ABSTRACT
The cell cortex is a dynamic and heterogeneous structure that governs cell identity and behavior. The ERM proteins (ezrin, radixin and moesin) are major architects of the cell cortex, and they link plasma membrane phospholipids and proteins to the underlying cortical actin cytoskeleton. Recent studies in several model systems have uncovered surprisingly dynamic and complex molecular activities of the ERM proteins and have provided new mechanistic insight into how they build and maintain cortical domains. Among many well-established and essential functions of ERM proteins, this Cell Science at a Glance article and accompanying poster will focus on the role of ERMs in organizing the cell cortex during cell division and apical morphogenesis. These examples highlight an emerging appreciation that the ERM proteins both locally alter the mechanical properties of the cell cortex, and control the spatial distribution and activity of key membrane complexes, establishing the ERM proteins as a nexus for the physical and functional organization of the cell cortex and making it clear that they are much more than scaffolds.
This article is part of a Minifocus on Establishing polarity. For further reading, please see related articles: ‘Establishment of epithelial polarity – GEF who's minding the GAP?’ by Siu Ngok et al. (J. Cell Sci. 127, 3205–3215). ‘Integrins and epithelial cell polarity’ by Jessica Lee and Charles Streuli (J. Cell Sci. 127, 3217–3225).
KEY WORDS: Actin cytoskeleton, Apical morphogenesis, Cell cortex, Ezrin, Moesin, Radixin
Introduction
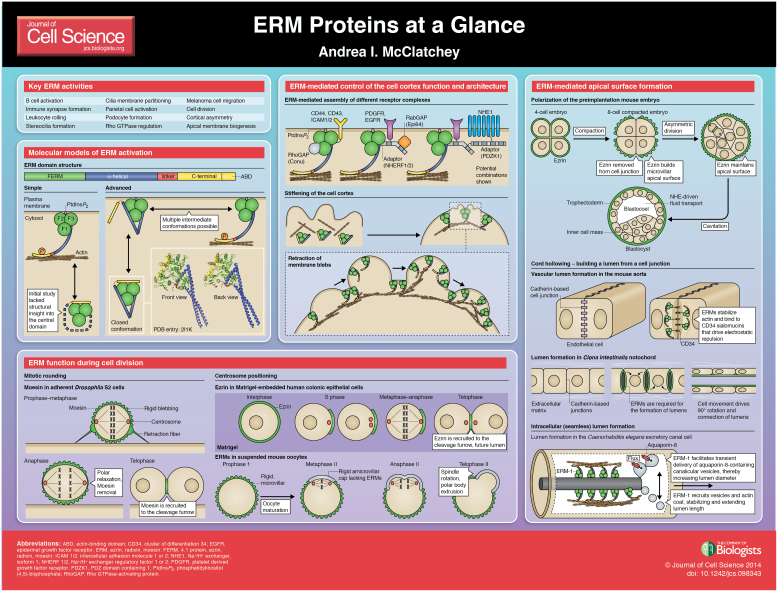
The ERM proteins (ezrin, radixin and moesin) form a highly conserved branch of the FERM (four-point-one, ezrin, radixin, moesin) domain superfamily of proteins and carry out many crucial cellular functions and carry out many crucial cellular functions (supplementary material Table S1). (Fehon et al., 2010). Important insight into ERM function has come from lower organisms, including the fruit fly Drosophila melanogaster, nematode Caenorhabditis elegans and tunicate Ciona intestinalis, the genomes of which harbor single ERM orthologs (Dong et al., 2011; Göbel et al., 2004; McCartney and Fehon, 1996; van Fürden et al., 2004). The three mammalian ERMs are widely expressed, and most cell types express multiple ERMs (Ingraffea et al., 2002). It is widely believed that mammalian ERMs perform at least overlapping functions, prompting the use of ‘dominant-active’ and ‘dominant-negative’ versions of ERMs in many studies. However, it is not clear that mammalian ERMs are truly functionally redundant. Moreover, recent studies have uncovered surprisingly complex and dynamic molecular properties of ERMs, suggesting that our understanding of the mechanisms of action of dominant-acting ERMs is incomplete.
