Abstract
Flow cytometric cell sorting of biological specimens has become prevalent in basic and clinical research laboratories. These specimens may contain known or unknown infectious agents, necessitating precautions to protect instrument operators and the environment from biohazards arising from the use of sorters. To this end the International Society of Analytical Cytology (ISAC) was proactive in establishing biosafety guidelines in 1997 (Schmid et al., Cytometry 1997;28:99–117) and subsequently published revised biosafety standards for cell sorting of unfixed samples in 2007 (Schmid et al., Cytometry Part A J Int Soc Anal Cytol 2007;71A:414–437). Since their publication, these documents have become recognized worldwide as the standard of practice and safety precautions for laboratories performing cell sorting experiments. However, the field of cytometry has progressed since 2007, and the document requires an update. The new Standards provides guidance: (1) for laboratory design for cell sorter laboratories; (2) for the creation of laboratory or instrument specific Standard Operating Procedures (SOP); and (3) on procedures for the safe operation of cell sorters, including personal protective equipment (PPE) and validation of aerosol containment.
Key terms: flow cytometry, occupational health, biohazards, cell sorting, biosafety, aerosol containment
Introduction
In 1994 the International Society of Analytical Cytology (renamed International Society for the Advancement of Cytometry in 2006; ISAC), an association representing researchers involved in cytometry, recognized the need to formulate safety guidelines for sorting and analysis of unfixed cells. ISAC initiated the formation of a Biohazard Working Group and the formation of a Biosafety committee, which published the official guidelines in 1997 (1). These guidelines provided recommendations for practices to reduce the potential for biohazard exposure of instrument operators. As stated in the preface to those guidelines, revisions were expected to occur at periodic intervals, and in 2007 the ISAC Biosafety Standards for sorting of unfixed cells was published (2). Although these standards reflect valid procedures and practices, an update is relevant at this time for the following reasons:
The ISAC Biosafety Committee disseminated an online survey to ISAC members that elicited responses on a variety of biosafety related topics. As a result of this survey, it became evident that certain areas in the current standards warranted clarification. For example, the appropriate biosafety procedures for sorting of human cell lines and of lentivirus-infected cells are often difficult to determine, hence leading to questionable sorting practices.
A more thorough characterization of aerosols capable of being produced by cell sorters has recently been published (3).
The National Institutes of Health (NIH) has recently instituted a Biosafety Policy for Cell Sorters for all NIH Intramural laboratories, which establishes procedures whose incorporation into the Standard were deemed important.
Instrument manufacturers responded to the need for improved operator protection, and have introduced instrumentation containing novel safety features including the availability of cell sorters contained within Class II Biological Safety Cabinets (BSC; also known as Microbiological Safety Cabinets).
The current ISAC Biosafety Committee (www.ISAC-net.org) was charged with this task and herein presents an updated standard that reflects the present knowledge and occupational safety practices. The purpose of this document is to update and expand the 2007 standard document for handling and sorting of potentially biohazardous specimens. In particular it provides guidance for laboratory directors, managers, and instrument operators on facility design and sorter placement, direction for the creation of appropriate standard operating procedures, and also provides guidance to instrument manufacturers for the design of instrument biosafety features.
Standards set forth in this document focus on cell sorting of live samples. However, it is important to note that performing analysis, without sorting, on a jet-in-air flow cytometer, has the same risk of aerosol exposure as cell sorting. Therefore, the standard applies to all procedures whenever samples are run through a jet-in-air flow cytometer or a sorter that combines a flow cell with jet-in-air sorting.
Laboratory-Associated Infections (LAI) and Aerosols
It is well documented that laboratory workers have a higher risk of acquiring infections than the general public (4–7). The latest review of laboratory associated infections reveal that the incidence of infections is approximately equal between clinical and research laboratories (4). The possible routes of infection are inhalation, ingestion, inoculation, and skin and/or mucous membrane contamination. While the documented source or route of infection has been determined for many LAIs, the source for 82% of documented LAIs is unknown (8). However, it is believed that aerosols are the probable source of many LAIs (9) and according to Collins and Kennedy (6), ideal conditions exist in laboratories for the spread of infection by airborne particles. Infections by airborne route have occurred in the laboratory with organisms not normally transmitted by aerosols in nature (10–12). This is due to increased pathogen concentration and aerosolization (during laboratory procedures) not seen in nature. As explained below, the potential of cell sorters to create aerosols during cell sorting procedures places cell sorter operators at high risk for LAI.
Aerosols containing infectious biological particles, a class of bioaerosols, are capable of initiating a disease process in a susceptible host. Infectivity is related to various microbial factors, host factors and to particle size and shape, or more specifically, its aerodynamic diameter. The aerodynamic diameter of aerosols is important in determining the probability of deposition within the respiratory tract. Specifically, aerosols in the range of 2 to 4 μm preferentially deposit in the alveolar region of the lung (13), but larger aerosols deposit in the upper respiratory tract and nasopharynx (reviewed in Ref. 14)). Alveolar deposition has been associated with increased infectivity for some pathogens, including Coxsackie virus, rhinovirus, and Influenza as shown by a decreased median dosage for lethality or infection (15–18). Therefore the potential for infection from inhalation of aerosols, especially aerosols that are less than 5μm, must be considered for laboratory procedures that generate aerosols.
Creation of Droplets and Aerosols During Cell Sorting
Jet-in-air technology used for fluorescence-activated cell sorting employs a liquid stream carrying the cells through a nozzle vibrating at a high frequency (50–100 kHz). At a given distance from the nozzle orifice, the stream is broken into individual, well-defined droplets. These droplets are then passed between two high voltage plates. Droplets containing the cells that were preselected based upon fluorescence and light scatter characteristics, are electrostatically charged and deflected into a variety of sample receptacles. Overall droplet size depends on the instrument operating pressure, the size of the nozzle orifice and its vibration frequency, but can range between 80 and 300 μm. High-speed cell sorters utilize higher system pressures and sort frequencies (19), and thus they produce a higher number of smaller droplets compared with older instruments designed for low speed separations (20). All sorters also generate smaller microdroplets, i.e. satellite droplets. Moreover, during sorting it is possible that the nozzle orifice can be partially obstructed due, in part to large cell aggregates, resulting in a disruption of this defined droplet pattern and trajectory. In this case, the stream may deviate and impact a hard surface such as the stream waste collection trough. This can result in unwanted aerosols being created that have recently been determined to be at concentrations as high as 1.8 × 104 particles per cubic centimeter (3). In this same study, a significant positive correlation was shown between aerosol concentration and sheath pressure. Therefore, high-speed cell sorters operating at pressures of 70 psi or higher have a greater potential for high concentration aerosol release. Significantly, the aerodynamic diameter of these aerosols was measured to be between 1.6 and 2.1 μm (3), a size range associated with increased alveolar deposition and infectivity (see above). The possible release of aerosols in high concentration that are within the respirable size range and are associated with infectivity by pathogens, mandates that safety procedures as outlined in this document be adopted in order to mitigate the risks of exposure to cell sorter operators.
Standard Precautions and Other Regulatory Requirements
In the United States, all laboratory personnel who handle human cells and other potentially infectious materials, such as specimens from experimentally infected animals, are required to follow Standard (Universal) Precautions. These procedures are outlined in the Occupational Safety and Health Administration document “Occupational Exposure to Bloodborne Pathogen Standard” (21) and must be adhered to in addition to specific local and institutional safety regulations. Laboratories also must comply with federal code regulations for possession, use, and transfer of select agents and toxins (22,23). All NIH funded non-exempt recombinant DNA experiments have to be performed in compliance with the specific NIH guidelines (24) and have to be approved by Institutional Biosafety Committees. All institutions receiving grant or contract awards from NIH are required to follow the current health and safety guidelines published at http://grants.nih.gov/grants/policy/policy.htm.
