Abstract
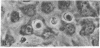
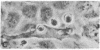
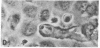
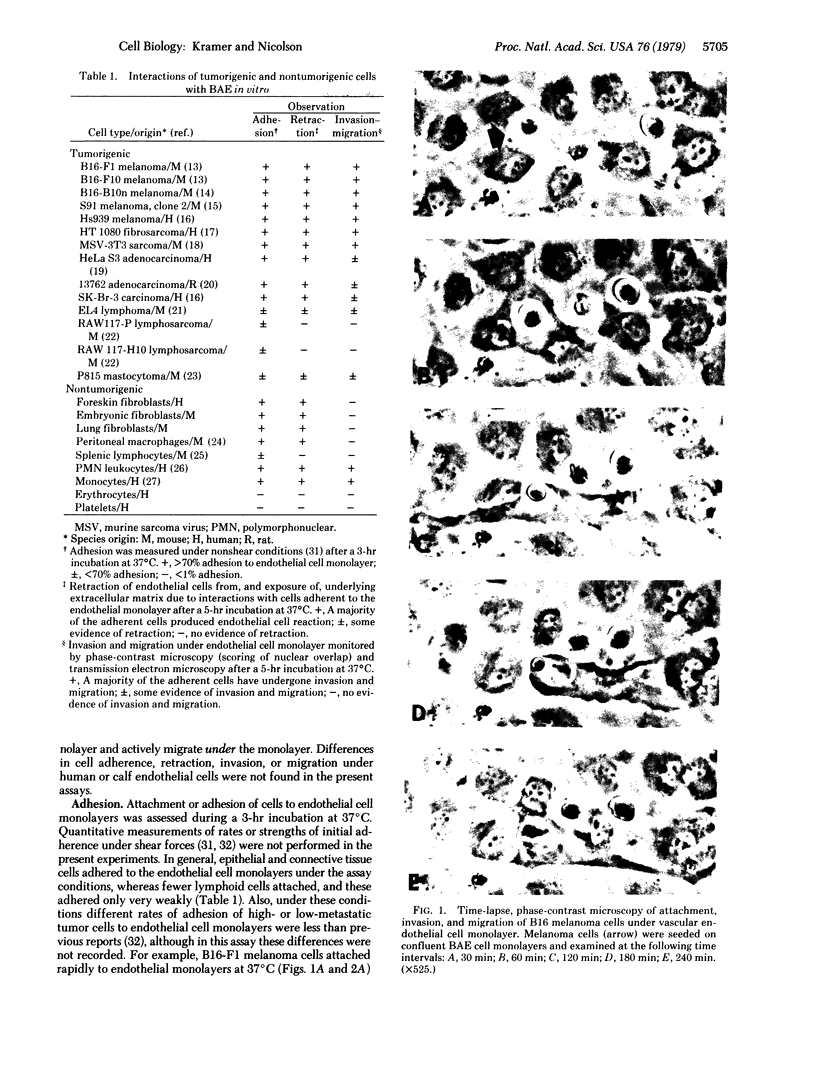
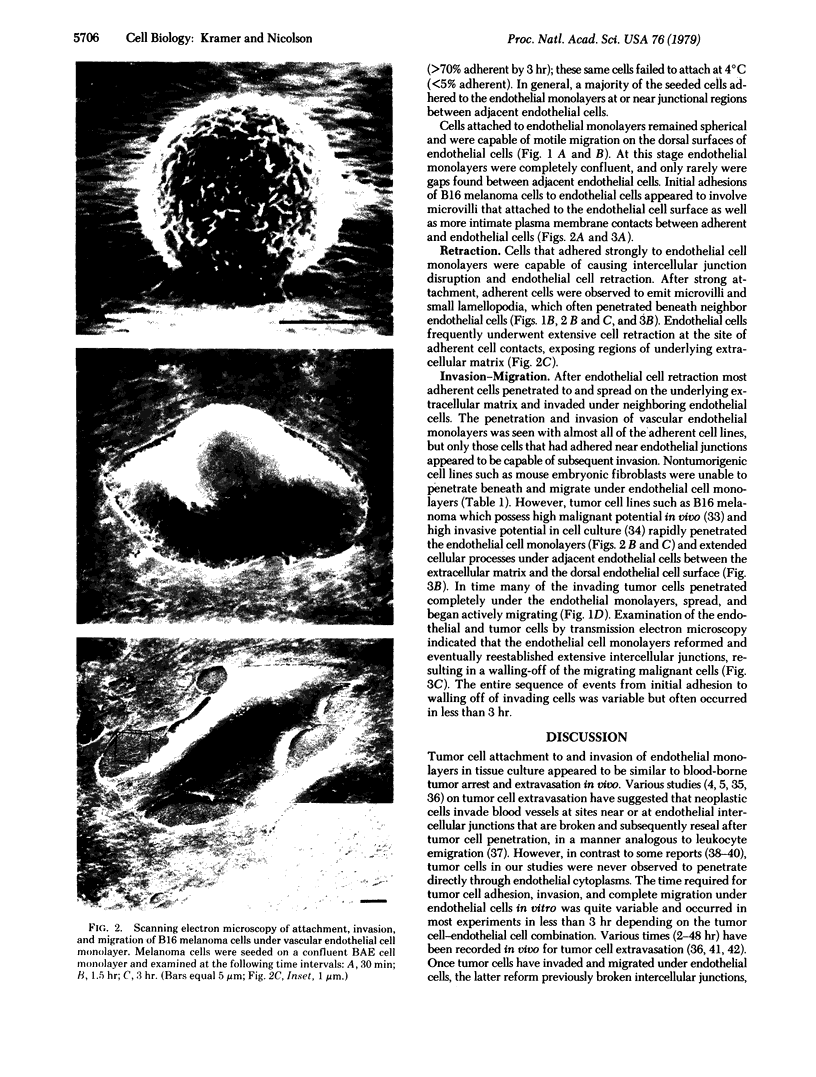
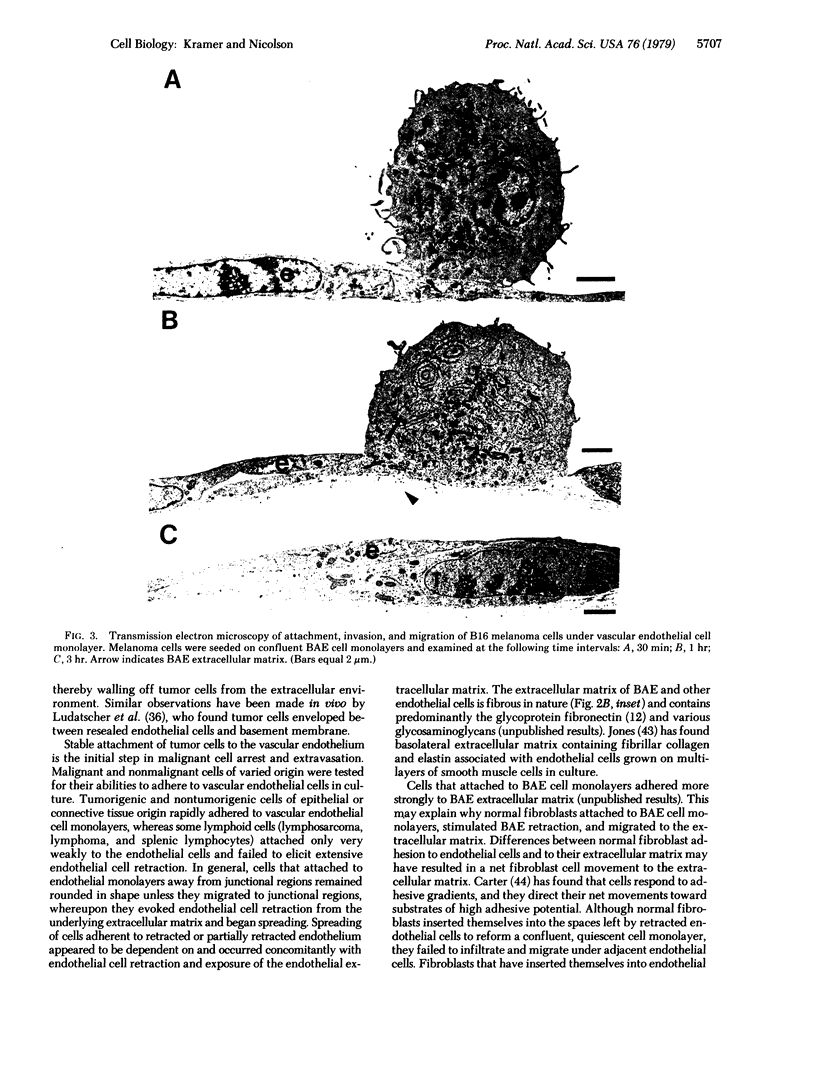
The interactions of tumorigenic and nontumorigenic human and rodent cells with vascular endothelial cells and their underlying extracellular matrix were studied in culture. The abilities of various cells to attach to endothelial monolayers and cause morphologic changes, such as rupture of endothelial-endothelial cell interactions leading to retraction of endothelial cells and exposure of extracellular matrix, as well as their propensities to invade and underlap retracted endothelial monolayers and continue migration were assessed by time-lapse and phase-contrast microscopy as well as scanning and transmission electron microscopy. In general, highly malignant or highly invasive cells in vivo were capable of attachment, invasion, and migration under endothelial cells in vitro. This system may be useful for elucidating mechanisms of tumor cell arrest and extravasation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., HEAYSMAN J. E. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res. 1953 Sep;5(1):111–131. doi: 10.1016/0014-4827(53)90098-6. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Birdwell C. R., Gospodarowicz D., Nicolson G. L. Identification, localization, and role of fibronectin in cultured bovine endothelial cells. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3273–3277. doi: 10.1073/pnas.75.7.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson K. W., Beattie G., Nicolsin G. L. Selection and altered properties of brain-colonising metastatic melanoma. Nature. 1978 Apr 6;272(5653):543–545. doi: 10.1038/272543a0. [DOI] [PubMed] [Google Scholar]
- Brunson K. W., Nicolson G. L. Selection and biologic properties of malignant variants of a murine lymphosarcoma. J Natl Cancer Inst. 1978 Dec;61(6):1499–1503. [PubMed] [Google Scholar]
