Abstract
Objective:
To investigate the relationship between older age and mean cerebral white matter fiber bundle lengths (FBLs) in specific white matter tracts in the brain using quantified diffusion MRI.
Methods:
Sixty-three healthy adults older than 50 years underwent diffusion tensor imaging. Tractography tracings of cerebral white matter fiber bundles were derived from the diffusion tensor imaging data.
Results:
Results revealed significantly shorter FBLs in the anterior thalamic radiation for every 1-year increase over the age of 50 years.
Conclusions:
We investigated the effects of age on FBL in specific white matter tracts in the brains of healthy older individuals utilizing quantified diffusion MRI. The results revealed a significant inverse relationship between age and FBL. Longitudinal studies of FBL across a lifespan are needed to examine the specific changes to the integrity of white matter.
Diffusion tensor imaging (DTI) is a noninvasive imaging technique frequently used to visualize age-related changes in white matter integrity. DTI measures water movement within a voxel and captures abnormalities in the underlying microstructural anatomy that change the direction and speed of diffusion in specific brain regions.1 Age effects demonstrated with DTI are regionally diverse and typically show an anterior-to-posterior pattern of age-related decline.2–4 In quantifying age-related decline, most DTI studies have examined white matter integrity in focal brain regions, utilizing region of interest (ROI) and voxel-based morphometry approaches.
Quantitative tractography based on DTI (qtDTI) combines traditional tractography methods4 with scalar metrics to detect region-specific microstructural changes in white matter fiber bundles.5 Specifically, the approach computes fiber bundle length (FBL) using fractional anisotropy (FA) to produce tract lines representing coherent bundles of nerve fibers. FBL constructed utilizing qtDTI provides additional detail about the direction and curvature of white matter pathways coursing throughout the brain.6 This method is sensitive to white matter changes within entire tracts and thus may be more beneficial than methods that involve placing ROIs on 2-dimensional scalar DTI parameter maps.6
In the present study, we utilized qtDTI tractography methods to examine the impact of aging on the white matter integrity in the brains among healthy older adults. All selected tracts have been previously associated with various factors related to age. We hypothesized that advanced age would negatively affect the microstructural integrity of white matter, resulting in shorter mean FBL in white matter tracts.
METHODS
Participants.
Sixty-three cognitively healthy individuals enrolled in a study of healthy aging were included in the present study. Eligible individuals included both male and female English-speaking adults older than 50 years. Participants were recruited from the local community using print, radio, and direct outreach. Additional participants were recruited from the Research Participant Registry of the Washington University Institute of Clinical and Translational Sciences. To be enrolled in the study, individuals were required to be English speakers, older than 50 years, and free of any medical or psychiatric condition that could reasonably affect cognition. Exclusion criteria included history of neurologic disease including dementia, stroke, Parkinson disease, or other neurologic condition that could affect mental status. Individuals were excluded if determined to be cognitively impaired by total score on the Mini-Mental State Examination7 (figure e-1 on the Neurology® Web site at Neurology.org). In addition, individuals with diabetes requiring treatment, head injury with loss of consciousness longer than 5 minutes, history of alcohol or drug abuse either past or present, or other poorly controlled axis I or II psychiatric condition (e.g., schizophrenia, bipolar disorder, current depression) were excluded. A physician evaluated all imaging scans to exclude individuals with gross radiologic abnormalities.
Standard protocol approvals, registrations, and patient consents.
All participants provided signed informed consent and were financially compensated for involvement in the study. Approval was obtained from the local institutional review boards of the corresponding institutions before collection of data.
Neuroimaging acquisition.
MRI acquisitions were obtained using a head-only Magnetom Allegra 3T MRI scanner (Siemens Healthcare, Erlangen, Germany) at Washington University in St. Louis. High-performance gradients (maximum strength 40 mT/m in a 100-μs rise time; maximum slew rate 400 T/m/s) were utilized to minimize scan times. Quality assurance was conducted daily to ensure data fidelity. For maximal stability, no scanner hardware or software modifications were made during the course of the study. Movement was limited by application of surgical tape across the forehead and use of a radiofrequency coil. The total time in the scanner was limited to less than 1 hour. Each scanning session began with the collection of a scout scan consisting of 3 orthogonal planes to confirm head positioning. Automated high-order shimming was utilized.