Other countries have developed their own stringent regulatory standards and/or have adopted aspects of regulations and guidelines for work with biological agents as mandated in the US. International biosafety regulations, guidelines, and information sources are available online through the European Biosafety Association at http://www.ebsaweb.eu/Home.html or through Biosafety-Europe at http://www.biosafety-europe.eu/index.html. Examples include, Directive 2000/54/EC—biological agents at work (https://osha.europa.eu/en/legislation/directives/exposure-to-biological-agents/77), “The Advisory Committee on Dangerous Pathogens: Protection against blood-borne infections in the workplace: HIV and Hepatitis” (http://www.hse.gov.uk/biosafety/diseases/bbv.pdf), the “Canadian Biosafety Guidelines, 3rd edition” (http://www.phac-aspc.gc.ca/lab-bio/res/blk-acb/lbg-ldmbl-eng.php), the “Handbook on the Regulation of Gene Technology in Australia” (http://www.svhm.org.au/research/governance/Documents/handbook%5B1%5D.pdf), and the World Health Organization (WHO) Laboratory Biosafety Manual, available on line at http://www.who.int/csr/resources/publications/biosafety/WHO_CDS_CSR_LYO_2004_11/en/index.html. Furthermore, guidelines for specimen handling based on US regulations that are focused on clinical settings are published in the document “M29-A3” by the Clinical and Laboratory Standards Institute (25) and also by the Morbility and Mortality Weekly Report (26). Relevant details for the preparation of infectious samples containing HIV for flow cytometry, such as shipping and receiving of specimens, local transport, staining, and disposal, were previously described (27). Each laboratory must develop or adapt a biosafety operations manual, which specifies practices designed to minimize risks and takes into account the biohazard potential of the specimens that are processed (28). Conformity to established safety regulations and practices are the responsibility of the laboratory director. Personnel must be trained in the required procedures and strict adherence to the techniques set forth is essential.
Biosafety Principles and Cell Sorting
The two fundamental principles of the practice of biosafety are risk management and risk assessment. Risk management encompasses protective equipment and engineering controls, personal protective clothing and equipment, safe laboratory procedures, and laboratory design for containment. Containment refers to safe methods in the laboratory designed to reduce or eliminate exposure of lab workers and the outside environment to potentially hazardous agents. Containment methods are based on the type of organism and the type of risk associated with the procedure. Risk assessment is defined as the identification of hazards and the measurement of the risk or probability that something will happen as a result of that hazard. Risk assessment takes into account the Risk Group (see below) of the agent and the procedures performed with the agent. The designation of safety measures is dependent upon the risk and the severity of the consequences if exposure occurs. Risk assessment is the responsibility of the directors and principal investigators of the laboratory, in conjunction with biosafety professionals, subject matter experts and members of safety committees such as Institutional Biosafety Committees. Specifically for cell sorting laboratories, the principal investigator or laboratory director must be knowledgeable in flow cytometric sorting technology in order to conduct an accurate risk assessment. The results of a comprehensive risk assessment determine the appropriate procedures and practices for cell sorting.
Risk assessment for cell sorting follows the general principles of risk assessment for other laboratory activities and is composed of five steps:
Identify and evaluate agent hazards: To aid in the identification of risks associated with biohazardous agents, microbiological agents have been classified into one of four Risk Groups (RG) by the WHO, Canada, Australia, the European Union and the NIH Recombinant DNA Advisory Committee (see http://www.absa.org/riskgroups/index.html). Although these classifications differ dependent upon the country or organization, they generally will take into account factors such as pathogenicity of the organism, virulence, mode of transmission, infectious dose, communicability and availability of effective vaccines or effective treatment. The CDC/NIH Biosafety in Microbiological and Biomedical Laboratories (BMBL; Ref. 9) guidelines do not use risk groups, but provide description of four biosafety containment levels that provide a starting point for the containment of biohazards within each of the four bands that mirror risk group classifications. These biosafety level designations (BSL1 through BSL4) will be used in this document. Within the UK, similar criteria is used but are designated CL1 through CL4 (http://www.hse.gov.uk/pubns/misc208.pdf). There is a wide range of risk within each risk group classification, underscoring the importance of conducting a risk assessment for each biohazard. Agent characteristics to consider may involve the degree of attenuation, fixatives used to inactivate the agent, route of infection, and how a pathogen may have been rendered defective. Other characteristics that could elevate risk include the use of strains for which immunization is not protective, prior LAIs with the agent via the airborne route, and agents with a high consequence of infection.
Identify laboratory procedure hazards: It is important to emphasize that the second major factor to consider in risk assessment are the laboratory procedures in agent handling. Procedures with biohazards involving the use of sharps, those involving research animals, and those that may generate splash, splatter or aerosols can elevate risk. For example, human pathogens that are designated as Risk Group 2 agents under normal laboratory procedures and practices may be classified at a higher biosafety containment level because of the potential for aerosol and/or splash exposure (29). Cell sorting is therefore considered a laboratory procedure hazard due its potential for aerosol production.
Make final determination of biosafety level and assign additional precautions as indicated by the risk assessment: Table 1 provides general guidelines for the determination of biosafety containment practices as applied to certain risk assessment conditions during cell sorting. Biosafety levels 2, 3, and 4 are listed in Table 1 together with BSL2 with enhanced precautions. BSL2 with enhanced precautions is not a biosafety level, but reflects procedures and practices at the BSL2 level together with additional procedures as specified in Table 1 and in Sections 2 and 4 of the ISAC Biosafety Standard for Cell Sorting (below). The guidelines for risk assessment in Table 1 take into account both agent hazards and sample origins for assignment of biosafety containment levels and procedures. This therefore combines agent Risk Group classifications (i.e. WHO and others http://www.absa.org/riskgroups/index.html) and OHSA Standard Precautions (21) and similar Standards for UK, Canada, Europe and the Far East: see Ref. 30), coupled with the recognition of cell sorting as a laboratory procedure hazard. In this regard, handling of all human and non-human primate specimens and primary human cell cultures as infectious is recommended. (See the Biosafety Standards for Cell Sorting Section below for further detail.) Although impractical for most cell sorting experiments, samples may be fixed in order to reduce the biocontainment level required. However, in this case, appropriate methods must be selected to reliably inactivate potentially biohazardous agents. Concerns exist about the effectiveness of standard fixation methods to reduce the level of infectivity in samples containing high titers of known viruses or unknown infectious agents resistant to inactivation (31,32). Fixation procedures must be performed carefully within well-defined standard operating procedures; otherwise, samples that are presumed inactivated, may not be and therefore could pose a serious health risk to laboratory personnel. Cell sorting operators or managers and IBCs may require proof of inactivation for higher risk biohazards to ensure that the biosafety level selected is appropriate for the proposed sorting experiment.