- Carr I., McGinty F., Norris P. The fine structure of neoplastic invasion: invasion of liver, skeletal muscle and lymphatic vessels by the Rd/3 tumour. J Pathol. 1976 Feb;118(2):91–99. doi: 10.1002/path.1711180205. [DOI] [PubMed] [Google Scholar]
- Carter S. B. Principles of cell motility: the direction of cell movement and cancer invasion. Nature. 1965 Dec 18;208(5016):1183–1187. doi: 10.1038/2081183a0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans K. P. Behavior of intravenously injected malignant lymphoma cells. A morphologic study. J Natl Cancer Inst. 1973 Dec;51(6):1883–1895. doi: 10.1093/jnci/51.6.1883. [DOI] [PubMed] [Google Scholar]
- Dingemans K. P., Roos E., van den Bergh Weerman M. A., van de Pavert I. V. Invasion of liver tissue by tumor cells and leukocytes: comparative ultrastructure. J Natl Cancer Inst. 1978 Mar;60(3):583–598. doi: 10.1093/jnci/60.3.583. [DOI] [PubMed] [Google Scholar]
- Fasske E., Fetting R., Rühland D., Schubert T., Themann H. Die Kolonisation transplantierter virusbildender Leukämiezellen in der Leber der Maus. Elektronenmikroskopische Untersuchungen. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1975 Nov 25;84(3):251–269. doi: 10.1007/BF00312247. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975 Jan;35(1):218–224. [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978 Sep;38(9):2651–2660. [PubMed] [Google Scholar]
- Fonck-Cussac Y., Delage J., Petit J. Observations ultrastructurales sur le mode d'implantation endovasculaire des métastases d'un cancer bronchique. Poumon Coeur. 1969;25(3):231–234. [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Bialecki H., Zetter B. R. Factors involved in the modulation of cell proliferation in vivo and in vitro: the role of fibroblast and epidermal growth factors in the proliferative response of mammalian cells. In Vitro. 1978 Jan;14(1):85–118. doi: 10.1007/BF02618177. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J., Braun D., Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975 Apr 10;250(7):2515–2520. [PubMed] [Google Scholar]
