship between telomere length of lymphocytes at diagnosis and the response to immunosuppressive therapy in 64 children with aplastic anemia, using flow fluorescence in situ hybridization. Median age of patients was ten years (range 1.5–16.2 years). Severity of the disease was classified as very severe in 23, severe in 21, and moderate in 20 patients. All patients were enrolled in multicenter studies using antithymocyte globulin and cyclosporine. The response rate to immunosuppressive therapy at six months was 52% (33 of 64). The probability of 5-year failure-free survival and overall survival were 56% (95% confidence interval (CI): 41–69%) and 97% (95%CI: 87–99%), respectively. Median telomere length in responders was −0.4 standard deviation (SD) (−2.7 to +3.0 SD) and −1.5 SD (−4.0 to +1.6 (SD)) in non-responders (P<0.001). Multivariate analysis showed that telomere length shorter than −1.0 SD (hazard ratio (HR): 22.0; 95%CI: 4.19–115; P<0.001), platelet count at diagnosis less than 25×109/L (HR: 13.9; 95%CI: 2.00–96.1; P=0.008), and interval from diagnosis to immunosuppressive therapy longer than 25 days (HR: 4.81; 95%CI: 1.15–20.1; P=0.031) were the significant variables for poor response to immunosuppressive therapy. Conversely to what has been found in adult patients, measurement of the telomere length of lymphocytes at diagnosis is a promising assay in predicting the response to immunosuppressive therapy in children with aplastic anemia.
Introduction
Aplastic anemia (AA) is defined as bone marrow aplasia and peripheral blood pancytopenia; disease pathogenesis is thought to involve immune-mediated processes. The first choice of treatment for severe AA in children is hematopoietic stem cell transplantation from a human leukocyte antigen (HLA)-matched sibling donor.1,2 However, 60–70% of children with severe AA have no matched sibling donor and receive immunosuppressive therapy (IST), consisting of antithymocyte globulin (ATG) and cyclosporine (CyA). According to previous studies in children, the response rate to IST at six months was 60–70%, with the probability of survival at five years being over 90%. On the other hand, relapses occur in 10–30% of patients who responded to IST and, overall, clonal evolution develops in 10–15% patients.3–5 In adults, several pre-treatment biomarkers have been proposed as promising tests for predicting favorable response to IST, including the presence of either human leukocyte antigen (HLA)-DR15 or a minor population of paroxysmal nocturnal hemoglobinuria (PNH)-type cells.6–9 However, we previously reported that neither test was useful to predict response to IST and that lower white blood cell count and shorter interval from diagnosis to IST were significant predictive markers of better response,10 on the other hand, a National Institutes of Health (NIH) study showed that higher base-line absolute reticulocyte and lymphocyte counts were highly predictive of response to IST in adult patients.11 These results suggest a difference in etiology of AA between adults and children.10
Dyskeratosis congenita (DC) is a rare inherited disease characterized by the classical mucocutaneous triad of abnormal skin pigmentation, nail dystrophy, and mucosal leukoplakia.12 Patients with DC are unable to maintain the telomere complex that are protein-DNA structures at the end of eukaryotic chromosomes that prevent degradation and aberrant recombination of the chromosome ends,13,14 and consequently have very short telomeres.15 Shortened telomeres can cause a wide variety of clinical features consisting not only of mucocutaneous abnormalities, but also other symptoms, including bone marrow failure, pulmonary fibrosis, hepatic fibrosis, and predisposition to malignancy.16 Several recent studies revealed cryptic forms of DC among patients with seemingly acquired AA who did not have apparent physical abnormalities.17,18 Failure of AA patients to respond to IST may be explained by the presence of cryptic inherited bone marrow failure syndromes (IBMFSs).
