Abstract
Background and Purpose
In humans, activin receptor-like kinase 1 (Alk1) deficiency causes arteriovenous malformations (AVMs) in multiple organs, including the brain. Focal Alk1 pan-cellular deletion plus vascular endothelial growth factor (VEGF) stimulation induces brain AVMs (bAVMs) in the adult mouse. We hypothesized that deletion of Alk1 in endothelial cell (EC) alone plus focal VEGF stimulation is sufficient to induce bAVM in the adult mouse.
Methods
Focal angiogenesis was induced in the brain of eight-week-old Pdgfb-iCreER;Alk12f/2f mice by injection of adeno-associated viral vectors expressing VEGF (AAV-VEGF). Two weeks later, EC-Alk1 deletion was induced by tamoxifen (TM) treatment. Vascular morphology was analyzed, and EC proliferation and Dysplasia Index (number of vessels with diameter >15μm per 200 vessels) were quantified10 days after TM administration.
Results
Tangles of enlarged vessels resembling AVMs were present in the brain angiogenic region of TM-treated Pdgfb-iCreER;Alk12f/2f mice. Induced bAVMs were marked by increased Dysplasia Index (P<0.001), and EC proliferation clustered within the dysplastic vessels. AVMs were also detected around the ear tag-wound and in other organs.
Conclusions
Deletion of Alk1 in EC in adult mice leads to an increased local EC proliferation during brain angiogenesis and de novo bAVM.
Keywords: animal model, AVM, EC proliferation, hereditary hemorrhagic telangiectasia-2
Introduction
Brain AVMs cause life-threatening intra-cranial hemorrhage, but bAVM pathobiology is poorly understood. Previously, we induced a bAVM phenotype in adult mice by focal pan-cellular Alk1 gene deletion and VEGF stimulation.1 Alk1 is predominantly expressed in the arterial EC.2 We tested the hypotheses that deletion of Alk1 in EC is sufficient to cause bAVM after angiogenic stimulation, and that increased growth of Alk1-deficient EC is a key mechanism underlying brain AVM development.
Methods
Detailed methods are described in the online-only Data Supplement. Experimental procedures for using laboratory animals were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco and conformed to the NIH Guidelines for the care and use of animals in research.
Alk12f/2f mice (Alk1 exons 4-6 flanked by loxP sites)3 were bred with Pdgfb-iCreER mice that express tamoxifen (TM)-inducible cre recombinase (iCreER) in EC.4 EC-Alk1 deletion was induced5 by intra-peritoneal injection of TM 14 days after intra-brain injection of AAV serotype 1-packaged AAV-VEGF with CMV promoter driving VEGF expression. Mice with NG2 promoter (NG2-iCreER) driving Cre expression were used to induce pericyte-Alk1 deletion. Vascular morphology was analyzed using latex casting and immunostaining 10 days after TM administration. EC proliferation and Dysplasia Index were quantified. Experimental design and groups are shown in Figure 1. Data are mean±SD.
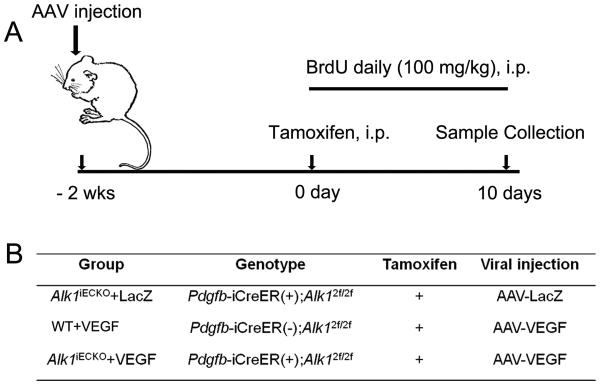
Figure 1. Design and groups.
(A) Design: Adult mice were injected with AAV-VEGF or AAV-LacZ into the brain. Two weeks later, TM was injected i.p. to induce Alk1 gene deletion. Samples were collected 10 days later. To track proliferating cells, BrdU was injected i.p. daily starting on the day of TM injection and continuing for 10 days. (B) Groups. iECKO: inducible EC Alk1 knockout. Twelve mice per group: 6 for latex perfusion and 6 for immunostaining.