- Granger G. A., Kolb W. P. Lymphocyte in vitro cytotoxicity: mechanisms of immune and non-immune small lymphocyte mediated target L cell destruction. J Immunol. 1968 Jul;101(1):111–120. [PubMed] [Google Scholar]
- Hoover R. L., Briggs R. T., Karnovsky M. J. The adhesive interaction between polymorphonuclear leukocytes and endothelial cells in vitro. Cell. 1978 Jun;14(2):423–428. doi: 10.1016/0092-8674(78)90127-7. [DOI] [PubMed] [Google Scholar]
- Jones P. A. Construction of an artificial blood vessel wall from cultured endothelial and smooth muscle cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1882–1886. doi: 10.1073/pnas.76.4.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J., Goldblatt P. J., Leighton J. Ultrastructural features of invasion in chick embryo liver metastasis of Yoshida ascites hepatoma. Cancer Res. 1970 Jun;30(6):1632–1644. [PubMed] [Google Scholar]
- Lotan R. Different susceptibilities of human melanoma and breast carcinoma cell lines to retinoic acid-induced growth inhibition. Cancer Res. 1979 Mar;39(3):1014–1019. [PubMed] [Google Scholar]
- Lotan R., Giotta G., Nork E., Nicolson G. L. Characterization of the inhibitory effects of retinoids on the in vitro growth of two malignant murine melanomas. J Natl Cancer Inst. 1978 May;60(5):1035–1041. doi: 10.1093/jnci/60.5.1035. [DOI] [PubMed] [Google Scholar]
- Ludatscher R. M., Luse S. A., Suntzeff V. An electron microscopic study of pulmonary tumor emboli from transplantable Morris hepatoma 5123. Cancer Res. 1967 Nov;27(11):1939–1952. [PubMed] [Google Scholar]
- MARCHESI V. T., FLOREY H. W. Electron micrographic observations on the emigration of leucocytes. Q J Exp Physiol Cogn Med Sci. 1960 Oct;45:343–348. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L. Specific partial depletion of graft-vs-host activity by incubation and centrifugation of mouse spleen cells on allogeneic spleen cell monolayers. J Immunol. 1975 Oct;115(4):911–913. [PubMed] [Google Scholar]