-
Evaluate proficiencies of staff and integrity of safety equipment: It is critical to evaluate the level of proficiency of the cell sorter operator in conducting a risk assessment. This includes an evaluation of cell sorter operating skills, as well as techniques for safe handling of specimens and use of any safety equipment. Proficiency in the operation of the cell sorter is particularly important in the event of a nozzle obstruction with subsequent aerosol production. In this case, an inexperienced operator will focus on instrument operation and thus will be more likely to ignore or circumvent biosafety features and procedures resulting in potential exposure. Training of cell sorter operators is therefore an essential component of the cell sorting laboratory’s operational procedures. The amount of training deemed sufficient for independent operation of a cell sorter is dependent upon several factors, but must include the results of the risk assessment process. Specifically, for sorts requiring higher biosafety containment levels (BSL2 with enhanced precautions, BSL3 or BSL4) the degree of training and experience must be correspondingly greater. For independent operation of cell sorters at these biosafety containment levels, a checklist of requirements of experience/training is essential to ensure safe operation. These must include required institutional biosafety training such as bloodborne pathogen training, BSL3/4-specific training, BSC training, etc. but also instrument experience, such as hours of supervised and independent cell sorting operation. Ideally, before sorting samples at a higher biosafety containment level, initial training should include sorting on cell sorters of similar design using non-infectious samples of the same type that will contain the known biohazard. In addition, when procedures are changed, all operators should be required to review these procedures and documentation of this review must be maintained.
All safety equipment must be inspected or tested to verify functionality. For cell sorters, evaluation of safety equipment includes visual inspection of sort/collection chamber doors to ensure integrity, or absence of dirt and/or salt crystals on seals; presence of an aerosol management system and validation of containment (see below) and verification that all other supplied safety features are intact.
Review risk assessment with biosafety professional: Table 2 shows examples of agents and their biosafety levels; however, as stated above the final determination of the biosafety level is dependent upon the result of the risk assessment by (a) the Principal Investigator, (b) lab director and operator of the cell sorting laboratory in conjunction with (c) biosafety specialists, (d) subject matter experts and (e) the institutional biosafety committee (IBC), or equivalent. This is not a comprehensive list of possible agents that may be encountered; see BMBL (9) and Canada Pathogen Safety Data Sheets (PSDS; http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/index-eng.php) for more information.
Table 1.
Biosafety level determination for cell sorting
| BSL2 | BSL-2 WITH ENHANCED PRECAUTIONS (DURING SORTING OPERATIONS) | BSL3 | BSL4 | |
|---|---|---|---|---|
| Risk Assessment Condition | Uninfected non-primate cells | Non-infectious Human/NHP cells; Infectious but with low risk assessment | Infectious samples with high risk assessment; All samples containing known aerosol pathogens | Extremely Dangerous Pathogens |
| Example sample type or agentsa | Normal murine cells third-generation Lentivirus (non-human cells) | Normal human blood; Human cell linesa; An example agent is: Influenza Aa; second-generation Lentivirus or third-generation in human cells | Example agents includea: Mycobacterium Tuberculosis, Monkeypox | Example agents includea: Ebola, Marburg |
| Containment System Validated | Periodically (monthly or with filter change)b | Periodically (monthly or with filter change)b | Weekly or before Every Sortb | Weekly or before Every Sortb |
| Aerosol Containment Operational | Required | Required | Required | Required |
| Respirator | Optional | N-95, FFP2 or betterc | PAPR | Special Suit |
| Eye protection | Safety Glasses | Face shield or safety goggles | N/A | N/A |
| Lab Coat | Front Closure lab coat | Wrap around, solid-front | Coveralls | Special suit |
| Separate Room and Environmental controls | Optional | Required or limited access to roomd | Requirede | Requirede |
Example Sample type or Agents—the samples and/or agents listed represent only a partial list of agents, which may be included in each category. A risk assessment should be conducted for all samples/agents before sorting, and the appropriate biosafety level determined in collaboration with safety specialists, cell sorter operators, subject matter experts and the Institution’s IBC or equivalent. For additional information please consult the following web sites: http://www.phac-aspc.gc.ca/msds-ftss/index-eng.php; http://www.cdc.gov/biosafety/publications/bmbl5/index.htm.
Frequency of testing will be dependent upon the risk assessment and consultation with biosafety professionals and/or the IBC or equivalent. For more detail see Section 3.1.1.1.
Respirators must remain on during all procedures associated with sample manipulation, including sample tube cap removal and loading of sample on instrument, or when removing collection tubes or other procedures where the sort or collection chamber is opened. Note that respirator protection may otherwise be removed during the sorting process providing the aerosol management system is active and all sort chamber and collection chamber doors are closed. For human pathogens, i.e. Risk Group 2 agents, which are classified as BSL2 and are not respiratory hazards, but which may pose a risk if exposed to mucous membranes, only mucous membrane protection is required. Examples of agents in this category include Leishmania and toxoplasmosis in murine cells.
Enclosure of the cell sorter within a certified (see Section 3.1.1.2) Class II BSC may abrogate the need to house the sorter in a separate room within the BSL2 lab space; PPE (as detailed above) is optional, but strongly encouraged for the operator during procedures requiring manipulation of instrument. Cell sorters located within a shared laboratory may be operated under BSL2 with enhanced precautions if during the operation of the sorter, access to the room is limited and PPE as detailed above is worn by all occupants.
Enclosure of cell sorter within a certified (see Section 3.1.1.2) Class II BSC required.
Table 2.
Recommended agent biosafety level for cell sortinga
| RECOMMENDED BIOSAFETY LEVEL | RESTRICTIONS OR COMMENTS | PSDSb LINK | |
|---|---|---|---|
| 1918 Influenza | BSL3 | Influenza (seasonal) vaccine required | |
| Avian influenza | BSL3 | Influenza (seasonal) vaccine required | |
| H1N1 | BSL3 | H1N1 vaccine required; | |
| Hepatitis C | BSL2 w/enhanced precautions | http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/msds77e-eng.php | |
| HIV | BSL2 w/enhanced precautions or BSL3 | http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/hiv-vih-eng.php | |
| Human Metapneumovirus | BSL2 w/enhanced precautions | ||
| Human Parainfluenza Virus type 3 | BSL2 w/enhanced precautions | ||
| Influenza A | BSL2 w/enhanced precautions | Influenza (seasonal) vaccine required | |
| Klebsiella pneumonia | BSL2 w/enhanced precautions | http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/kleb-siella-eng.php | |
| LaCrosse virus | BSL2 w/enhanced precautions | ||
| LCMV | BSL2 w/enhanced precautions or BSL3 | Ensure that HVAC system does not exhaust near vivarium housing mice; BSL dependent upon strain and days post infection; pregnant women should consult Occupational Medicine or their personal physician before prior to performing a procedure with this agent. | http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/lympcho-eng.php |
| Leishmania | BSL2 w/enhanced precautionsc | http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/leishmania-eng.php | |
| Malaria | BSL2 w/enhanced precautionsc | ||
| Monkeypox | BSL3 | vaccinia vaccine required, every 3 years | |
| PVM (Pneumonia Virus of Mice) | BSL2 w/enhanced precautions | ||
| Respiratory Syncytial Virus | BSL2 w/enhanced precautions | http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/pneumovirus-eng.php | |
| Toxoplasma gondii | BSL2 w/enhanced precautions | Pregnant women should consult Occupational Medicine or their personal physician before performing a procedure with this agent. | http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/msds153e-eng.php |
| Vaccinia | BSL2 w/enhanced precautions | Vaccine required | http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/msds160e-eng.php |
| TB, Mycobacterium tuberculosis | BSL3 | http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/msds103e-eng.php |
This list represents examples of biosafety level determination for cell sorting of specific agents. The final determination of the biosafety level is dependent upon the risk assessment conducted in collaboration with safety specialists, subject matter experts, and the IBC or equivalent.
Pathogen Safety Data Sheets from the Public Health Agency of Canada.
Respirator PPE optional for this agent, except where the sample also contains human/NHP blood cells or fluids. Note: mucous membrane protection required.
The above risk assessment guidelines can be applied to all samples/agents, but two sample/agent types are discussed in more detail below because of their widespread use in biomedical cell sorting laboratories.