Diffusion-weighted imaging acquisition.
Axial diffusion-weighted imaging (DWI) was acquired using a customized single-shot multislice echo-planar tensor-encoded pulse sequence designed and implemented in-house for this study. Thirty-one noncollinear diffusion-encoded directions were used in the acquisition consisting of 24 main directions (diffusion weighting of b = 996 s/mm2). In addition, we used a “core” of tetrahedral-perpendicular directions8 (b = 1,412 and 680 s/mm2, respectively) and 5 I0 acquisitions (b approximately 0) for wide directional coverage and signal-to-noise ratio (SNR) efficiency. The encoding scheme was ordered to cancel eddy-current effects. Pulse sequence and acquisition parameters were optimized for tractography, and attention was given to high SNR to reduce track foreshortening.9 The echo time was 86.2 milliseconds with full-Fourier acquisition (maximizing SNR and minimizing artifact). In a repetition time of 7.82 seconds, 64 contiguous 2.0-mm slices were acquired for each contrast. The acquisition matrix was 128 × 128 with a 256 × 256 mm field of view (isotropic 2.0 × 2.0 × 2.0 mm voxels). Signal averaging (72 total acquisitions) was acquired over 2 scan repeats, and all unedited data were included in the analyses. Raw data were saved to the operating system disk, and floating-point DWIs were custom reconstructed using a SunFire V880 computer server. This methodology has been used in prior studies.10–12
Quantitative diffusion tractography.
Each individual's DWIs were registered to the I0 image using FSL's FLIRT (mutual information metric) to correct for subject motion, and the b-vectors were rotated to account for the rotation induced by the registration.13 Brain tissue was extracted automatically using FSL's Brain Extraction Tool. Tensors and FA values were reconstructed by linear least squares with trilinear interpolation of the diffusion-weighted signal. Whole-brain streamline tractography was deterministic and performed with the principal eigenvector, one random seed per voxel, second-order Runge-Kutta integration, an angle threshold of 35°, an FA threshold of 0.15, and a minimum-length threshold of 10 mm.
To examine average FBL in each white matter tract of interest, we mapped the volumetric Johns Hopkins University (JHU)-DTI white matter atlas14 to each subject's FA image using affine registration with mutual information in FSL's FLIRT.15 The following bundles were included in the analysis: uncinate fasciculus, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, anterior thalamic radiation, cingulum of the cingulate, cingulum of the hippocampus, and the corticospinal tract. These were modeled separately for each hemisphere for a total of 16 distinct bundles. For each of the bundles in the atlas, track lines were selected that were contained in the volumetric ROI of the associated bundle. Specifically, from all fibers found by whole-brain tractography, a fiber was included in a bundle if 80% or more of its arc length was contained in the associated mask. Because the complexity of the tracts make the specificity of fiber extraction difficult without probabilistic tractography maps, the JHU-DTI atlas was used to ensure that no other surrounding fiber bundles would be included in our measurements. For each JHU fiber bundle, streamline fibers were culled with an interfiber of 0.8 mm, a process that is described in detail in previous work.16 The average length of each bundle was then estimated and normalized by dividing by the total brain volume.6 FBL was averaged over the population.
Statistical analyses.
All statistical analyses were conducted using R version 15.2. Three outliers were removed before analysis (see table e-1 and figure e-2). A multivariate linear model was used to test the main hypothesis that advanced age predicts FBL reduction in the uncinate fasciculus, superior longitudinal fasciculus, inferior longitudinal fasciculus, cingulum of the hippocampus, cingulum of the cingulate cortex, anterior thalamic radiation, inferior fronto-occipital fasciculus, and corticospinal tract. Univariate linear models were used as follow-up analyses to determine the impact of age on individual white matter tracts. In both multivariate and univariate models, age was entered as the predictor, and FBL values were entered jointly in the multivariate model and separately in the univariate model. Years of education were also added to the univariate models to assess its effects. Analyses were conducted to examine the average decrease in each 1-year increment in our cross-sectional sample. Univariate linear models were used to examine the relationship between age and FA to examine FBL in the context of other DTI metrics.