Several investigators have demonstrated that telomere lengths of leukocytes in patients with AA vary widely, with an increased proportion of the patients having shorter telomeres than healthy individuals.19,20 It is known that not only patients with typical DC, but also those with cryptic DC have very short telomeres.21 Moreover, the telomere length in leukocytes is decreased in subsets of patients with other IBMFSs including Fanconi anemia,22 Diamond-Blackfan anemia,23 and Schwachman-Diamond syndrome.24 Therefore, measuring telomere length of patients with AA at diagnosis may be useful in detecting patients with cryptic type of IBMFSs.
Recently, Scheinberg et al. reported that the telomere length of peripheral blood leukocytes was associated with risk of hematologic relapse, clonal evolution to myelodysplastic syndrome, and overall survival (OS), but not related to hematologic response to IST in patients with severe AA.25 Because there was no study to validate their observation, we evaluated the relationship between telomere length in hematopoietic cells before IST and the response to IST in children with AA.
Methods
Patients
Peripheral blood samples at diagnosis and clinical records were obtained from 64 children who fulfilled entry criteria and enrolled in two prospective studies conducted by the Japan Childhood Aplastic Anemia Study Group.26,27 Patients with acquired AA were eligible if selection criteria were satisfied (see Online Supplementary Appendix for details). Thirty-eight patients received horse ATG (Lymphoglobulin; Genzyme, Cambridge, MA, USA) at 15 mg/kg/day for five days and 26 received rabbit ATG (Thymoglobulin, Genzyme, Cambridge, MA, USA) at 3.75 mg/kg/day for five days. CyA (6 mg/kg/day, orally) was started on Day 1 and continued to at least Day 180. The dose was adjusted to achieve a whole blood trough level of 100–200 ng/mL. Standard supportive care was supplied in each institute. Response to IST was evaluated according to previously described criteria.3 We defined patients with complete response or partial response at six months after IST as responders, and the other patients as non-responders. Relapse was defined by conversion to no response from a partial or complete response and/or the requirement for blood transfusions.
All samples and clinical records were collected after written informed consent had been obtained according to protocols approved by the Ethics Review Committee, Nagoya University Graduate School of Medicine (Research n. 732).
Measurements of telomere length and population of PNH clones
The average relative telomere length (RTL) of peripheral lymphocytes was measured by flow fluorescence in situ hybridization (flow-FISH), using a Telomere PNA kit (Dako Cytomation, Glostrup, Denmark).28 Lymphocytes were derived from fresh peripheral blood in 38 cases and from frozen stored peripheral blood in 26 cases. We used delta RTL to compare patients’ telomere length with that of age-matched healthy controls. Details of methods for measuring telomere length and definition of delta RTL are described in the Online Supplementary Methods. A minor population of paroxysmal nocturnal hemoglobinuria (PNH)-type granulocytes and red blood cells were also evaluated by flow cytometry according to a previously described method.10
Statistical analysis
We analyzed predictive variables associated with response to IST, failure-free survival (FFS; in which relapse, clonal evolution, second IST, HSCT, and death were censored), transplantation-free survival (TFS; in which HSCT and death were censored), and OS. Pre-treatment variables included patient’s sex, age, etiology, disease severity, interval from diagnosis to IST, leukocyte count, lymphocyte count, neutrophil count, hemoglobin (Hb) level, platelet count, reticulocyte count, presence of HLA-DR15, presence of minor PNH clone, and delta RTL. Differences in these variables between responders and non-responders were assessed using the Mann-Whitney U-test and Fisher’s exact probability test. Predictive factors with P<0.10 in the univariate analyses were set in the multivariate analysis (logistic regression modeling). P<0.05 was considered statistically significant. Measures of association were expressed as hazard ratios (HR) with 95% confidence intervals (CI). All tests were two-tailed with a type I error of less than 0.05 considered as statistically significant. All analyses were performed using STATA12.0 software (STATA, College Station, TX, USA).
Results
Pre-treatment patients’ characteristics and clinical outcomes
A total of 64 patients with AA were included in this study. Patients’ characteristics are shown in Table 1. The median age at IST was 10.0 years (range 1.5–16.2 years). Disease severity was assessed as very severe in 23 patients, severe in 21 patients, and moderate in 20 patients. Causes of AA were idiopathic in 60 patients and hepatitis in 4 patients. Median follow-up time from the time of IST was 35 months (range 6–132 months).