Human Cell Lines
Although the 1991 OSHA Bloodborne Pathogen Standard (BPS) clearly defined biosafety requirements for handling of human blood and body fluids and other potentially infectious material, the appropriate biosafety containment level was ambiguous for established human cell lines that were in vitro or animal-passaged human explanted tissues transformed by spontaneous mutation or a natural or laboratory infection with an immortalization agent, e.g., Epstein–Barr virus. To clarify this issue, OSHA released in 1994 an Interpretation of the Standard for established human cell lines (http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=INTERPRETATIONS&p_id=21519). In this interpretation, human cell lines are considered to be potentially infectious and therefore covered by the BPS unless the established cell line has been characterized to be free of bloodborne pathogens including HIV, Hepatitis B and C virus, Plasmodium falciparum, and pathogens used to transform cells such as EBV and human papilloma viruses. In keeping with this interpretation, cell lines from the American Type Culture Collection (ATCC) and other sources bear warnings that they may contain blood-borne pathogens, and ATCC recommends that universal precautions according to 29 CFR 1910.1030 (21) should be followed. Since it is not possible to test cell lines for every possible human pathogen or to assert that they are pathogen-free, and due to the potential for aerosol exposure during cell sorting, human cell lines are to be sorted using biosafety precautions appropriate for human blood and body fluids, i.e. BSL2 with enhanced precautions.
Organisms Containing Recombinant DNA Including Lentiviral Vectors
Risk assessment is likely to be most difficult for samples containing recombinant DNA molecules (33). In recent years, technologies have evolved that lead to the generation of modified viruses, bacteria, yeast, and other microorganisms. Common recombinant viruses include lentiviruses, adenoviruses, alphaviruses, retroviruses, vaccinia, and herpesviruses designed to express heterologous gene products. The challenge faced when selecting the appropriate biosafety level for such work begins by establishing the classification of the nonmodified virus and then proceeds to an evaluation for a possible increase in hazard potential associated with a given genetic alteration. Of particular concern are modifications that result in expression of a toxin or a known oncogene, or of sequences that alter the host range or cell tropism, or allow the virus to integrate into the host genome. If required, advice from a virologist or the local IBC should be sought to determine the proper BSL for planned flow cytometric experiments, but the NIH has also published guidelines for research involving recombinant or synthetic nucleic acid molecules that classify recombinant agents into risk groups (Ref. 24; http://oba.od.nih.gov/rdna/nih_guidelines_oba.html). Lentiviruses have become one of the most practical gene transfer vectors because of their stable integration and long-term gene expression. They belong to the Retroviridae family, which can deliver a large amount of genetic information into the DNA of host cells and have the unique ability among retroviruses of being able to replicate in non-dividing cells. However, since these vectors are derived from viruses that may be pathogenic to humans, in particular HIV-derived vectors, the biosafety of these vectors is of utmost concern. To address safety issues, the NIH Recombinant Advisory Committee has developed guidelines (http://oba.od.nih.gov/rdna_rac/rac_guidance_lentivirus.html) which provide criteria important for risk assessment of lentiviral vectors including the nature of the vector system and the nature of the transgene insert. Researchers have made various modifications to the HIV-based vector and packaging systems in order to improve biosafety. These have included modifications to the packaging plasmid, which have been termed first-, second-, and third-generation vectors; and modification to the transfer plasmid in order to create self-inactivating vectors (34). Hence, the modifications present in the third generation, self-inactivating vectors are considered to confer a higher level of biosafety (34). Table 1 therefore indicates a recommended biosafety containment level of BSL2 for third-generation lentiviruses in transduced murine cells, whereas first- and second-generation lentiviruses would demand BSL2 with enhanced precautions. However, human cell lines or primary human cells transduced with lentiviruses, even if they are third-generation, would be sorted at BSL2 with enhanced precautions as outlined above and in Table 1 and Sections 2 and 4. Caution should be exercised however, when performing the risk assessment on lentiviral vectors, since the cell sorting lab director may not be able to obtain all of the necessary documentation relevant to the vector system. For example, information as to whether a lentiviral vector mix obtained commercially is a true third-generation system may not be provided either by the commercial vendor or the investigator. Therefore, consultation with biosafety professionals and the Institutional Biosafety Committee or equivalent is an important adjunct for risk assessment of these vector systems.
Standard Operating Procedure Development
An important outcome of any risk assessment process is the creation of SOPs. The SOP takes into account hazards (agent and laboratory procedure) and specifies practices and procedures designed to minimize or eliminate exposures to these hazards. For cell sorters, the design of the instrument, especially containment or aerosol evacuation components, must be considered in the development of the SOP. Each instrument must be evaluated for deficiencies in containment or aerosol evacuation design and appropriate procedures adopted to minimize risk. An important example of this is to consider an interlock designed to prevent the operator from opening the sort chamber after a nozzle obstruction with subsequent stream deviation. Unfortunately, most cell sorters do not possess this system and therefore, the SOP should clearly address the procedures for evacuating the sort chamber of aerosols before opening the sort chamber, including a stated time period to wait after a clog induced stream deviation. Development of the SOP should also include consultation with safety specialists who can provide guidance on general biosafety procedures. Examples of SOPs for cell sorters are included in Appendix D to serve as templates for development of individual laboratory SOPs. Finally, the SOP should be reevaluated at least on an annual basis or whenever there is a change in instrument configuration that may affect biosafety.
General Considerations for SOP Development and Specific Recommendations
-
Preparation before the sort
-
Check fluids, empty waste
Set up a rigorous sorter preventive maintenance schedule either as part of a service contract offered by the manufacturer of the instrument or performed by laboratory personnel. Routinely perform leak checks on the fluid lines of the cell sorter. To do this, gain access to the fluidic lines. Carefully check for wet areas, indicating leaks in the tubing. Inspect tubing for cracks and signs of stress, particularly around the fittings and valve junctions. Also, inspect sheath lines and waste lines, and replace any leaking tubing.
-
Use protective covers for computer control surfaces, including keyboards and computer mice
Alternatively, cover control surfaces with plastic wrap, or use washable computer keyboards and mice.
-
Perform containment testing (see Section 3.1 and Appendix C)
-
As previously recommended (2) validation of containment and evacuation of aerosols is essential for operator safety. Testing on individual cell sorters may differ due to variation in cell sorter design among the available models, but it is essential that the following considerations are incorporated into the SOP:
Fail mode testing: test is designed to mimic a nozzle obstruction with stream deviation and the subsequent generation of aerosols.
Testing frequency: dependent upon risk assessment, and biosafety containment level. See Section 3.1.1.1.
Containment testing for sorter in BSC: aerosol containment and evacuation of sorter independent of BSC operation must be performed.
Record keeping.
-
-
Verify sorting operation and sample introduction system
Before each sort, verify the proper operation of the sort mechanism and the stability of the sort streams and droplet break-off. If the streams and the droplet break-off do not remain stable during the sort setup, correct the problem before sorting.
Cell sorters pressurize the sample tube once it is secured on the sample introduction port. While newer generation instruments are equipped with completely enclosed sample introduction chambers for operator safety, some older sorters have an open port requiring careful operator handling. Each time a sample tube is placed on the instrument, the operator must check the tube seal and its secure fit onto the sample introduction port. Otherwise, once the sample tube is pressurized, it could blow off and splash sample onto the operator or others involved in the experiment. Make sure that the tube material provides sufficient strength to tolerate high instrument pressure. On some instruments, when the tube is removed, the sample line back-drips, creating a potential biohazard through splattering of sample droplets on hard surfaces. To avoid this hazard, allow the back-drip to go into a tube until the sample is flushed out of its introduction line to avoid splashing of sample droplets. Alternatively, a soft absorbent pad soaked in disinfectant can collect the back drip without splattering. Installation of a plastic shield around the sample introduction port can block droplet spraying from the sample back-drip. The catch tray or trough should be decontaminated carefully after each sort.