As shown in the accompanying poster, initial molecular models depict a simple conformational switch that controls ERM ‘activity’, usually held as the ability to link the plasma membrane to the underlying actin cytoskeleton (Fehon et al., 2010). In the cytosolic state, intramolecular self-association masks protein interaction sites on both the N-terminal FERM domain and the C-terminal actin-binding domain (ABD). This ‘dormant’ conformation is relieved by phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2]-binding of the FERM domain and/or C-terminal phosphorylation, prompting translocation of the ‘active’ protein to the membrane–cytoskeleton interface. This simple model was based, in part, on early structural studies (Pearson et al., 2000). Crystallization of the full-length ERM protein is difficult, and this study analyzed the interaction between isolated FERM and C-terminal domains but lacked insight into the contribution of the intervening α-helical domain. Subsequent crystallization of a full-length insect ERM revealed that the α-helical domain can mask additional sites on the FERM domain, including the PtdIns(4,5)P2-binding site (Li et al., 2007). This might help to explain why PtdIns(4,5)P2-association is essential for subsequent C-terminal ERM phosphorylation in many contexts (Coscoy et al., 2002; Roch et al., 2010). This also suggests that the FERM domain might be regulated in modular fashion, yielding intermediate conformational states. Indeed, a recent proteomic study supports the existence of multiple conformational states for ezrin and has even identified proteins that specifically interacted with the closed, assumed-to-be dormant, form of ezrin (Viswanatha et al., 2013). Thus the early model is significantly oversimplified; instead, the ERMs likely adopt multiple ‘activation states’ at the plasma membrane.
The ERMs can be phosphorylated on several serine/threonine and tyrosine residues (Clucas and Valderrama, 2014; Parameswaran and Gupta, 2013). Best-studied is the conserved C-terminal threonine that lies at the interface between the C-terminal and FERM domains in the self-associated conformation (T567 in mammalian ezrin; Pearson et al., 2000). ERMs harboring phosphomimetic or non-phosphorylatable residues are often used as dominant ‘active’ and ‘inactive’ proteins. However, recent studies have revealed that dephosphorylation of this site is important in many contexts, and in some cases, a cycle of phosphorylation and de-phosphorylation is actually essential to ERM activity (Kunda et al., 2012; Roubinet et al., 2011; Viswanatha et al., 2012). Such ‘phosphocycling’ of ezrin has recently been shown to be necessary for the formation of apical microvilli (Viswanatha et al., 2012). In fact, expression of the phosphomimetic ezrinT567D can lead to both loss and exaggerated stabilization of apical microvilli (Brown et al., 2003; Göbel et al., 2004; Parameswaran et al., 2011; Saotome et al., 2004). Many kinases have been reported to phosphorylate this site, and particularly strong evidence implicates SLK family kinases in both flies and mammalian cells. In flies, the phosphatase PP1-87B/Sds22 (also known as PPP1R7 in mammals) can reverse that phosphorylation (Grusche et al., 2009; Kunda et al., 2012; Parameswaran and Gupta, 2013; Roubinet et al., 2011). Mounting evidence suggests that dephosphorylation and likely active phospho-cycling of ERMs is important, but little is known with regard to how it is spatiotemporally regulated in vivo. Even less is known about the dynamic phosphorylation of other residues, including a conserved threonine in the FERM domain that is predicted to directly appose T567 in the self-associated conformation (Yang and Hinds, 2003).
The cloverleaf-shaped FERM domain can interact with numerous membrane-associated proteins, including transmembrane receptors, small GTPase regulators and adapters (Fehon et al., 2010; McClatchey and Fehon, 2009; Neisch et al., 2013). Recent studies of just two of these interacting partners highlight the molecular complexity of ERM-associations. The ERM FERM domain binds with high affinity to the tandem PDZ-domain-containing adaptors Na+/H+ exchanger regulatory factor 1 and 2 (NHERF1 and NHERF2; also known as EBP50 and E3KARP, respectively) (Fehon et al., 2010). This interaction impedes ERM self-association, alters FERM domain conformation in a manner that reduces its affinity for other transmembrane proteins and prevents self-association of NHERF1 itself. NHERF1 and NHERF2 can, in turn, associate with other membrane proteins, including receptors, such as Na+/H+ exchanger 3 (NHE3) and epidermal growth factor receptor (EGFR), and regulatory factors, such as the RabGAP EBP50-PDZ interactor of 64 kDa (Epi64) and the NHERF1/2 homolog PDZK1 (NHERF3) that itself has four PDZ domains (Fehon et al., 2010). Therefore, the ERMs can assemble a diverse and highly regulated array of multiprotein complexes at the cell cortex.