Table 1.
Patients’ characteristics.
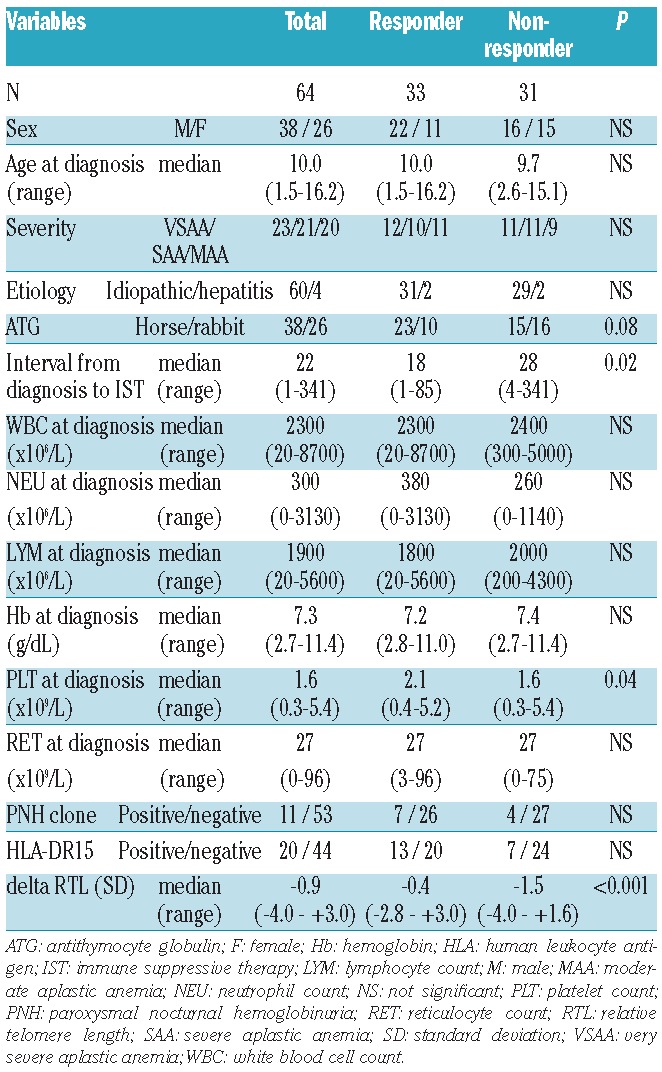
Overall, 33 of 64 patients (52%) responded to IST at six months after administration of ATG. Of the 33 responders, 4 children relapsed at 6, 34, 66, and 91 months after IST, respectively. The probability of 5-year cumulative incidence of relapse was 8% (95%CI: 2–28%). Nineteen transplantations were carried out for non-responders or patients with relapse. Of 64 children with AA, only one patient developed clonal evolution at 23 months after IST. During the observation period, 2 patients died; both of them had shown no response to IST, one suffered from lethal cerebral hemorrhage at six months, and the other underwent bone marrow transplantation from an HLA-matched unrelated donor at 12 months after IST and died of transplantation-related hepatic failure. The probability of 5-year FFS, TFS, and OS were 56% (95%CI: 41–69%), 63% (95%CI: 48–75%), and 97% (95%CI: 87–99%), respectively.
Telomere length of children with AA
Comparing SD calculated in 71 healthy individuals, median telomere length was −0.9SD (range −4.0 to +3.0SD) in all patients (n=64), −0.4SD (range −2.7 to +3.0SD) in responders (n=33), and −1.5SD (range −4.0 to +1.6SD) in non-responders (n=31) (Figure 1A and B). There was a significant difference in telomere length between responders and non-responders (P<0.001). We evaluated the effects of age-adjusted telomere length quartiles on the response rate. There was a significant relationship between hematologic response and telomere length. The response rates at six months were 12.5% in the first (the shortest), 37.5% in the second, 75% in the third, and 81.3% in the fourth (the longest) quartiles of telomere length (Figure 2). The most powerful cut-off point for dividing responders and non-responders by telomere length was −1.0 SD (P=6.9×10−6). There was no statistical tendency between relapse rate / clonal evolution / overall survival and telomere length.