Select the appropriate nozzle size for the cell size to be sorted. Smaller nozzle sizes provide optimal signal resolution and easy sort setup, however, to avoid clogs, it is recommended that the nozzle orifice be at least four times larger than the cell diameter (35), but ideally it should be at least six times larger.
-
Verify any automated decontamination functions
If these systems are not used on a regular basis, it is possible that valves, connectors or pumps may fail due to buildup of salts, etc.
-
Preparation of disinfectant solutions
Disinfectants should be made before starting the sort, especially for those that have limited shelf life, such as solutions of sodium hypochlorite.
Sample preparation, i.e. staining, centrifugation, pipetting, or manipulations that may generate aerosols should be performed in a manner to maximize containment and protect the worker (see Section 1 of Biosafety Standards for Cell Sorting below).
-
-
Nozzle obstruction and decontamination
-
Procedures in the event of a nozzle obstruction
-
Turn off stream
Many models of newer sorters have safety devices that will stop the sorting process as soon as a clog develops and cover the collection vessels. The stream must be turned off manually if the automated function fails.
-
Evacuate sort chamber before opening;
Increase Aerosol Management System (AMS) evacuation rate to maximum for 2 min or more.
Attempt to clear nozzle clog by stream flush routines, with sort chamber door closed. If clog is not cleared, remove the nozzle and dependent upon sample risk assessment, decontaminate nozzle before sonication
-
-
Decontamination procedures
All decontamination procedures should be validated and documented per Institution guidelines.
Decontaminate and clean sample lines, sort chamber and collection chamber
Decontaminate and clean surfaces around cytometer, especially near the sort chamber after each sort, the instrument should be decontaminated with a disinfecting agent, taking into account the biohazards under study. Sort collection tube holders are heavily exposed to sample droplets and must be carefully decontaminated before handling. Before designing a cell sorter-specific decontamination protocol, the operator or laboratory manager should consult the instrument manufacturer for compatible disinfectants and refer to more complete resources for decontamination (9,36). Two disinfectants commonly used in cell sorters are alcohols and bleach. Alcohols are not classified as high-level disinfectants, because they cannot inactivate bacterial spores and penetrate protein-rich materials, and isopropanol is not able to kill hydrophilic viruses. Aqueous solutions of sodium hypochlorite are widely used because they have a broad spectrum of antimicrobial activity, are inexpensive, fast acting, are unaffected by water hardness and do not leave a toxic residue. They can be corrosive to metals and therefore should be rinsed with water following decontamination. All surfaces inside the sort chamber, the sample introduction port and holder, are wiped down with appropriate disinfectant. Disinfectant is also run through the instrument for the appropriate exposure time and then followed with distilled water to completely remove the disinfectant as some disinfectants are corrosive to instrument components (consult manufacturer), and residual disinfectant solution can affect the viability of sorted samples. Make sure that the water used for removal of the disinfectant is sterile and does not introduce new contaminants into the instrument.
-
-
Stream cameras and remote sorting
Viewing cameras focused on sort streams are standard on newer sorters. They create distance between the sorter operator and the area of the sorter that poses the greatest potential biohazard. Viewing systems that illuminate the center stream and the deflected streams near the sort collection are recommended as they allow the operator to monitor increased aerosol production because of shifting stream positions and fanning.
Remote computer control. For BSL3/4 labs, it is desirable that the cell sorter be controlled remotely or outside of the BSL-3/4 facility. Together with cameras or indicators that sense sample and collection tube status, software is available for remotely viewing and controlling the cell sorter acquisition computer. These programs allow users to monitor all vital functions of the sort operation with the maximum safety factor (37).
Biosafety Standards for Cell Sorting
The following Standards provide requirements for the operation of jet-in-air cell sorters, including sample preparation, disinfection, room design, cell sorter-specific biosafety equipment, and user-specific safety equipment. The intent of these Standards is to augment existing laboratory biosafety policies and practices (see “Standard Precautions and Other Regulatory Requirements,” above) for the class of instruments known as cell sorters, or fluorescence activated cell sorters and does not reduce or alter the requirements of these other policies. These Standards should be incorporated into the SOP of the cell sorter laboratory.
-
1
General Considerations
-
1.1
Specimen processing before cell sorting should ideally be performed in a BSC. If a BSC is unavailable, universal precautions procedures should be followed and appropriate additional PPE used (such as respirator and/or splash shield) as determined by the risk assessment. Capped tubes or microtiter plates with sealed covers should be used as sample containers. For local transport, place primary collection tubes or sample tubes into a secondary container that is able to contain the specimen in case of breakage of the primary container, e.g., a plastic carrier with a secure lid. Place absorbent between the primary and secondary containers to collect any liquid if leakage does occur. Place a label containing the universal biohazard symbol accompanied by the word “Biohazard” on the exterior of the container. For specimen centrifugation, use sealed vessels or safety carriers.
-
1.2
Avoid use of “sharps”: Avoid the use of needles, glass pipettes, glass transfer pipettes, or glass containers or tubes whenever possible for handling or transferring any biological material, and use suitable replacements. For disposal of any “sharps” a leak proof, puncture-resistant container as specified by local biosafety regulations must be used.
-
1.3
No mouth pipetting: No mouth pipetting is allowed. Manual pipetting devices must be used and must be equipped with filters to prevent infectious liquid from contaminating the pipetting device.
-
1.4
Sample preparation steps to minimize potential aerosol formation: Samples that are to be sorted need to be prepared as single cell suspensions because aggregated cells can partially or completely clog sort nozzles. Partial nozzle obstruction with subsequent stream deviation has the highest potential to create aerosols. Furthermore, any interruption of a potentially biohazardous sort increases the risk of operator exposure to pathogens contained in the sort sample because of an increased probability for splashes and escape of sort aerosols during the manipulations required to continue sorting. To reduce the formation of cell aggregates during sample preparation, samples should be centrifuged gently, e.g., at 300g for 5 to 10 min. Higher centrifugation speeds can damage cells and compact them so densely that they are difficult to break apart. Samples with low viability such as frozen cell samples that are thawed may release DNA into the media. DNA binds to the surface of live cells, and after centrifugation these samples form solid aggregates leading to nozzle clogging problems and excessive aerosol formation. In these situations, addition of 20 μg/ml of RNase-free DNAse for 10 min at 37°C will prevent aggregation (38). Just before sorting, it is recommended that samples be filtered through nylon mesh filters, e.g., different pore size meshes, tubes with cell strainer caps, or individual cell strainers to remove any remaining cell aggregates and debris. Selection of an optimal solution for sample resuspension to maintain cell viability is important. Highly concentrated cell suspensions have an increased tendency to clump, therefore, dilute them to the lowest possible density for the sort speed used. Other sample preparation guidelines for high speed sorting have been published (39).
-
1.5
Work area clean-up: Work areas must be cleaned routinely. Discard all contaminated materials, e.g., sample and collection tubes, pipettes, pipette tips, gloves, laboratory coats, into appropriate biohazard containers. Follow the established procedures at your institution for storage and disposal of biomedical/hazardous waste. Generally, this involves either autoclaving or decontamination with a 1/10 volume dilution of 0.71 M sodium hypochlorite (undiluted household bleach) before waste disposal. Wipe off all work surfaces with an appropriate disinfectant solution, taking into account the potential biohazard. As per regulations outlined in the Blood-borne Pathogen Standard (21) appropriate disinfectants for decontamination of equipment and exposed work surfaces include diluted bleach, Environmental Protection Agency (EPA) registered tuberculocides, EPA-registered sterilants, and products registered to be effective against HIV or HBV as listed online at http://www.epa.gov/oppad001/list_d_hepatitisbhiv.pdf. Summary information on the survival and disinfectant inactivation of HIV has been reviewed (27). More complete information on general disinfectant choices and procedures has been published (9,36).