RESULTS
Participant demographics and mean FBL values are listed in table 1. The results of the multivariate linear model revealed that age predicted significant variation in mean FBL, with significantly shorter lengths evident with advanced age (Pillai-Bartlett = 0.30, F8,52 = 2.84, p = 0.01). Results of the follow-up univariate linear models (table 2) revealed that for every 1-year increase in age, there was a corresponding 0.65-mm reduction in FBL in the anterior thalamic radiation (p < 0.05) with the mean starting length of 139 mm (figure 1) after Bonferroni correction. Education significantly predicted FBL at a trend level (p > 0.07). However, when education was added, the annual decrease in FBL for the anterior thalamic radiation was nearly unchanged (−0.65 mm, p < 0.05). Results of the univariate linear models examining the relationship between age and FA revealed that for every 1-year increase, there was a marginally nonsignificant reduction in FA in the right anterior thalamic radiation, left inferior fronto-occipital fasciculus, right inferior fronto-occipital fasciculus, left anterior thalamic radiation, left inferior longitudinal fasciculus, and right uncinate fasciculus after Bonferroni correction (table e-2). Further information regarding the relationship between mean FBL and white matter integrity is provided in figures e-3 and e-4.
Table 1.
Demographic information and mean fiber bundle length (mm) (N = 60 participants)
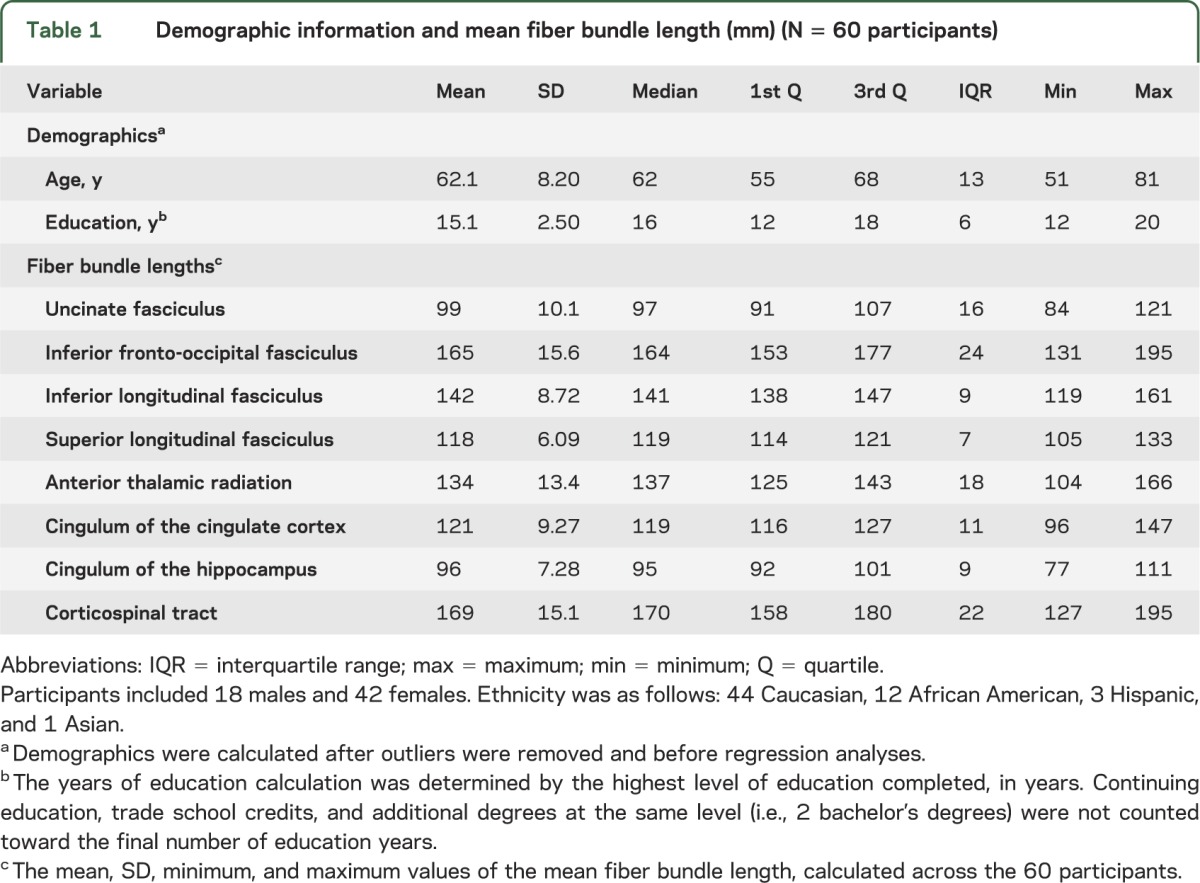
Table 2.
