Abstract
Bilateral thalamic inflammation in the presence of a clinical picture suggestive of viral encephalitis should raise concern for West Nile virus infection.
PEARLS
Bilateral thalamic inflammation in the presence of a clinical picture suggestive of viral encephalitis should raise concern for West Nile virus infection.
Other members of the Japanese encephalitis serocomplex, particularly Japanese encephalitis, may give a similar radiologic appearance.
Other causes of bilateral thalamic injury include influenza A and ischemic infarction involving the artery of Percheron.
OY-STERS
Initial serology for West Nile infection frequently returns false-negative results, particularly in patients who are immunosuppressed.
PATIENT 1
A 46-year-old man with a history of pancreas and kidney transplants presented after 3 weeks of progressive abdominal pain, emesis, fever, and diffuse weakness. He was receiving mycophenolate mofetil and tacrolimus for immunosuppression related to organ transplant. He developed worsening weakness and altered mental status, eventually becoming comatose. CSF collected 3 days after admission showed 69 nucleated cells (85% lymphocytes, 2% neutrophils, 13% monocytes), 7 red blood cells, a glucose concentration of 53 mg/dL (appropriate for serum glucose concentration), and a protein level of 100 mg/dL, consistent with viral encephalitis. Initial ELISA was negative for West Nile virus (WNV) immunoglobulin G (IgG) and immunoglobulin M (IgM). Brain MRI showed signal hyperintensity in bilateral thalamic nuclei on fluid-attenuated inversion recovery (FLAIR), extending to the lower cervical spinal cord (figure, A). Foci of restricted diffusion were seen in bilateral thalamic nuclei, the medulla, and the tegmentum (figure, B). CSF collected 11 days after admission subsequently returned positive for WNV infection on ELISA IgM, microsphere immunofluorescence assay IgM (MIA), and real-time qualitative PCR.
Figure. MRI.
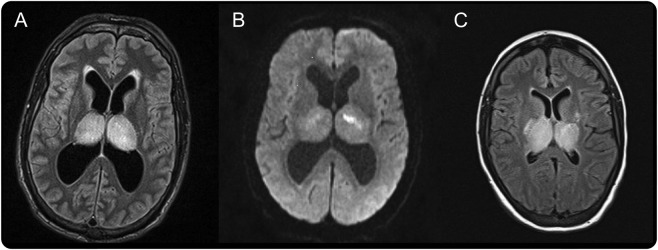
MRI shows fluid-attenuated inversion recovery (FLAIR) hyperintensity (A) and diffusion restriction on diffusion-weighted imaging (B) in bilateral thalamic nuclei in a 46-year-old man, and FLAIR hyperintensity (C) in bilateral thalamic nuclei in a 23-year-old woman.
PATIENT 2
A 23-year-old woman with a history of cardiac and renal transplants presented with several days of progressive abdominal pain, emesis, and diarrhea. She was receiving mycophenolate mofetil and tacrolimus for immunosuppression related to organ transplant. She gradually became comatose and developed status epilepticus. CSF collected on admission showed 160 nucleated cells (90% lymphocytes, 6% neutrophils, 4% monocytes), 2,350 erythrocytes, a glucose concentration of 76 mg/dL (appropriate for serum glucose concentration), and a protein level of 153 mg/dL, consistent with viral encephalitis. WNV ELISA returned positive at one laboratory for IgG and IgM and negative at a second. MIA was positive for IgM on initial CSF, and repeat CSF collected 14 days after admission was positive for IgG and IgM. Brain MRI also showed signal hyperintensity in bilateral thalamic nuclei on FLAIR imaging extending to the brainstem (figure, C).