-
1.6
Disinfection of spills: After any spill of biological material, the protection of personnel is the first priority. In general, for small spills on a non-permeable surface, a disinfecting agent, e.g., a 1/10 volume dilution of 0.71 M sodium hypochlorite (undiluted household bleach) is applied to a paper towel, placed on the spill, and allowed to make contact for an appropriate time to inactivate any biological organisms. Rapid cleanup of contamination should be an established laboratory practice. For the handling of larger spills or spills on a non-smooth or permeable surface, refer to the Clinical and Laboratory Standards Institute document (25) or the biosafety office of your institution. Note: spill response procedures commensurate with the biohazard risk presented by the agent and potential for release must be established in advance of the sort. Spill response procedures must be approved by the Cell Sorting Facility Manager, the Biosafety Officer and the Institutional Biosafety Committee, or equivalent. The procedures must also be practiced and understood before sorting biohazardous samples. Procedures must also take into account the risk of aerosol contamination of the operator or individual in the spill at the time of release. Immediate evacuation of the area is recommended to minimize contamination of personnel and lower risk of secondary exposure when removing personal protective clothing and equipment.
-
1.7
Preparation for instrument service: The cell sorter must be decontaminated following the established laboratory SOP decontamination procedures before allowing access to service engineers. Service engineers must comply with entry and PPE requirements of the laboratory. Minimally, gloves, safety glasses, and lab coat should be worn. If manipulation of the aerosol management system is necessary, components of this system must be assumed to be contaminated. Appropriate PPE should be worn when components are removed or disconnected (see procedure as outlined in Appendix D, 2.b.).
-
1.1
Special Operator Specific Recommendations
Consultation with environmental health and safety specialist or employee medical services should be consulted regarding serum banking policies and the availability of vaccines for specific agents, including, but not restricted to Hepatitis B vaccines. In addition, identifiable subpopulations of workers having a higher risk, such as pregnant women and workers with immunodeficiency should consult occupational health staff.
-
2
Room design (Adapted and Extended from bmbl, 5th Ed., Section IV)
-
2.1
Biosafety Level 2 Laboratory—General
-
2.1.1
The laboratory must meet all criteria for BSL2 containment as established by local, institutional or national regulatory agencies.
-
2.1.2
A sign incorporating the universal biohazard symbol must be posted at the entrance to the laboratory when infectious agents are present. Posted information must include: the laboratory’s biosafety level, the supervisor’s name (or other responsible personnel), telephone number, and required procedures for entering and exiting the laboratory. Agent information should be posted in accordance with the institutional policy.
-
2.1.3
It is recommended that air flow in the room is balanced to create negative airflow into the room. It is recommended that a visual or audible monitoring device be located at the door to measure negative airflow.
-
2.1.4
Laboratories must have a sink for hand washing. The sink may be manually, hands-free, or automatically operated. It should be located near the exit door.
-
2.1.5
The laboratory should be designed so that it can be easily cleaned and decontaminated. Carpets and rugs in laboratories are not permitted.
-
2.1.6
Vacuum lines should be protected with High Efficiency Particulate Air (HEPA) filters, or their equivalent. Filters must be replaced as needed. Liquid disinfectant traps may be required.
-
2.1.7
An eyewash station must be readily available.
-
2.1.1
-
2.2
Biosafety Level 2 with Enhanced Precautions
-
2.2.1
The laboratory must meet all criteria for BSL2 containment as outlined above, but in addition meet the following criteria:
-
2.2.2
Ideally, the cell sorter is located in a separate, lockable room where no other laboratory activity is performed. If the cell sorter is enclosed within a certified1 Class II BSC, the requirement for placement of the cell sorter in a separate room may be abrogated, dependent upon the overall risk assessment.
-
2.2.3
If the sorter is located in shared laboratory space, all PPE requirements (as outlined in Section 4 and in Table 1) should be followed by all personnel during sorting procedures. The cell sorter should be placed in a location in the lab so that directional air flow is toward the cell sorter and away from other areas of the lab.
-
2.2.4
If possible, air flow in the room is balanced to create negative airflow into the room. It is recommended that a visual monitoring device be located at the door to measure negative airflow. Your Institution’s biosafety committee may require that cell sorters be placed in a laboratory with negative pressure, due to the high risk of aerosol generation.
-
2.2.5
The sorting room is locked to restrict access and allow the operator to concentrate on the sort and to maintain regular air flow and negative air pressure in the room.
-
2.2.6
A sign incorporating the universal biohazard symbol must be posted at the entrance to the laboratory when infectious agents are present. Posted information must include: the laboratory’s biosafety level, the supervisor’s name (or other responsible personnel), telephone number, and required procedures for entering and exiting the laboratory. Agent information should be posted in accordance with the institutional policy. During sorting procedures, a sign should be placed on the outside of the door to indicate that a potentially biohazardous sort is in progress, and include a description of the PPE required (see Section 4).
-
2.2.1
-
2.3
Biosafety Level 3 Laboratory
-
2.3.1
The laboratory must meet all criteria for BSL3 containment as established by local, institutional, or national regulatory agencies.
-
2.3.2
The cell sorter must be located within a certified1 BSC or equivalent primary containment device. Enclosure in a Class II BSC is recommended; however, if permitted by local Institutional biosafety regulations, a certified1 Class I BSC may be used providing that the airflow is verified to be have an inflow velocity of 100 fpm, and procedures are established for annual leakage testing of HEPA filters.
-
2.3.1
-
2.4
Biosafety Level 4 Laboratory
-
2.4.1
The laboratory must meet all criteria for BSL4 containment as established by local, institutional or national regulatory agencies.
-
2.4.2
The cell sorter must be located within a certified1 Class II BSC or equivalent containment device.
-
2.4.1
-
2.1
-
3
Cell Sorter Specific Safety Equipment and Practices
-
3.1
Aerosol Containment
-
3.1.1
Aerosol management System (AMS): All cell sorters must be equipped with an aerosol management or evacuation system which is designed to evacuate the sort chamber and sort collection area of the cytometer. It consists of an evacuator that creates negative pressure within those chambers and transports aerosols through a HEPA or ULPA filter before exhausting to the room. The AMS should be operated during sorting operations and under all biosafety levels, BSL2, BSL2 with enhanced precautions, BSL3 and BSL4.
-
3.1.1.1
Validation of aerosol containment systems
Currently, the most widely accepted method of containment testing utilizes fluorescent plastic particles, which are run on the instrument using normal sample testing conditions (40,41). The AMS must be tested under simulated worse case “failure mode”. In this mode, the instrument is set to high pressure (usually 70 psi) and fluorescent particles are concentrated to approach speeds of approximately 20,000 to 50,000 particles/s. The stream is forced to glance off of the waste catcher shield (or the waste catcher is covered with tape or a piece of tubing) to create a large plume of aerosols and aerosols concentrated on a slide for subsequent analysis on a microscope. Tolerance of aerosol escape is zero particles when the AMS is active and sort chamber door is closed. Frequency of testing will be dependent upon the risk assessment and consultation with biosafety professionals and/or the IBC or equivalent. However, containment testing must be performed in the following circumstances:
Following instrument service or maintenance involving the sort chamber and/or AMS hose connections.