Controlling the mechanical properties of the cell cortex
In addition to assembling membrane complexes, the ERMs provide spatially controlled links between the membrane and underlying actin cytoskeleton. This function of ERMs in membrane–cytoskeleton crosslinking has long been appreciated, but recent studies of ERM function during cell division have provided crucial insights into how cells exploit this activity to organize the mechanical properties of the cortex. Insight into the multiple functions of ERMs during cell division comes from studies of both Drosophila and mammalian cells. For example, in adherent Drosophila S2 cells Moesin is necessary and sufficient for mitotic rounding, during which the cell cortex assumes a more rigid conformation (Carreno et al., 2008; Kunda et al., 2008; Thery and Bornens, 2008). Atomic force microscopy has revealed that Moesin stiffens the cortex of S2 cells during mitotic rounding; in the absence of Moesin the cortex remains floppy and unstable. Drosophila Moesin is also important for the retraction of membrane blebs that form to accommodate the reduction in membrane area that occurs during mitotic rounding. Similarly, ezrin is required for bleb retraction in constitutively blebbing mammalian M2 macrophages (Charras et al., 2006). A key function of mitotic rounding is to provide a mechanically stable structure that guides the formation and functional integrity of the mitotic spindle (Thery and Bornens, 2008). Indeed, loss of Moesin in S2 cells yields multiple defects in spindle morphogenesis and function that appear to be driven by the defective interaction of astral microtubules with an unstable cortex (Carreno et al., 2008; Kunda et al., 2008). In fact, recent studies suggest that Drosophila Moesin can also associate directly with microtubules, albeit with low affinity, and that this activity is essential for communication between the mitotic spindle and cortical actin cytoskeleton during cell division (Solinet et al., 2013). In normal cells, the subsequent removal of ERMs from the cell poles during anaphase relaxes the rigid cortex, guiding cell elongation and chromosome segregation. Recent studies suggest that this is driven by the PP1-87B/Sds22 phosphatase, which reverses C-terminal Moesin phosphorylation specifically at the polar cortex (Kunda et al., 2012; Roubinet et al., 2011). The ERMs then become progressively restricted to the contracting cleavage furrow where they may have additional mechanical functions during cytokinesis (see poster).
Cortical ezrin also undergoes a dynamic cell cycle-dependent redistribution in mammalian colonic epithelial (Caco2) cells; studies of this phenomenon uncovered a direct role for ezrin in orienting the centrosome and mitotic spindle (Hebert et al., 2012). When embedded in Matrigel, individual Caco2 cells undergo a well-studied program of polarized cyst formation (Jaffe et al., 2008). Recent studies have revealed that in single embedded Caco2 cells, ezrin is progressively restricted from a uniform cortical distribution into a discrete actin-rich cap-like structure in the absence of external spatial cues (Hebert et al., 2012). The ezrin ‘cap’ forms just prior to S phase and instructively positions the interphase centrosome and one pole of the ensuing mitotic spindle, likely providing a stiff platform for astral microtubule attachment. At the metaphase-to-anaphase transition, ezrin vanishes from the polar cortex and reappears at the cleavage furrow, which becomes the nascent apical lumen around which cells divide to form a polarized cyst (Hebert et al., 2012).
An intriguing comparison is also provided by studies of cortical ERM function during oocyte meiosis (Larson et al., 2010). The immature mammalian oocyte cortex is covered with ERM-containing microvilli. Meiotic maturation is characterized by the formation of a more rigid amicrovillar ‘cap’ that is rich in actin and myosin II, lacks ERMs and is surrounded by the ERM-containing microvillar cortex. The amicrovillar cap sequesters the meiotic spindle, guiding its rotation to produce the second polar body. Interfering with ERM function reduces the effective tension across the cortex and blocks spindle rotation, impairing normal meiosis (Larson et al., 2010).
Studies in S2 cells, Caco2 cells and oocytes all conclude that cortical ERMs play a crucial role in the formation, orientation and function of the mitotic spindle, but feature distinct patterns of cortical ERM distribution across the cell cycle. These differences likely reflect the different physical environments that the cells experience, that is, attached ventrally to a rigid substrate versus embedded in a soft substrate versus suspended in a fluidic milieu. Thus, although cultured S2 cells have to completely transform their cortex during mitotic rounding, embedded Caco2 cells and oocytes are already spherical and do not ‘round-up’; this unmasks their ability to intrinsically establish polarity by restricting cortical ERMs in a manner that correlates with centrosome or spindle position. Cells in tissues divide and orient their spindles within an existing mechanical environment that includes both cell–substrate and cell–cell attachments. These studies of individual cells lay the foundation for understanding how the dynamic spatial distribution of cortical ERMs guides the mechanical properties of cells that move and divide within multicellular tissues.
Building an apical surface
Studies of ERMs in single cells illustrate the importance of ERM activity in controlling the mechanical properties of the cell cortex and in establishing cortical asymmetry. This provides a new framework for considering the well-known function of ERMs in establishing apical polarity in multicellular contexts. Although it might be possible to establish apicobasal polarity in the absence of ERM activity in some contexts, mounting evidence indicates that the ERMs are fundamental drivers of polarity. In fact, the asymmetric restriction of ezrin to the outer, apical surface of the compacting eight-cell mouse embryo is one of the earliest indications of polarity during mammalian development (Louvet et al., 1996). During compaction, ezrin is removed from cell–cell contacts, which is essential for their maturation, and concentrated at the outer surface, where it is required for the formation of a primitive, microvillus-containing apical pole. This pole, in turn, orients the ensuing mitotic spindle, guiding an asymmetric division that distinguishes outer trophectoderm cells from unpolarized inner cell mass (ICM) cells and defines the first lineage segregation in the embryo (Dard et al., 2008). Apical Na+ influx, mediated by the ezrin-regulated Na+/H+ exchanger NHE3, then drives vectorial fluid transport across the trophectoderm, expanding the blastocoel cavity (Dard et al., 2008; Hayashi et al., 2013). Thus ezrin drives the formation of the first functional apical surface in the developing embryo.