Results
EC-Alk1 deletion resulted in AVM in the brain angiogenic region and other organs
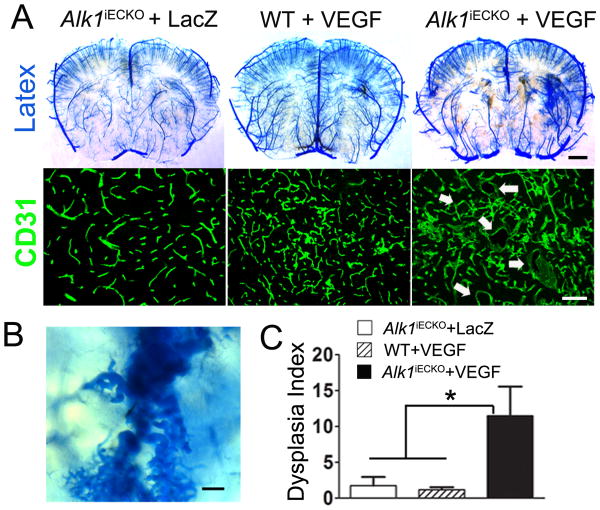
Alk1iECKO+VEGF mice developed tangles of irregular vessels in the brain angiogenic region, whereas Alk1iECKO+LacZ or WT+VEGF did not (Figures 2A & B). The Dysplasia Index was higher in the Alk1iECKO+VEGF group (11.5±4.1) than in the WT+VEGF (1.2±0.4, P<0.001) and Alk1iECKO+LacZ (1.8±1.2, P<0.001) groups (Figure 2C). Macrophage infiltration and microhemorrhage were observed around the malformed vessels (Supplementary Figure I). EC-specificity of PDGFb-iCreER was confirmed in the brain of Alk1iECKO+VEGF mice (Figure 1B) using Ai14 cre reporter (Supplementary Figure II).
Figure 2. Malformed vessels in the brain of Alk1iECKO+VEGF mice.
(A) Latex-casted vessels and CD31-stained brain sections. Arrows indicate dilated dysplasia vessels. Scale bar: 1 mm (upper panel) and 100 μm (lower panel). (B) Enlarged image shows tortuous and dilated abnormal vessels. Scale bar: 100 μm. (C) Quantifications of dysplasia index.
AVMs also developed in the intestine, lung, and around ear-tag wounds of Alk1iECKO+VEGF and Alk1iECKO+LacZ mice. Alk1iECKO mice had pale paws and darkened feces indicating anemia and gastrointestinal bleeding, and died 6-13 days after TM administration (Supplementary Figures III and IV).
EC proliferation increased in bAVM
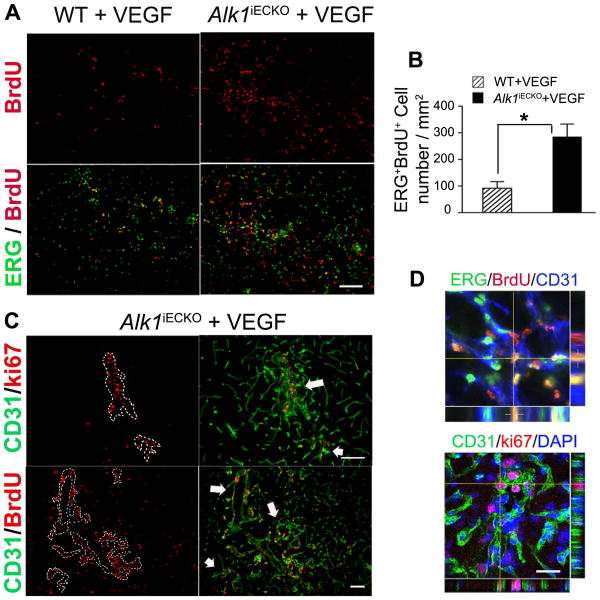
Compared to WT+VEGF mice, Alk1iECKO+VEGF mice had more newly proliferated ECs (BrdU+/ERG+, 285±48 vs. 91±25 cells/mm2, P<0.001, Figure 3) and a higher vessel density in the brain angiogenic region (P<0.001, Supplementary Figure V). The newly proliferated (BrdU+) and proliferating (ki67+) ECs were clustered around dysplastic vessels (Figure 3).
Figure 3. Increased EC proliferation in the angiogenic foci of Alk1iECKO+VEGF mice.
(A) BrdU+ (red) EC nuclei (ERG+, green) in the angiogenic foci of WT+VEGF and Alk1iECKO+VEGF mice. Scale bar: 100 μm. (B) Quantification of ERG+/BrdU+ ECs. (C) Proliferating ECs (Ki67+ or BrdU+) are clustered on the dysplastic vessels (outlined by dotted lines in the images on the left and indicated by arrows in the images on the right). Scale bar: 100 μm. (D) Confocal image showing colocalization of BrdU (red) and ERG (green, top), and ki67 (red) and CD31 (green, bottom) positive staining. The nuclei were counterstained with DAPI (blue). Scale bar: 20 μm.