Following initial instrument installation or relocation.
Following change out of the standalone AMS filter.
-
For BSL3/4 labs:
Before every sort if the frequency of sorting is once/week or less
Weekly, if the frequency of sorting is multiple sorts/week
Details of the validation procedure are in Appendix A.
-
3.1.1.2
Cell sorters in biological safety cabinets
Class II BSCs enclosing cell sorters must be manufactured to meet functional certification criteria for personnel and product protection as defined by NSF 49 (US (42)) or CSN EN 12469 (Europe (43)) or JIS K 3800: 2009 (Japan (44)) or AS 2252.2 (Australia (45)). Class I BSCs enclosing cell sorters must be manufactured to meet functional certification criteria for personnel protection as defined by the BMBL (9) or EN 12469, although it is recommended that the inward airflow velocity be 100 linear feet per minute or greater; HEPA filters must be tested for leakage annually. Cell sorters placed in BSCs must have an AMS in which aerosol containment validation can be performed independent of the BSC blowers, i.e. with the BSC directional air current system turned off. This is done to provide greater sensitivity when performing the cell sorter AMS containment tests. The BSC must be validated initially at installation. Frequent retesting and monitoring proper functioning of the cabinet is mandatory, as per NSF 49 or equivalent requirements.
-
3.1.1.1
-
3.1.1
-
4
User Specific Safety Equipment
-
4.1
Personal Protective Equipment (PPE) for Biosafety Level 2 Laboratory
-
4.1.1
Front closure lab coat and gloves
-
4.1.2
Eye Protection: Safety glasses
-
4.1.1
-
4.2
Personal Protective Equipment (PPE) for Biosafety Level 2 with Enhanced Precautions:
-
4.2.1
Isolation style solid-front or wrap around gown and gloves
-
4.2.2
Eye protection: Safety goggles, face shield, splatter guard, or integral respirator/face shield which provide mucous membrane protection as required for anticipated splashes or sprays of infectious or other hazardous materials.
-
4.2.3
Respirators:
-
4.2.3.1
Filtering face piece respirators must be worn by all personnel in the cell sorter laboratory during operation of the cell sorter at this biosafety level. Approved respirators include NIOSH-approved N-95, N-99, or N-100 or EN-certified FFP2 or FFP3 filtering face piece respirators (or equivalent) or powered air purifying respirators (PAPR) with integral face shield. Note that fit testing must be performed for all personnel wearing respirators in compliance with OSHA (46), CSA (47), AS/NZS (48), or EN529 (49) or other applicable regulations. Respirators must remain on during all procedures associated with sample manipulation, including sample tube cap removal and loading of sample on instrument, or when removing collection tubes or other procedures where the sort or collection chamber is opened. Note that respirator protection may otherwise be removed during the sorting process providing the aerosol management system is active and all sort chamber and collection chamber doors are closed. For non-primate samples containing Risk Group 2 agents that do not pose respiratory risk, mucous membrane protection may be substituted for respirators. For example, the human pathogens leishmania and mouse models of toxoplasmosis are included in this category.
-
4.2.3.2
For Cell Sorters enclosed in a certified1 BSC, use of respirators as outlined above is recommended during instrument/sample manipulation within the BSC but can otherwise be removed during sorting procedures providing the BSC is operational, aerosol management system is active and all sort chamber and collection chamber doors are closed. If the BSC-enclosed Cell Sorter is in a shared laboratory, respirators are not required for other laboratory personnel.
-
4.2.3.1
-
4.2.1
-
4.3
Personal Protective Equipment (PPE) for Biosafety Level 3 Laboratory:
-
4.3.1
Liquid-resistant scrub suits or coveralls gloves (double pair). After working with infectious samples in a BSC decontaminate outer gloves with bleach or appropriate disinfectant and replace with a clean pair of gloves.
-
4.3.2
Gloves and protective clothing must not be worn outside the laboratory and must be disposed of with other contaminated waste.
-
4.3.3
Eye protection/respirator:
-
4.3.3.1
NIOSH-approved or EN146 & EN12941-certified powered air purifying respirators (PAPR) with integral face shield must be worn at all times when in the laboratory. Eye and face protection must be disposed of with other contaminated waste or decontaminated before reuse.
-
4.3.3.1
-
4.3.1
-
4.1
-
3.1
Note that given the combination of engineering controls, aerosol evacuation system and instrument located within a certified1 BSC may, dependent upon a risk assessment, allow for alternate combination of PPE. This requires approval by the cell sorting operator/facility director, biosafety professional and the Institution’s biosafety committee.
Procedures Before Initiation of New Cell Sorting Experiment
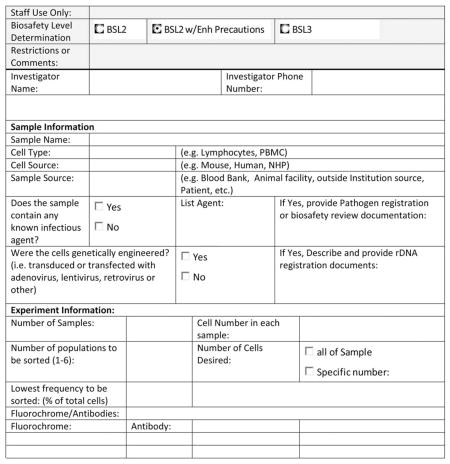
Before the initiation of cell sorting, it is important that the procedures as detailed in this document be instituted. Specifically, SOPs for various biosafety levels are written and approved by appropriate biosafety committees or professionals, appropriate engineering controls are operational and tested (including aerosol management systems) and risk assessment procedures established. Risk assessment procedures may include the establishment of ad hoc review committees, which may consist of the sorter operator or facility head, a biosafety officer, and a scientist not involved with the protocol under consideration. To facilitate the process of risk assessment, documentation of the experimental parameters should be provided by the investigator. Furthermore, it is essential to have a standardized sample information form that solicits information regarding the experimental needs as well as the sample origins and possible infectious agents being used. The information in this form is a critical component of the risk assessment process. Appendix C provides an example “Sample Information Form” that can be used or modified.
Future Updates to the ISAC Cell Sorter Biosafety Standards
It is recognized that as new technologies and methods become available, updates to this Standard will become necessary. In order to more rapidly adapt to these inevitable changes, the ISAC Cell Sorter Biosafety Standard will be updated by the standing ISAC Biosafety Committee and be made available on the ISAC web site when necessary. Future updates to this Standard can therefore be obtained at this site: www.ISAC-net.org.
Alternate combinations of engineering controls, personal protective equipment and biosafety procedures that do not perfectly match the recommended BSLs may be selected. The final risk management SOP should be selected based on risk assessment and endorsed by the cell sorting facility manager, biosafety professionals and the IBC.
Figure B.
Flow Meter connections (Cole Parmer Flow Meter: Catalog Number EW-32460-52 (meter). Catalog number EG-32462-50 (Stand).
Acknowledgments
The authors thank Geoffrey Lyon for comments and review of this article.
Grant sponsor: Intramural Research Program of the NIH, NIAID
Appendix A: Procedure For Validation of Aerosol Containment for BD FACs Aria (41)
General Considerations
This procedure is for containment validation of the BD FACS Aria cell sorter. Specific procedures for containment testing of other models of cell sorters will be posted when available on the ISAC web site (www.ISAC-net.org). However, the following general considerations apply to containment validation for all cell sorters:
Containment tests should be designed to test for containment during worse case fail mode condition, i.e. maximum aerosol concentration mimicking stream deviation due to a partial nozzle obstruction. This can be accomplished by either deviation of the stream to impact the center stream waste trough or by placing an object, such as a piece of tubing, in the path of the center stream. The instrument should be set at the highest sheath pressure that can be used for sorting.