Univariate analyses of age and fiber bundle length (mm)
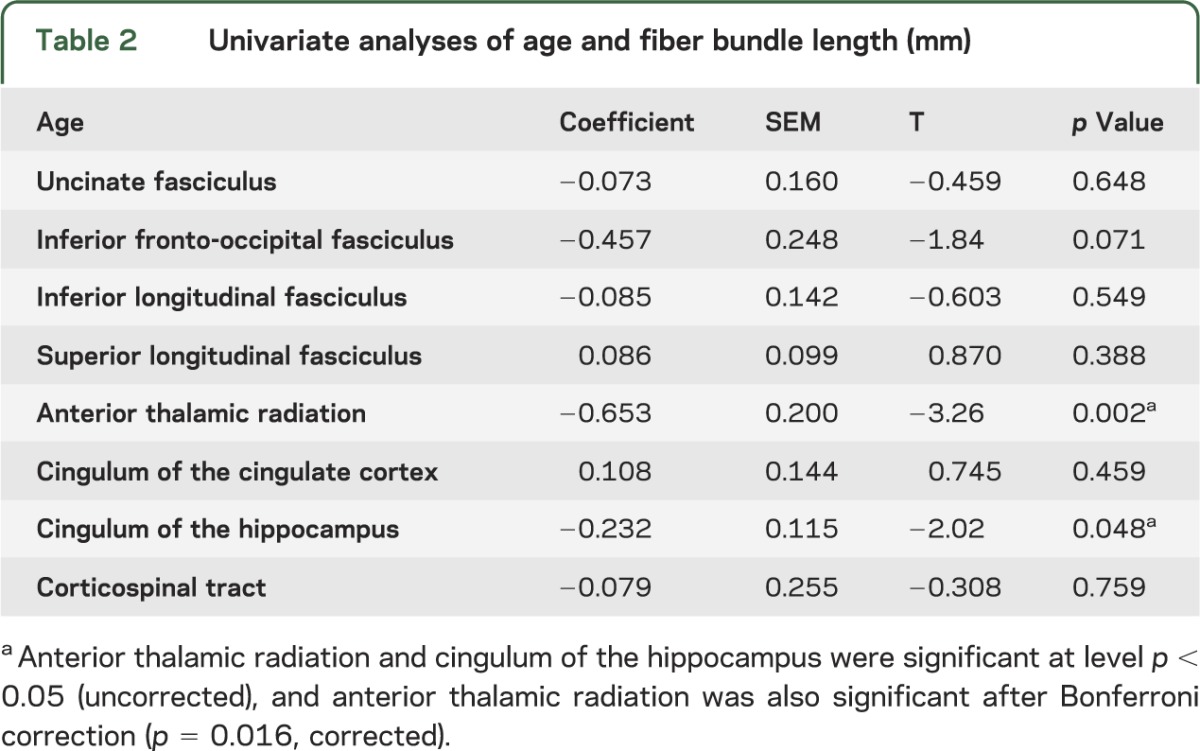
Figure 1. Regression analysis between age and mean FBL in the anterior thalamic radiation.