The ERM proteins localize to the apical surfaces of many epithelia and are essential for establishing apical identity and architecture across many organisms. This is best illustrated by studies of the conserved role of ERMs in building an apical lumen during tubulogenesis. In contrast to the convex outer apical surface of the blastocyst, tubulogenesis is driven by the formation of a concave inner apical lumen. Lumens can form through several different cellular mechanisms, including cell rearrangement (i.e. invagination of an existing epithelium), cavitation involving the death of inner nonpolarized cells, or the de novo creation or ‘hollowing’ of apical surface either within the junctional surface between two or more cells (cord hollowing) or intracellularly (cell hollowing) (Andrew and Ewald, 2010; Datta et al., 2011; Iruela-Arispe and Beitel, 2013). ERM function has been implicated in all of these, but studies of ERM function in cord or cell hollowing have yielded the most insight into ERM function in apical domain formation.
During cord hollowing, cadherin-based cell junctions are converted into apical surfaces by a mechanism that is thought to involve vesicle delivery (Andrew and Ewald, 2010; Datta et al., 2011). The ERMs are central to this basic molecular principle, which is used in several different geometric settings. For example, development of the mouse aorta involves the moesin-dependent formation of extracellular lumens along the longitudinal cadherin-based junctions between paired endothelial cells (Strilić et al., 2009). During this process, moesin coordinates the recruitment of F-actin and myosin, which drives apical shape change, with that of CD34 sialomucins, ERM-associated transmembrane proteins whose negatively charged extracellular glycosyl residues are thought to be antiadhesive (Strilic et al., 2010). Moesin1 promotes lumen formation in zebrafish intersegmental vessels by a similar process, suggesting a broad role for moesin in vascular lumen morphogenesis (Wang et al., 2010). Alternatively, notochord tubulogenesis in the ascidian Ciona intestinalis involves the formation of lumens along each of many lateral junctions between notochord cells, followed by rotation and fusion of the lumens to create a continuous tube; lumens do not form in the absence of Ci-ERM (Dong et al., 2011). In another example, the first division of single Caco2 cells in 3D culture features the redistribution of ezrin from the polar ‘cap’ to the cleavage furrow, which is converted into a cadherin-based junction and then into an apical lumen; cells subsequently divide symmetrically around the new central lumen to form a multicellular cyst (Hebert et al., 2012; Jaffe et al., 2008). Ezrin is required for lumen formation in this setting and ectopic cortical ezrin yields ectopic lumens. Thus, the plane of division can provide an instructive cue for apical membrane formation, a mechanism that contributes to ezrin-mediated secondary lumen expansion during villus morphogenesis in the developing mouse intestine (Grosse et al., 2011; Saotome et al., 2004).
The importance of ERM function in apical membrane biogenesis is highlighted by studies of intracellular lumen formation where apical membrane formation occurs in the absence of cell junctions. For example, the Caenorhabditis elegans excretory cell assembles an internal actin-supported lumenal canal that spans the length of the worm (Zhang et al., 2013). During canal formation, ERM-1 coordinates the actin-based extension of apical surface with the transient delivery of aquaporin-8 that drives transluminal flux, thereby enhancing both lumen length and diameter (Khan et al., 2013). Both steps involve the permanent or transient delivery of vesicles to the growing lumen, suggesting that there is a direct role for ERM-1 in executing vesicle-to-plasma-membrane interactions. Such a model could also apply to the conversion of cell junction into apical membrane during cord hollowing, which is known to involve vesicle delivery; perhaps in that setting ERMs coordinate the delivery of apical components with the removal of junctional components through vesicular trafficking.