Figure 1.
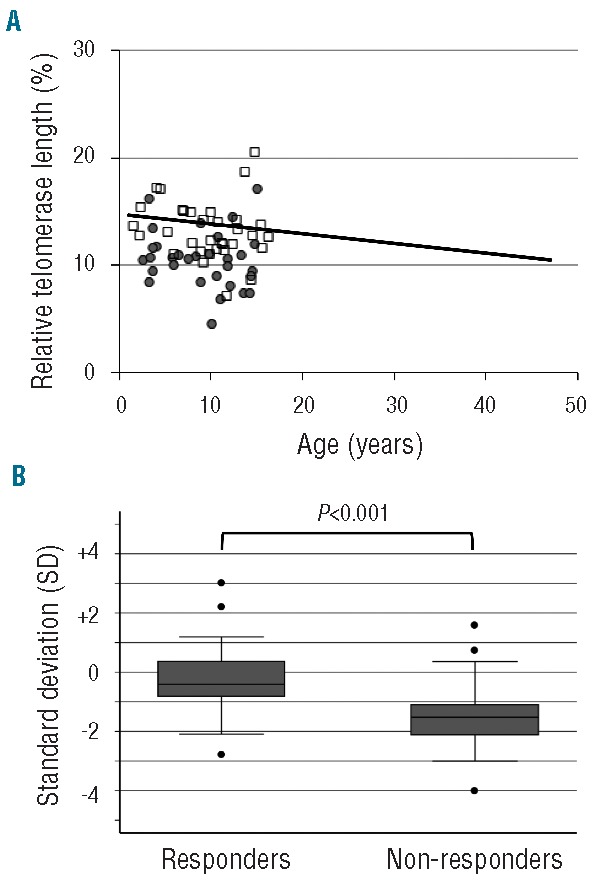
Relative telomere length in responders and non-responders. (A) Scatter plot of relative telomere length (RTL) versus age in patients with aplastic anemia (AA). The regression line for healthy individuals is shown as a solid line (Y= −0.0907X + 14.751). Results for AA patients were shown for responders (n=33; open squares) and non-responders (n=31; closed circles). (B) Comparison for telomere length between responders and non-responders. Box plots representing the distribution of telomere length in responders (n=33) and non-responders (n=31). The upper and lower limits of the boxes represent the 75th and 25th percentiles, respectively; the horizontal bar across the box indicates the median and the ends of the vertical lines indicate the minimum and maximum data values. Dots indicate outliers.
Figure 2.
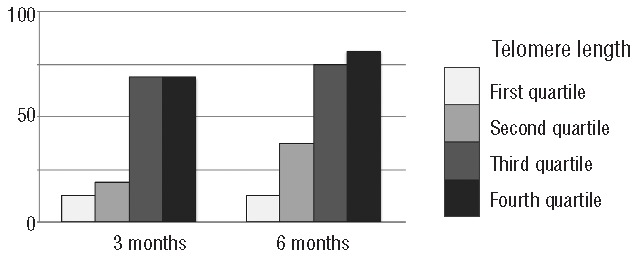
Response rates for immune suppressive therapy at three and six months according to telomere length. A poorer response rate was observed with each quartile as the telomere length shortened from the fourth to the first quartile.