DISCUSSION
WNV encephalitis (WNVE) outbreaks are increasingly common in the United States. According to the Centers for Disease Control and Prevention, in 2012, 2,873 cases of neuroinvasive WNV were reported in the United States, with over 5,600 total infections and 243 reported deaths. This incidence is dramatically increased from 2011, when 486 cases of neuroinvasive WNV were reported.1 The signs and symptoms of WNVE are generally nonspecific and can make diagnosis challenging. The most common symptoms found are fever, diffuse weakness, fatigue, headache, and confusion.2 When found, acute flaccid paralysis due to myelitis, cranial neuropathies, myoclonus, and meningitis may aid in diagnosis. Radiologic findings can also be important in the diagnosis of WNVE because initial serology is often falsely negative.2–4 False-negative results may be particularly common when patients are immunosupressed.2–4
WNV may preferentially affect the thalamic nuclei.2–6 This finding is similar to other members of the Japanese encephalitis serocomplex, which includes Japanese encephalitis, Murray Valley encephalitis, Kunjin encephalitis, and St. Louis encephalitis.7–9 However, this finding is not always present.2,6 Neuroimaging is frequently normal, with normal MRI findings in up to 30% of cases. Hyperintensity seen on T2 and FLAIR imaging with normal intensity on T1 is typical of WNVE, and diffusion restriction can be seen in up to 50% of cases. A predilection for the thalami, basal ganglia, brainstem, and cerebellum has been reported.2 While bilateral thalamic inflammation occurs in other diseases, identification of this radiologic pattern should raise suspicion for WNVE.10 Repeat CSF testing may be indicated when initial serology is negative.
AUTHOR CONTRIBUTIONS
Dr. Guth: drafting/revising the manuscript for content, study concept, analysis of data. Dr. Futterer: drafting/revising the manuscript for content. Dr. Hijaz: drafting/revising the manuscript for content. Dr. Liotta: drafting/revising the manuscript for content. Dr. Rosenberg: drafting/revising the manuscript for content. Dr. Naidech: drafting/revising the manuscript for content. Dr. Maas: drafting/revising the manuscript for content.
STUDY FUNDING
This study was departmentally funded.
DISCLOSURE
J. Guth, S. Futterer, T. Hijaz, E. Liotta, and N. Rosenberg report no disclosures relevant to the manuscript. A. Naidech was a medical safety monitor for 2 unrelated NIH funded trials and received unrelated research funding from the Northwestern Memorial Foundation. M. Maas receives support from the NIH grant L30 NS080176. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Centers for Disease Control and Prevention. Statistics and maps. Available at: http://www.cdc.gov/westnile/statsMaps/. Accessed June 11, 2013
- 2.Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol 2006;60:286–300 [DOI] [PubMed] [Google Scholar]
- 3.Kleinschmidt-DeMasters BK, Marder BA, Levi ME, et al. Naturally acquired west Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Arch Neurol 2004;61:1210–1220 [DOI] [PubMed] [Google Scholar]
- 4.Kumar D, Prasad GV, Zaltzman J, Levy GA, Humar A. Community-acquired west Nile virus infection in solid-organ transplant recipients. Transplantation 2004;77:399–402 [DOI] [PubMed] [Google Scholar]
- 5.Bosanko CM, Gilroy J, Wang AM, et al. West Nile virus encephalitis involving the substantia nigra: neuroimaging and pathologic findings with literature review. Arch Neurol 2003;60:1448–1452 [DOI] [PubMed] [Google Scholar]
- 6.Rosas H, Wippold FJ., II West Nile virus: case report with MR imaging findings. AJNR Am J Neuroradiol 2003;24:1376–1378 [PMC free article] [PubMed] [Google Scholar]
- 7.Abe T, Kojima K, Shoji H, et al. Japanese encephalitis. J Magn Reson Imaging 1998;8:755–761 [DOI] [PubMed] [Google Scholar]
- 8.Burrow JN, Whelan PI, Kilburn CJ, Fisher DA, Currie BJ, Smith DW. Australian encephalitis in the northern territory: clinical and epidemiological features, 1987–1996. Aust NZ J Med 1998;28:590–596 [DOI] [PubMed] [Google Scholar]
- 9.Reyes MG, Gardner JJ, Poland JD, Monath TP. St Louis encephalitis. quantitative histologic and immunofluorescent studies. Arch Neurol 1981;38:329–334 [DOI] [PubMed] [Google Scholar]
- 10.Iijima H, Wakasugi K, Ayabe M, Shoji H, Abe T. A case of adult influenza A virus–associated encephalitis: magnetic resonance imaging findings. J Neuroimaging 2002;12:273–275 [PubMed] [Google Scholar]