Apical elaboration
Beyond establishing apical domain identity, ERM function is essential for building specialized appendages that are elaborated from the apical surface. Best-studied are microvilli, finger-like actin-based structures that project from the apical surface of most epithelia, including the intestine and kidney, where they form exquisitely ordered arrays known as brush borders. Ezrin was originally identified as a microvillar component and microvilli do not form properly in its absence in vivo (Bonilha et al., 2006; Bretscher, 1983; Göbel et al., 2004; Saotome et al., 2004). Microvilli are traditionally thought of as static structures that serve to increase membrane surface area and receptor content. However, mounting evidence suggests that they are very dynamic and, at least in the intestine, can even act as conveyor belts that shed functional vesicles into the luminal space (McConnell et al., 2009). In fact, an important series of recent studies have yielded key insights into both the molecular function of ezrin and the unexpectedly dynamic molecular properties of microvilli. A key discovery was that ezrin undergoes constant phosphocycling and is regulated spatially within the microvillus through restricted localization and activation of the SLK and LOK kinases at the microvillus tip (Hanono et al., 2006; Viswanatha et al., 2012). Moreover, the association between ezrin and NHERF1, which is also required for microvillus formation, is highly regulated and unexpectedly dynamic within the microvillus, dashing the prevailing assumption that NHERF1 is a simple scaffold that tethers membrane proteins to the cytoskeleton through ezrin (Garbett and Bretscher, 2012; Garbett et al., 2010). Together with the discovery that NHERF1, like the ERMs, undergoes regulated intramolecular and intermolecular interactions (LaLonde et al., 2010), these studies suggest that ezrin and NHERF1 orchestrate the dynamic and spatially regulated assembly of perhaps many different membrane protein complexes along the length of the microvillus. This, in turn, likely governs the architecture and biology of the microvillus (Garbett and Bretscher, 2012).
Studies of ERM function in the rhabdomere, the expanded light-sensitive apical surface of the Drosophila photoreceptor, also suggest that there is a dynamic role for ERMs in functional apical membrane elaboration (Chorna-Ornan et al., 2005; Karagiosis and Ready, 2004). The rhabdomere is a microvillus-dense membrane that forms through the periscopic extension and 90-degree rotation of the photoreceptor apical membrane; the amplified membrane houses the light-sensitive signaling apparatus and holds it perpendicular to incoming light. The single Drosophila ERM, Moesin, localizes to the rhabdomere terminal web and is required for rhabdomere architecture and function (Karagiosis and Ready, 2004; Sengupta et al., 2013). Moesin associates with photosensitive transient receptor potential (TRP) channels only in the dark; this association is transiently relieved by light-induced dephosphorylation and translocation of Moesin from the cell membrane to the cytoplasm (Chorna-Ornan et al., 2005). Recent studies suggest that light-induced control of membrane PtdIns(4,5)P2 levels downstream of TRP channel activation is essential for maintaining the membrane localization of Drosophila Moesin and preventing degeneration of the rhabdomere microvillar membrane (Sengupta et al., 2013), providing an elegant example of how the ERMs can act at the nexus of receptor activity and membrane architecture.
Perspectives
The ERMs are clearly more dynamic and versatile than previously thought. Studies in lower organisms and single cells are providing crucial new insights into the fundamental mechanisms by which cells exploit the ERMs to establish molecular and mechanical asymmetry. These basic principles will provide a crucial foundation for understanding the role of ERM-mediated cortical organization in more complex multicellular contexts, including tissue morphogenesis, homeostasis and collective migration. A key goal of future studies will be to carefully study the three mammalian ERM proteins and determine whether they carry out distinct functions and/or are differentially regulated. This will require the development of unique tools that detect and interfere with the function of each ERM individually. An understanding of the molecular and cellular functions of mammalian ERMs will yield new insights into fundamental aspects of mammalian development and into established links between aberrant ERM activity and human disease processes, including pathogen–host interactions, deafness, microvillus inclusion disease, and cancer development and metastasis (Clucas and Valderrama, 2014; Dhekne et al., 2014; Hebert et al., 2012; Khan et al., 2007; Kubo et al., 2008; Skoudy et al., 1999).
Supplementary Material
Acknowledgments
I would like to thank all members of the McClatchey laboratory for discussion, comments and suggestions.
Footnotes
Competing interests
The author declares no competing interests.
Funding
A.I.M is funded by the National Institutes of Health; MGH Research Scholars Program; Ellison Foundation; O'Brien Trust; Children's Tumor Foundation; and Advocure. Deposited in PMC for release after 12 months.