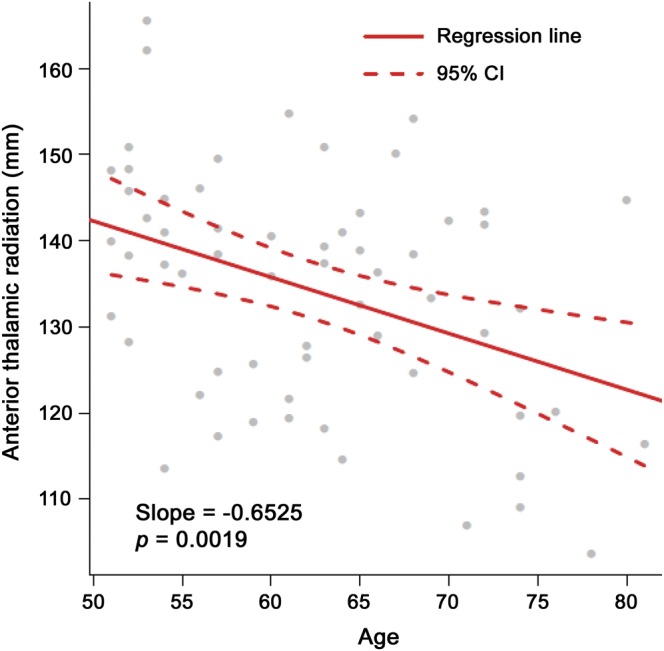
Significant inverse relationship (p < 0.05) between age and mean fiber bundle length (FBL) in the anterior thalamic radiation. Line of best fit (solid line) and 95% confidence interval (CI) (dashed lines).
DISCUSSION
In the present study, we investigated the effects of age on FBL in specific white matter tracts in the brains of healthy older individuals utilizing qtDTI. To understand the relationship between age and FBL decline, we examined the decrease in FBL for age difference measured in 1-year increments. Analyses revealed an overall decline in FBL with older age, and these age-related reductions were specific to the anterior thalamic radiation (see figures e-5 to e-8). We also observed reductions in the uncinate fasciculus, cingulum of the hippocampus, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, and corticospinal tract, although these reductions were not statistically significant. Furthermore, we observed marginally nonsignificant age-related changes in FA that were specific to the anterior thalamic radiation, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, and uncinate fasciculus. Collectively, these findings provide evidence that age is associated with deterioration of FBL in specific white matter tracts in healthy older adults. In addition, our results may help identify region-specific patterns of shorter FBL that are characteristic of normal brain aging.
Previously, white matter changes have been observed with increasing age. Overall, these studies have demonstrated that the aging brain exhibits extensive micro- and macroscopic changes that ultimately result in cognitive and functional decline. This research has demonstrated significant myelin and nerve fiber degradation in the white matter of the aging brain.17 Damage to the myelin takes the form of lamellar separations, formation of balloons, redundant myelination, and circumferential splits in the thick sheaths16 that are believed to reduce the conduction velocity along nerve fibers.16
In addition to myelin damage, postmortem examinations have revealed significantly shorter neuronal fiber lengths among older adults compared with younger adults, with short-range association fibers demonstrating enhanced vulnerability to aging processes.18,19 Histologic studies have shown that the total number of glial cells is not significantly different between younger and older individuals; however, myelinated fiber lengths are significantly shorter by as much as 50% with age.20 This loss is believed to be gradual, with a 10% loss in length of myelinated fibers per decade, equivalent to a 45% loss of fiber length over the lifespan beginning at age 20 years.18 Reduced FA has also been observed in the frontal lobe and this anatomical change has been hypothesized to represent a potential mechanism underlying cognitive aging.21 However, these studies lack a comprehensive model to examine underlying deficits observed in white matter integrity that can be observed using qtDTI. Our indices span from quantitative tractography metrics that describe fiber length and number and scalar anisotropy metrics that provide information about intravoxel structural integrity.6
White matter volume declines in an anterior-to-posterior pattern, with the greatest volumetric loss in the frontal and temporal lobes of the brain.22 In particular, there are significant age-related decreases in white matter integrity with age in the anterior thalamic radiation, which is formed by fibers interconnecting, via the anterior limb of the internal capsule, the anterior and medial thalamic nuclei and the cerebral cortex of the frontal lobe.23 A decline in this region has been associated with deficits in processing speed.24 Results from the present study show that there is significant decline in white matter integrity, utilizing qtDTI, in the anterior thalamic radiation that links the frontal lobe to various areas of the brain.
One possible mechanism that may result in decreased white matter FBL in older individuals is inflammation. Inflammation increases with advancing age, and this process may trigger structural changes in the aging brain25 with significant impact on the myelin and axonal integrity; disruption in either structural element of the neuron could reduce FBL. CNS inflammatory events may have an important role in affecting neuronal and behavioral deficits in aging.26 Therefore, age-dependent enhanced neuroinflammatory processes may generate cytotoxic properties that cause death or dysfunction of neurons in older individuals. In the present study, we did not examine inflammatory markers, but this represents an important area of future research.