Pericyte-Alk1 deletion did not cause AVM
NG2-iCreER was used to delete pericyte-Alk1. The cre activity in pericytes was confirmed using Ai14 reporter (Supplemental Figure VI-A). No AVM was detected in adult NG2-iCreER;Alk12f/2f mice (Supplementary Figure VI-B).
Discussion
Previously, we reported that injection of AAV-VEGF into the brain of adult Alk1+/- mice induces a capillary level of dysplasia,6 which served as a surrogate model for bAVM until we developed a macroscopic level of a bAVM model through focal homozygous Alk1 deletion plus VEGF stimulation.1 In that model, an adenoviral vector that has CMV promoter driving cre expression (Ad-Cre) was used to induce focal pan-cellular Alk1 deletion.1 Here, we show that deletion of Alk1 in adult EC results in a bAVM phenotype similar to that in the pan-cellular Alk1 deletion model. Both have macrophage infiltration and microhemorrhage.7 EC-specific Alk1 deletion in the adult mouse has also resulted in AVM in the small intestine and lung and around the skin wound, which recapitulated the phenotype of global Alk1 deletion in adult mice.5 Thus, deletion of Alk1 in adult EC is sufficient to induce AVMs.
SM22α—Cre-driven Alk1 deletion has been reported to result in brain and spinal cord AVMs,8 suggesting that loss of ALK1 from pericytes/vascular smooth-muscle cells can cause AVMs. In contrast, we find that conditional deletion of Alk1 in adult pericytes did not trigger AVMs in any organ, around the ear-tag wound, or in the VEGF-stimulated brain. As revealed by Rosa-LacZ cre reporters, SM22α promoter driving cre expression results in non-specific recombination in EC-lineage.8 Therefore, we propose that Alk1 deletion in pericytes alone is not sufficient and that gene deletion in EC is required to initiate AVM development.
We found that deletion of Alk1 in ECs in the adult brain increased EC proliferation in response to VEGF stimulation. Although the expression of Ai14 reporter indicated that cre was activated in ECs of all vessels in the angiogenic region, only a few dysplasia vessels were formed. The dysplastic vessels have more proliferating ECs than the surrounding normal capillaries. As it is statistically unlikely that the dominance of the proliferating cells in dysplastic vessels is the result of multiple independent Cre recombination events, we propose that ECs with homozygous Alk1 deletion undergo clonal expansion and have a higher proliferation rate than WT EC or Alk1+/- EC, which results in unevenly enlarged abnormal vessels. This hypothesis needs to be further validated.
In summary, deletion of Alk1 in EC leads to increased focal EC proliferation during brain angiogenesis and de novo AVM development. Knowledge of the importance of EC in AVM development will help in understanding AVM pathogenesis and in designing specific therapies.
Supplementary Material
Acknowledgments
We thank Voltaire Gungab for assistance with manuscript preparation, S. Paul Oh at the University of Florida for providing Alk12f/2f mice, and members of the UCSF BAVM Study Project (http://avm.ucsf.edu) for their support.
Sources of Funding: Supported by grants to H.S. from the National Institutes of Health (R01NS027713 and P01NS044155), and from the Leslie Munzer Foundation. Additional support was provided by a grant to W.L.Y. from the Michael Ryan Zodda Foundation.
Footnotes
Disclosures: None
References
- 1.Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol. 2011;69:954–962. doi: 10.1002/ana.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93:682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- 3.Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, et al. ALK5- and TGFBR2- independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2 (HHT2) Blood. 2008;111:633–642. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claxton S, Kostourou V, Jadeja S, Chambon P, Hodivala-Dilke K, Fruttiger M. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis. 2008;46:74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
- 5.Park SO, Wankhede M, Lee YJ, Choi EJ, Fliess N, Choe SW, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest. 2009;119:3487–3496. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hao Q, Zhu Y, Su H, Shen F, Yang GY, Kim H, et al. VEGF induces more severe cerebrovascular dysplasia in Endoglin+/- than in Alk1+/- mice. Transl Stroke Res. 2010;1:197–201. doi: 10.1007/s12975-010-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Guo Y, Walker EJ, Shen F, Jun K, Oh SP, et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler Thromb Vasc Biol. 2013;33:305–310. doi: 10.1161/ATVBAHA.112.300485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milton I, Ouyang D, Allen CJ, Yanasak NE, Gossage JR, Alleyne CH, Jr, et al. Age-dependent lethality in novel transgenic mouse models of central nervous system arteriovenous malformations. Stroke. 2012;43:1432–1435. doi: 10.1161/STROKEAHA.111.647024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.