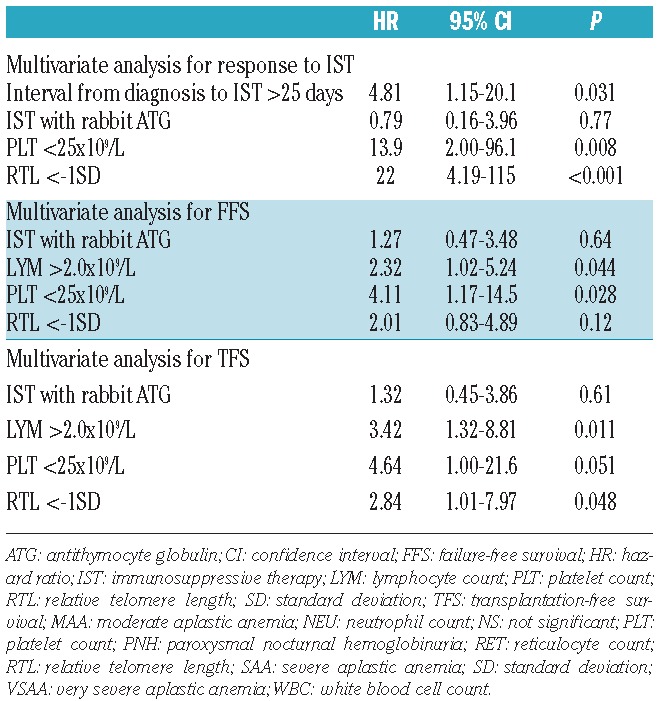
We evaluated the pre-treatment variables for predicting response to IST in 64 children with AA (Table 1). Univariate analysis showed that interval from diagnosis to IST longer than 25 days (P=0.01), platelet count at diagnosis less than 25×109/L (P=0.01), and telomere length shorter than −1SD (P<0.001) were the variables statistically significant for poor response to IST, while there were no significant differences between responders and non-responders in terms of patient age, sex, disease severity, WBC count, neutrophil count, lymphocyte count, reticulocyte count, presence of HLA-DR15, and presence of minor PNH clones. Patients with rabbit ATG showed a tendency of poorer response to IST than patients with horse ATG (P=0.08).
Multivariate analysis confirmed that telomere length shorter than −1.0SD (HR 22.0; 95%CI: 4.19–115; P<0.001), platelet count at diagnosis less than 25×109/L (HR 13.9; 95%CI: 2.00–96.1; P=0.008), and interval from diagnosis to IST longer than 25 days (HR 4.81; 95%CI: 1.15–20.1; P=0.031) were the significant predictive variables for poor response to IST (Table 2).
Table 2.
Multivariate analyses for poor response to IST, failure-free survival, and transplantation-free survival.
Discussion
Our study demonstrated that the measurement of telomere length of lymphocytes is useful for predicting the response to IST in patients with AA. Recently, the NIH group reported that the telomere length of peripheral blood leukocytes was not related to hematologic response to IST, but was associated with the high risk of hematologic relapse, clonal evolution to myelodysplastic syndrome, and OS.25 Several reasons may explain the conflicting results of the two studies. To begin with, there are several differences between the current study and the NIH study, including the methods of telomere length measurement and patients’ characteristics. In the NIH study, the telomere length of pre-treatment total leukocytes was assessed by quantitative polymerase chain reaction (PCR). We measured the telomere length of lymphocytes using flow-FISH, which enabled us to measure median telomere length in the subpopulations of blood cells. Alter et al. compared the diagnostic sensitivity and specificity of short telomeres in different subpopulations of blood cells.29 Their results indicated that lymphocytes were more suitable for diagnosis of DC than total leukocytes, which were a heterogeneous mixture of cell populations. The proportions of each cell population were different in each patient. The use of total leukocytes is suspected to provide less consistent results than analyses of defined leukocyte subpopulations.
Another difference between the two studies was the distribution of patients’ age. Patients in our study were much younger (mean age 10 years) than those in the NIH study (mean age 35 years). Because telomeres shorten with age,30 the differences in telomere length between patients and healthy individuals may become smaller in adults than in children. Moreover, in the NIH study, the cohort was restricted to patients with severe AA, and patients with moderate AA were not included. In contrast, 20 of 64 AA patients in our study had moderate disease. We could not estimate the frequency of clonal evolution since in our cohort there was only one patient who evolved into myelodysplastic syndrome during the observation period.