A few limitations of the present study should be considered. The sample may not be highly generalizable to the population because it consists predominantly of highly educated Caucasians and African Americans. In addition, there are methodologic concerns that should be considered when interpreting these findings.6 For example, steps were taken to ensure accuracy of the metrics; however, proper FBL measurements are complex. Theoretically, the length of a tract derived from deterministic qtDTI represents the true biological path length of an ordered fiber bundle. However, it is important to note that a large percentage of axons in the fiber bundle would have to shorten in order to yield a shorter qtDTI track because the DWI signal arises from large ensembles of axons. The observed qtDTI tract length also depends on factors unrelated to actual FBL, namely, the pathway anisotropy and the SNR level of the MRI data. qtDTI tracts will generally be shorter in conditions of lower pathway anisotropy and lower SNR because these effects increase the likelihood that the tract derivation algorithm will encounter a voxel with subthreshold anisotropy (thus stopping the tract), or will deviate out of the pathway because of a high angular shift.8 This effect is referred to as foreshortening and is not significant if SNR is sufficient and if the pathway anisotropy is well above the stopping threshold. Therefore, we do not expect tract foreshortening due to low SNR. Because SNR would not be expected to vary significantly with age or white matter tracts, SNR effects cannot explain the findings herein. Also, any age-related anisotropy changes across the age range in the study would not be expected to have a significant effect on tract foreshortening, because even the weak anisotropy in association areas was approximately 2.5 times the tractography stopping threshold used in this study (i.e., FA = 0.15).
In the present study, we investigated the age-related microstructural decline in white matter FBL in a healthy aging population. The functional significance of this shortening is currently unknown. Among individuals with cerebrovascular disease, significant relationships have been observed between these metrics and cognitive performance on tests sensitive to subcortical brain structures whereas no such relationships were evident between these metrics and cognitive tests sensitive to cortical brain regions in the same population.6 Future studies should investigate whether age-dependent loss in white matter FBL is related to cognitive decline in a normal aging population. It is possible that white matter FBLs represent a meaningful biomarker of cognitive aging. White matter tracts serve as connections between brain regions, and likely have an important role in coordinating complex cognition. Information is required to transfer quickly between different brain regions and age-related damage to any part of these white matter connections could lead to changes in cognitive performance.27 Longitudinal studies using a broader sample, comparing FBL over the age span, are necessary to further characterize these findings and account for the variability observed in our results.
Supplementary Material
GLOSSARY
- DTI
diffusion tensor imaging
- DWI
diffusion-weighted imaging
- FA
fractional anisotropy
- FBL
fiber bundle length
- FLIRT
FMRIB's Linear Image Registration Tool
- FSL
FMRIB's Software Library
- JHU
Johns Hopkins University
- qtDTI
quantitative tractography based on diffusion tensor imaging
- ROI
region of interest
- SNR
signal-to-noise ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Ms. Baker: drafting/revising the manuscript, acquisition of data, study concept and design, and interpretation of data. Dr. Laidlaw: analysis and interpretation of data and revising the manuscript. Dr. Conturo: analysis and interpretation of data. Dr. Hogan, Ms. Zhao, and Dr. Luo: statistical analysis, interpretation of data, and revising the manuscript. Dr. Correia and Mr. Cabeen: analysis and interpretation of data and revising the manuscript. Dr. Lane: study supervision, acquisition of data, and drafting/revising the manuscript. Dr. Heaps and Mr. Bolzenius: study supervision, acquisition of data, and drafting/revising the manuscript. Ms. Salminen: drafting/revising the manuscript and acquisition of data. Dr. Akbudak: analysis and interpretation of data. Ms. McMichael: acquisition of the data and study supervision. Ms. Usher and Ms. Behrman: drafting/revising the manuscript and acquisition of data. Dr. Paul: study concept and design, study supervision, obtaining funding, acquisition of data, and drafting/revising the manuscript.