Cell science at a glance
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.098343/-/DC2
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.098343/-/DC1
References
- Andrew D. J., Ewald A. J. (2010). Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev. Biol. 341, 34–55 10.1016/j.ydbio.2009.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha V. L., Rayborn M. E., Saotome I., McClatchey A. I., Hollyfield J. G. (2006). Microvilli defects in retinas of ezrin knockout mice. Exp. Eye Res. 82, 720–729 10.1016/j.exer.2005.09.013 [DOI] [PubMed] [Google Scholar]
- Bretscher A. (1983). Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J. Cell Biol. 97, 425–432 10.1083/jcb.97.2.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. J., Nijhara R., Hallam J. A., Gignac M., Yamada K. M., Erlandsen S. L., Delon J., Kruhlak M., Shaw S. (2003). Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERM proteins, which facilitates loss of microvilli and polarization. Blood 102, 3890–3899 10.1182/blood-2002-12-3807 [DOI] [PubMed] [Google Scholar]
- Carreno S., Kouranti I., Glusman E. S., Fuller M. T., Echard A., Payre F. (2008). Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J. Cell Biol. 180, 739–746 10.1083/jcb.200709161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras G. T., Hu C. K., Coughlin M., Mitchison T. J. (2006). Reassembly of contractile actin cortex in cell blebs. J. Cell Biol. 175, 477–490 10.1083/jcb.200602085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorna-Ornan I., Tzarfaty V., Ankri-Eliahoo G., Joel-Almagor T., Meyer N. E., Huber A., Payre F., Minke B. (2005). Light-regulated interaction of Dmoesin with TRP and TRPL channels is required for maintenance of photoreceptors. J. Cell Biol. 171, 143–152 10.1083/jcb.200503014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clucas J., Valderrama F. (2014). ERM proteins in cancer progression. J. Cell Sci. 127, 267–275 10.1242/jcs.133108 [DOI] [PubMed] [Google Scholar]
- Coscoy S., Waharte F., Gautreau A., Martin M., Louvard D., Mangeat P., Arpin M., Amblard F. (2002). Molecular analysis of microscopic ezrin dynamics by two-photon FRAP. Proc. Natl. Acad. Sci. USA 99, 12813–12818 10.1073/pnas.192084599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dard N., Breuer M., Maro B., Louvet-Vallée S. (2008). Morphogenesis of the mammalian blastocyst. Mol. Cell. Endocrinol. 282, 70–77 10.1016/j.mce.2007.11.004 [DOI] [PubMed] [Google Scholar]
- Datta A., Bryant D. M., Mostov K. E. (2011). Molecular regulation of lumen morphogenesis. Curr. Biol. 21, R126–R136 10.1016/j.cub.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhekne H. S., Hsiao N. H., Roelofs P., Kumari M., Slim C. L., Rings E. H., van Ijzendoorn S. C. (2014). Myosin Vb and Rab11a regulate phosphorylation of ezrin in enterocytes. J. Cell Sci. 127, 1007–1017 10.1242/jcs.137273 [DOI] [PubMed] [Google Scholar]
- Dong B., Deng W., Jiang D. (2011). Distinct cytoskeleton populations and extensive crosstalk control Ciona notochord tubulogenesis. Development 138, 1631–1641 10.1242/dev.057208 [DOI] [PubMed] [Google Scholar]
- Fehon R. G., McClatchey A. I., Bretscher A. (2010). Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11, 276–287 10.1038/nrm2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett D., Bretscher A. (2012). PDZ interactions regulate rapid turnover of the scaffolding protein EBP50 in microvilli. J. Cell Biol. 198, 195–203 10.1083/jcb.201204008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett D., LaLonde D. P., Bretscher A. (2010). The scaffolding protein EBP50 regulates microvillar assembly in a phosphorylation-dependent manner. J. Cell Biol. 191, 397–413 10.1083/jcb.201004115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel V., Barrett P. L., Hall D. H., Fleming J. T. (2004). Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev. Cell 6, 865–873 10.1016/j.devcel.2004.05.018 [DOI] [PubMed] [Google Scholar]
- Grosse A. S., Pressprich M. F., Curley L. B., Hamilton K. L., Margolis B., Hildebrand J. D., Gumucio D. L. (2011). Cell dynamics in fetal intestinal epithelium: implications for intestinal growth and morphogenesis. Development 138, 4423–4432 10.1242/dev.065789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusche F. A., Hidalgo C., Fletcher G., Sung H. H., Sahai E., Thompson B. J. (2009). Sds22, a PP1 phosphatase regulatory subunit, regulates epithelial cell polarity and shape [Sds22 in epithelial morphology]. BMC Dev. Biol. 9, 14 10.1186/1471-213X-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanono A., Garbett D., Reczek D., Chambers D. N., Bretscher A. (2006). EPI64 regulates microvillar subdomains and structure. J. Cell Biol. 175, 803–813 10.1083/jcb.200604046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Tamura A., Krishnan D., Tsukita S., Suzuki Y., Kocinsky H. S., Aronson P. S., Orlowski J., Grinstein S., Alexander R. T. (2013). Ezrin is required for the functional regulation of the epithelial sodium proton exchanger, NHE3. PLoS ONE 8, e55623 10.1371/journal.pone.0055623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert A. M., DuBoff B., Casaletto J. B., Gladden A. B., McClatchey A. I. (2012). Merlin/ERM proteins establish cortical asymmetry and centrosome position. Genes Dev. 26, 2709–2723 10.1101/gad.194027.