The causes of the difference in telomere length between responders and non-responders remain unknown. The short telomere length in non-responders may be ascribed to the presence of cryptic forms of IBMFS in the study cohort. Alter et al. reported that nearly all of the patients with both typical and cryptic DC have very short telomeres, as low as the first percentile of normal controls.25 In our previous study, the RTLs of lymphocytes were below the 5% of normal controls in all of 6 DC patients and 2 AA patients harboring TERT mutation.31 In the current study, there were 10 AA patients with shorter telomere length than the −2.0 SD of the cohort of healthy controls, but none of them showed clinical features of DC or had any mutation in DKC1, TERC, TERT, NOP10, TINF2, and TCAB1. It is unlikely that short telomeres in non-responders are to be ascribed to the presence of a cryptic form of DC.
Another possibility is that short telomere length may be a surrogate marker for longer disease duration that damages the hematopoietic stem cells and causes a higher number of compensatory stem cell divisions. We recently reported a significant inverse correlation between response rate to IST and interval between diagnosis and treatment in a large cohort of 312 children with newly diagnosed AA.32 It is often difficult to determine the exact date of onset of the disease in patients with AA, especially in patients with moderate AA. The shorter telomere length may simply reflect longer duration of the disease in non-responders.
However, our study has several limitations, including a heterogeneous study population, a relatively small number of patients and a short follow-up period. To validate the results, we are conducting a prospective study to determine the optimal use of rabbit ATG for severe AA, in which we evaluate the relationship between telomere length of lymphocytes at diagnosis and the response to IST.
In conclusion, measurement of the telomere length in lymphocytes by flow-FISH is a promising assay, not only for identifying cryptic DC, but also for predicting the response to IST of patients with AA.
Acknowledgments
The authors would like to thank all contributors associated with the Japan Childhood Aplastic Anemia Study Group.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
The authors supported by a grant from the Research Commitee for the dyskeratosis congenita, Ministry of Halth, and Walfare of Japan (H23-Nanchi-Japan-099) and a grant from Sanofi K.K..
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org
References
- 1.Kojima S, Horibe K, Inaba J, Yoshimi A, Takahashi Y, Kudo K, et al. Long-term outcome of acquired aplastic anaemia in children: comparison between immunosuppressive therapy and bone marrow transplantation. Br J Haematol. 2000;111(1):321–8 [DOI] [PubMed] [Google Scholar]
- 2.Young NS. Acquired aplastic anemia. JAMA. 1999;282(3):271–8 [DOI] [PubMed] [Google Scholar]
- 3.Kojima S, Hibi S, Kosaka Y, Yamamoto M, Tsuchida M, Mugishima H, et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood. 2000;96(6):2049–54 [PubMed] [Google Scholar]
- 4.Fuhrer M, Rampf U, Baumann I, Faldum A, Niemeyer C, Janka-Schaub G, et al. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood. 2005;106(6):2102–4 [DOI] [PubMed] [Google Scholar]
- 5.Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2007;92(1):11–8 [DOI] [PubMed] [Google Scholar]
- 6.Nakao S, Takamatsu H, Chuhjo T, Ueda M, Shiobara S, Matsuda T, et al. Identification of a specific HLA class II haplotype strongly associated with susceptibility to cyclosporine-dependent aplastic anemia. Blood. 1994;84(12):4257–61 [PubMed] [Google Scholar]
- 7.Maciejewski JP, Follmann D, Nakamura R, Saunthararajah Y, Rivera CE, Simonis T, et al. Increased frequency of HLA-DR2 in patients with paroxysmal nocturnal hemoglobinuria and the PNH/aplastic anemia syndrome. Blood. 2001;98(13):3513–9 [DOI] [PubMed] [Google Scholar]
- 8.Oguz FS, Yalman N, Diler AS, Oguz R, Anak S, Dorak MT. HLA-DRB1*15 and pediatric aplastic anemia. Haematologica. 2002;87(7):772–4 [PubMed] [Google Scholar]