STUDY FUNDING
Supported by NIH/NINDS grants R01 NS052470 and R01 NS039538, and NIH/NIMH grant R21 MH090494. Recruitment database searches were supported in part by NIH/NCRR grant UL1 TR000448.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson 1996;111:209–219 [DOI] [PubMed] [Google Scholar]
- 2.Kochunov P, Thompson PM, Lancaster JL, et al. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage 2007;35:478–487 [DOI] [PubMed] [Google Scholar]
- 3.Salat DH, Tuch DS, Greve DN, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 2005;26:1215–1227 [DOI] [PubMed] [Google Scholar]
- 4.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: evidence from diffusion tensor imaging. Neuroimage 2005;26:891–899 [DOI] [PubMed] [Google Scholar]
- 5.Conturo TE, Lori NF, Cull TS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci USA 1999;96:10422–10427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correia S, Lee SY, Voorn T, et al. Quantitative tractography metrics of white matter integrity in diffusion-tensor MRI. Neuroimage 2008;42:568–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical model for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 8.Conturo TE, McKinstry RC, Akbudak E, Robinson BH. Encoding of anisotropic diffusion with tetrahedral gradients: a general mathematical diffusion formalism and experimental results. Magn Reson Med 1996;35:399–412 [DOI] [PubMed] [Google Scholar]
- 9.Lori NF, Akbudak E, Shimony JS, et al. Diffusion tensor fiber tracking of human brain connectivity: acquisition methods, reliability analysis and biological results. NMR Biomed 2002;15:494–515 [DOI] [PubMed] [Google Scholar]
- 10.Tate DF, Conley J, Paul RH, et al. Quantitative diffusion tensor imaging tractography metrics are associated with cognitive performance among HIV-infected patients. Brain Imaging Behav 2010;4:68–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salminen LE, Schofield PR, Lane EM, et al. Neuronal fiber bundle lengths in healthy adult carriers of the ApoE4 allele: a quantitative tractography DTI study. Brain Imaging Behav 2013;7:274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolzenius JD, Laidlaw DH, Cabeen RP, et al. Impact of body mass index on neuronal fiber bundle lengths among healthy older adults. Brain Imaging Behav 2013;7:300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and acute linear registration and motion correction of brain images. Neuroimage 2002;17:825–841 [DOI] [PubMed] [Google Scholar]
- 14.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980 [DOI] [PubMed] [Google Scholar]
- 15.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract based atlas of human white matter anatomy. Radiology 2004;230:77–87 [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Demiralp C, Laidlaw DH. Visualizing diffusion tensor MR images using streamtubes and streamsurfaces. IEEE Trans Vis Comput Graph 2003;9:454–462 [Google Scholar]
- 17.Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol 2002;31:581–593 [DOI] [PubMed] [Google Scholar]
- 18.Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiol Aging 1997;18:609–615 [DOI] [PubMed] [Google Scholar]
- 19.Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol 2003;462:144–152 [DOI] [PubMed] [Google Scholar]
- 20.Pakkenberg B, Pelvig D, Marner L, et al. Aging and the human neocortex. Exp Gerontol 2003;38:95–99 [DOI] [PubMed] [Google Scholar]
- 21.Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magn Reson Med 2003;49:953–961 [DOI] [PubMed] [Google Scholar]
- 22.Brickman AM, Zimmerman ME, Paul RH. Regional white matter and neuropsychological functioning across the adult lifespan. Biol Psychiatry 2006;60:444–453 [DOI] [PubMed] [Google Scholar]
- 23.Giorgio A, Santelli L, Tomassini V, et al. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage 2010;51:943–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deuring M, Zieren N, Herve D, et al. Strategic role of frontal white matter tracts in vascular cognitive impairment: a voxel-based lesion-symptom mapping study in CADASIL. Brain 2001;134:2366–2375 [DOI] [PubMed] [Google Scholar]
- 25.Floyd RA, Hensley K. Oxidative stress in brain aging: implications for therapeutics of neurodegenerative diseases. Neurobiol Aging 2002;23:795–807 [DOI] [PubMed] [Google Scholar]
- 26.Boldes AM, Barger SW. Cytokines and the aging brain: what we don't know might help us. Trends Neurosci 2004;27:621–626 [DOI] [PubMed] [Google Scholar]
- 27.Charlton RA, Barrick TR, McIntyre DJ, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology 2006;66:217–222 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