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraffea J., Reczek D., Bretscher A. (2002). Distinct cell type-specific expression of scaffolding proteins EBP50 and E3KARP: EBP50 is generally expressed with ezrin in specific epithelia, whereas E3KARP is not. Eur. J. Cell Biol. 81, 61–68 10.1078/0171-9335-00218 [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Beitel G. J. (2013). Tubulogenesis. Development 140, 2851–2855 10.1242/dev.070680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe A. B., Kaji N., Durgan J., Hall A. (2008). Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J. Cell Biol. 183, 625–633 10.1083/jcb.200807121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiosis S. A., Ready D. F. (2004). Moesin contributes an essential structural role in Drosophila photoreceptor morphogenesis. Development 131, 725–732 10.1242/dev.00976 [DOI] [PubMed] [Google Scholar]
- Khan S. Y., Ahmed Z. M., Shabbir M. I., Kitajiri S., Kalsoom S., Tasneem S., Shayiq S., Ramesh A., Srisailpathy S., Khan S. N. et al. (2007). Mutations of the RDX gene cause nonsyndromic hearing loss at the DFNB24 locus. Hum. Mutat. 28, 417–423 10.1002/humu.20469 [DOI] [PubMed] [Google Scholar]
- Khan L. A., Zhang H., Abraham N., Sun L., Fleming J. T., Buechner M., Hall D. H., Gobel V. (2013). Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat. Cell Biol. 15, 143–156 10.1038/ncb2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y., Yoshii H., Kamiyama H., Tominaga C., Tanaka Y., Sato H., Yamamoto N. (2008). Ezrin, Radixin, and Moesin (ERM) proteins function as pleiotropic regulators of human immunodeficiency virus type 1 infection. Virology 375, 130–140 10.1016/j.virol.2008.01.047 [DOI] [PubMed] [Google Scholar]
- Kunda P., Pelling A. E., Liu T., Baum B. (2008). Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr. Biol. 18, 91–101 10.1016/j.cub.2007.12.051 [DOI] [PubMed] [Google Scholar]
- Kunda P., Rodrigues N. T., Moeendarbary E., Liu T., Ivetic A., Charras G., Baum B. (2012). PP1-mediated moesin dephosphorylation couples polar relaxation to mitotic exit. Curr. Biol. 22, 231–236 10.1016/j.cub.2011.12.016 [DOI] [PubMed] [Google Scholar]
- LaLonde D. P., Garbett D., Bretscher A. (2010). A regulated complex of the scaffolding proteins PDZK1 and EBP50 with ezrin contribute to microvillar organization. Mol. Biol. Cell 21, 1519–1529 10.1091/mbc.E10-01-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson S. M., Lee H. J., Hung P. H., Matthews L. M., Robinson D. N., Evans J. P. (2010). Cortical mechanics and meiosis II completion in mammalian oocytes are mediated by myosin-II and Ezrin-Radixin-Moesin (ERM) proteins. Mol. Biol. Cell 21, 3182–3192 10.1091/mbc.E10-01-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. L., Streuli C. H. (2014). Integrins and epithelial cell polarity. J. Cell Sci. 127, 3217–3225 10.1242/jcs.146142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Nance M. R., Kulikauskas R., Nyberg K., Fehon R., Karplus P. A., Bretscher A., Tesmer J. J. (2007). Self-masking in an intact ERM-merlin protein: an active role for the central α-helical domain. J. Mol. Biol. 365, 1446–1459 10.1016/j.jmb.2006.10.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvet S., Aghion J., Santa-Maria A., Mangeat P., Maro B. (1996). Ezrin becomes restricted to outer cells following asymmetrical division in the preimplantation mouse embryo. Dev. Biol. 177, 568–579 10.1006/dbio.1996.0186 [DOI] [PubMed] [Google Scholar]
- McCartney B. M., Fehon R. G. (1996). Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J. Cell Biol. 133, 843–852 10.1083/jcb.133.4.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey A. I., Fehon R. G. (2009). Merlin and the ERM proteins – regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 19, 198–206 10.1016/j.tcb.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R. E., Higginbotham J. N., Shifrin D. A., Jr, Tabb D. L., Coffey R. J., Tyska M. J. (2009). The enterocyte microvillus is a vesicle-generating organelle. J. Cell Biol. 185, 1285–1298 10.1083/jcb.200902147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisch A. L., Formstecher E., Fehon R. G. (2013). Conundrum, an ARHGAP18 orthologue, regulates RhoA and proliferation through interactions with Moesin. Mol. Biol. Cell 24, 1420–1433 10.1091/mbc.E12-11-0800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngok S. P., Lin W. H., Anastasiadis P. Z. (2014). Establishment of epithelial polarity - GEF who's minding the GAP? J. Cell Sci. 127, 3205–3225 10.1242/jcs.153197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N., Gupta N. (2013). Re-defining ERM function in lymphocyte activation and migration. Immunol. Rev. 256, 63–79 [DOI] [PubMed] [Google Scholar]