- 9.Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, et al. Minor population of CD55–CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood 2006;107:1308–14 [DOI] [PubMed] [Google Scholar]
- 10.Yoshida N, Yagasaki H, Takahashi Y, Yamamoto T, Liang J, Wang Y, et al. Clinical impact of HLA-DR15, a minor population of paroxysmal nocturnal haemoglobinuria-type cells, and an aplastic anaemia-associated autoantibody in children with acquired aplastic anaemia. Br J Haematol. 2008;142(3):427–35 [DOI] [PubMed] [Google Scholar]
- 11.Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol. 2009;144(2):206–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walne AJ, Dokal I. Advances in the understanding of dyskeratosis congenita. Br J Haematol. 2009;145:164–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43(2 Pt 1):405–13 [DOI] [PubMed] [Google Scholar]
- 14.Szostak JW, Blackburn EH. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982;29(1):245–55 [DOI] [PubMed] [Google Scholar]
- 15.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–5 [DOI] [PubMed] [Google Scholar]
- 16.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352(14):1413–24 [DOI] [PubMed] [Google Scholar]
- 18.Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–5 [DOI] [PubMed] [Google Scholar]
- 19.Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JC, Gordon-Smith EC. Progressive telomere shortening in aplastic anemia. Blood. 1998;91(10):3582–92 [PubMed] [Google Scholar]
- 20.Brummendorf TH, Maciejewski JP, Mak J, Young NS, Lansdorp PM. Telomere length in leukocyte subpopulations of patients with aplastic anemia. Blood. 2001;97(4):895–900 [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, Young NS. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102(3):916–8 [DOI] [PubMed] [Google Scholar]
- 22.Leteurtre F, Li X, Guardiola P, Le Roux G, Sergère JC, Richard P, et al. Accelerated telomere shortening and telomerase activa tion in Fanconi’s anaemia. Br J Haematol. 1999;105(4):883–93 [DOI] [PubMed] [Google Scholar]
- 23.Pavesi E, Avondo F, Aspesi A, Quarello P, Rocci A, Vimercati C, et al. Analysis of telomeres in peripheral blood cells from patients with bone marrow failure. Pediatr Blood Cancer. 2009;53(3):411–6 [DOI] [PubMed] [Google Scholar]
- 24.Thornley I, Dror Y, Sung L, Wynn RF, Freedman MH. Abnormal telomere shortening in leucocytes of children with Shwachman-Diamond syndrome. Br J Haematol. 2002;117(1):189–92 [DOI] [PubMed] [Google Scholar]
- 25.Scheinberg P, Cooper JN, Sloand EM, Wu CO, Calado RT, Young NS. Association of Telomere Length of Peripheral Blood Leukocytes With Hematopoietic Relapse, Malignant Transformation, and Survival in Severe Aplastic Anemia. JAMA. 2010;304(12):1358–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosaka Y, Yagasaki H, Sano K, Kobayashi R, Ayukawa H, Kaneko T, et al. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem-cell transplantation from an alternative donor as second-line treatment for children with severe and very severe aplastic anemia. Blood. 2008;111:1054–9 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi Y, Muramatsu H, Sakata N, Hyakuna N, Hamamoto K, Kobayashi R, et al. Rabbit antithymocyte globulin and cyclosporine as first-line therapy for children with acquired aplastic anemia. Blood. 2013;121:862–3 [DOI] [PubMed] [Google Scholar]
- 28.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc. 2006;1(5):2365–76 [DOI] [PubMed] [Google Scholar]
- 29.Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110(5):1439–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–60 [DOI] [PubMed] [Google Scholar]
- 31.Nishio N, Kojima S. Recent progress in dyskeratosis congenita. Int J Hematol. 2010;92(3):419–24 [DOI] [PubMed] [Google Scholar]
- 32.Yoshida N, Yagasaki H, Hama A, Takahashi Y, Kosaka Y, Kobayashi R, et al. Predicting response to immunosuppressive therapy in childhood aplastic anemia. Haematologica. 2011;96(5):771–4 [DOI] [PMC free article] [PubMed] [Google Scholar]