- Nicolson G. L., Birdwell C. R., Brunson K. W., Robbins J. C., Beattie G., Fidler I. J. Cell interactions in the metastatic process: some cell surface properties associated with successful blood-borne tumor spread. Soc Gen Physiol Ser. 1977;32:225–241. [PubMed] [Google Scholar]
- Nicolson G. L., Smith J. R., Poste G. Effects of local anesthetics on cell morphology and membrane-associated cytoskeletal organization in BALB/3T3 cells. J Cell Biol. 1976 Feb;68(2):395–402. doi: 10.1083/jcb.68.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Sindelar W. F., Tralka T. S., Ketcham A. S. Electron microscopic observations on formation of pulmonary metastases. J Surg Res. 1975 Feb;18(2):137–161. doi: 10.1016/0022-4804(75)90010-4. [DOI] [PubMed] [Google Scholar]
- Stewart C. C., Lin H. S., Adles C. Proliferation and colony-forming ability of peritoneal exudate cells in liquid culture. J Exp Med. 1975 May 1;141(5):1114–1132. doi: 10.1084/jem.141.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taptiklis N. Penetration of the vascular endothelial barrier by non-neoplastic thyroid cells in circulation. Eur J Cancer. 1969 Nov;5(5):445–457. doi: 10.1016/0014-2964(69)90098-x. [DOI] [PubMed] [Google Scholar]
- Vlaeminck M. N., Adenis L., Mouton Y., Demaille A. Etude expérimentale de la diffusion métastatique chez l'oeuf de poule embryonné. Répartition, microscopie et ultrastructure des foyers tumoraux. Int J Cancer. 1972 Nov;10(3):619–631. doi: 10.1002/ijc.2910100322. [DOI] [PubMed] [Google Scholar]
- WOOD S., Jr Pathogenesis of metastasis formation observed in vivo in the rabbit ear chamber. AMA Arch Pathol. 1958 Oct;66(4):550–568. [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelhake J. L., Nicolson G. L. Determination of adhesive properties of variant metastatic melanoma cells to BALB/3T3 cells and their virus-transformed derivatives by a monolayer attachment assay. J Natl Cancer Inst. 1976 Feb;56(2):285–291. doi: 10.1093/jnci/56.2.285. [DOI] [PubMed] [Google Scholar]
- ZEIDMAN I. Metastasis: a review of recent advances. Cancer Res. 1957 Apr;17(3):157–162. [PubMed] [Google Scholar]