- Parameswaran N., Matsui K., Gupta N. (2011). Conformational switching in ezrin regulates morphological and cytoskeletal changes required for B cell chemotaxis. J. Immunol. 186, 4088–4097 10.4049/jimmunol.1001139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M. A., Reczek D., Bretscher A., Karplus P. A. (2000). Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101, 259–270 10.1016/S0092-8674(00)80836-3 [DOI] [PubMed] [Google Scholar]
- Roch F., Polesello C., Roubinet C., Martin M., Roy C., Valenti P., Carreno S., Mangeat P., Payre F. (2010). Differential roles of PtdIns(4,5)P2 and phosphorylation in moesin activation during Drosophila development. J. Cell Sci. 123, 2058–2067 10.1242/jcs.064550 [DOI] [PubMed] [Google Scholar]
- Roubinet C., Decelle B., Chicanne G., Dorn J. F., Payrastre B., Payre F., Carreno S. (2011). Molecular networks linked by Moesin drive remodeling of the cell cortex during mitosis. J. Cell Biol. 195, 99–112 10.1083/jcb.201106048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome I., Curto M., McClatchey A. I. (2004). Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev. Cell 6, 855–864 10.1016/j.devcel.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Sengupta S., Barber T. R., Xia H., Ready D. F., Hardie R. C. (2013). Depletion of PtdIns(4,5)P2 underlies retinal degeneration in Drosophila trp mutants. J. Cell Sci. 126, 1247–1259 10.1242/jcs.120592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoudy A., Nhieu G. T., Mantis N., Arpin M., Mounier J., Gounon P., Sansonetti P. (1999). A functional role for ezrin during Shigella flexneri entry into epithelial cells. J. Cell Sci. 112, 2059–2068 [DOI] [PubMed] [Google Scholar]
- Solinet S., Mahmud K., Stewman S. F., Ben El Kadhi K., Decelle B., Talje L., Ma A., Kwok B. H., Carreno S. (2013). The actin-binding ERM protein Moesin binds to and stabilizes microtubules at the cell cortex. J. Cell Biol. 202, 251–260 10.1083/jcb.201304052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilić B., Kucera T., Eglinger J., Hughes M. R., McNagny K. M., Tsukita S., Dejana E., Ferrara N., Lammert E. (2009). The molecular basis of vascular lumen formation in the developing mouse aorta. Dev. Cell 17, 505–515 10.1016/j.devcel.2009.08.011 [DOI] [PubMed] [Google Scholar]
- Strilic B., Eglinger J., Krieg M., Zeeb M., Axnick J., Babal P., Muller D. J., Lammert E. (2010). Electrostatic cell-surface repulsion initiates lumen formation in developing blood vessels. Curr. Biol. 20, 2003–2009 [DOI] [PubMed] [Google Scholar]
- Thery M., Bornens M. (2008). Get round and stiff for mitosis. HFSP J. 2, 65–71 10.2976/1.2895661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Fürden D., Johnson K., Segbert C., Bossinger O. (2004). The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodelling and tubulogenesis in the intestine. Dev. Biol. 272, 262–276 10.1016/j.ydbio.2004.05.012 [DOI] [PubMed] [Google Scholar]
- Viswanatha R., Ohouo P. Y., Smolka M. B., Bretscher A. (2012). Local phosphocycling mediated by LOK/SLK restricts ezrin function to the apical aspect of epithelial cells. J. Cell Biol. 199, 969–984 10.1083/jcb.201207047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanatha R., Wayt J., Ohouo P. Y., Smolka M. B., Bretscher A. (2013). Interactome analysis reveals ezrin can adopt multiple conformational states. J. Biol. Chem. 288, 35437–35451 10.1074/jbc.M113.505669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kaiser M. S., Larson J. D., Nasevicius A., Clark K. J., Wadman S. A., Roberg-Perez S. E., Ekker S. C., Hackett P. B., McGrail M. et al. (2010). Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development 137, 3119–3128 10.1242/dev.048785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. S., Hinds P. W. (2003). Increased ezrin expression and activation by CDK5 coincident with acquisition of the senescent phenotype. Mol. Cell 11, 1163–1176 10.1016/S1097-2765(03)00135-7 [DOI] [PubMed] [Google Scholar]
- Zhang H., Kim A., Abraham N., Khan L. A., Göbel V. (2013). Vesicular sorting controls the polarity of expanding membranes in the C. elegans intestine. Worm 2, e23702 10.4161/worm.23702 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.