The impactor should be placed near the potential sources of aerosol generation and/or escape, that is near the sort chamber and collection chambers and perhaps near the sample introduction area.
Five minute collection times are the minimum; increasing collection time may improve sensitivity.
Positive controls should be the last sample collected since escaped bead-laden aerosols might contaminate subsequent test samples.
Respiratory protection should be worn during testing procedures.
BD FACS Aria Containment Test
-
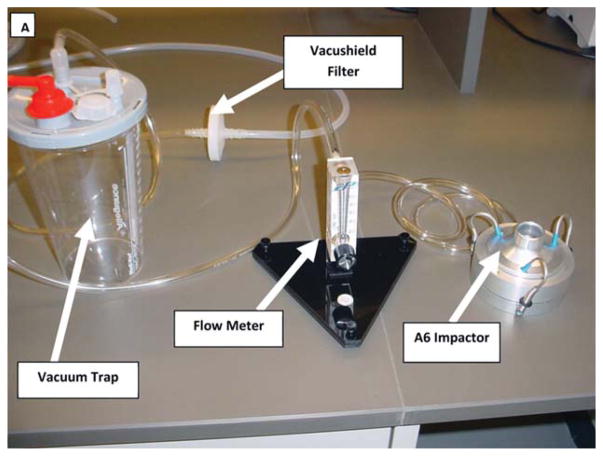
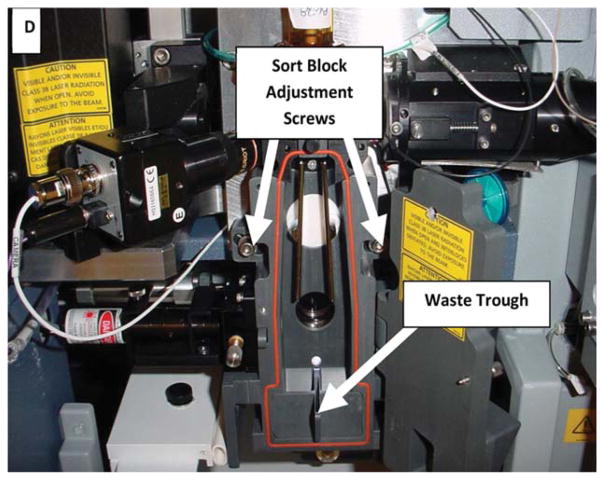
Assemble multiple-jet single-stage impactor, flow meter and vacuum trap (Fig. A–C):
Turn AMS on (20%) and check for proper vacuum function (<2.4 inches of H2O).
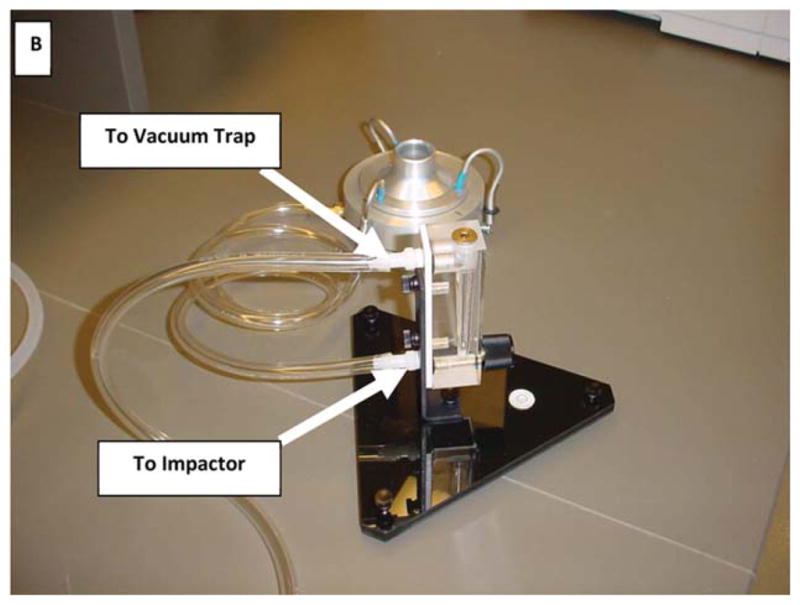
The AMS must be tested under simulated worse case failure mode. In this mode the instrument is set to 70 psi with the stream glancing off of waste-trough to create large aerosols. This is done by first adjusting the retaining screws on either side of the sort block chamber (see Fig. D) followed by moving the chamber until stream deflection is observed on the accudrop monitor. Alternatively, the waste trough can be covered with a piece of tubing or tape to generate aerosols.
Close the Sort Block Chamber door. Add a glass slide (in a 100 mm Petri dish) to the impactor (see Fig. C) and place directly on top of collection chamber (see Fig. E). Adjust the vacuum flow to the impactor to 28.3 LPM (liters per minute). Open the Aspirator Door using the software control but do not install tube holders.
Place Glo-Germ™ particles onto the sample station and adjust either the particle concentration or the flow rate to achieve a particle rate of at least 50,000 particles per second.
Begin acquiring Glo-Germ™ particles and allow collection for 5 min (Sample 1). If the sorter is housed in a Class II biological safety cabinet, the BSC blower must NOT be turned on in order to more accurately assess containment of the AMS.
Turn off vacuum to impactor and remove slide from inside. Put in a fresh slide and locate the impactor in front of the collection chamber (Fig. F). Turn on the vacuum, adjust to 28.3 LPM and collect for 5 min (Sample 2).
Turn off vacuum to impactor and remove slide from inside. Put in a fresh slide and locate the impactor under the sample station (Fig. G). Turn on the vacuum, adjust to 28.3 LPM and collect for 5 min (Sample 3).
Turn off vacuum to impactor and remove slide from inside. Put in a fresh slide and locate the impactor on top of collection chamber and open the Sort Block Chamber Door partially (Fig. H). Turn AMS off, close the aspirator door using the software control. Turn on the vacuum, adjust to 28.3 LPM and collect for 5 min (Sample 4). This is the Positive Control. Turn off vacuum, close front cover and verify that particles are still at or above the 50,000/s flow rate.
Examine glass slides for bright green fluorescence using a fluorescent microscope equipped with a FITC filter (520–640 nm). See Figure I.
Scan the entire slide on 10X and count all Glo-Germ™ particles. The positive control slide can be used as a reference if the slide reader needs help to distinguish between fluorescent debris and actual Glo-Germ™ particles. Record all data, see Appendix B.
Acceptable Tolerance: No Glo-Germ™ particles detected after 5 min of active air sampling in Samples 1, 2, and 3. The positive control slide (Sample 4) must contain greater than 100 particles after 5 min of active air sampling with the AMS turned off and no tube holder in place.
Figure A.
Picture showing containment test setup, consisting of impactor, flowmeter and vacuum trap.
Figure C.
Disassembled impactor showing slide placed inside Petri dish. See http://www.skcinc.com/prod/225-9611.asp)
Figure D.
Sort Block Chamber Adjustment for AMS Tests on BD FACS Aria. To create a “failure mode” condition, in order to perform the Glo-Germ™ AMS testing procedure, adjust hex screws as shown in order to direct center stream to hit the edge of the waste trough.
Figure E.

Impactor placement. Top of collection chamber.
Figure F.

Impactor placement. In front of collection chamber.
Figure G.

Impactor placement. Under sample station.
Figure H.

Impactor placement. On top of collection chamber, door partially open.
Figure I.
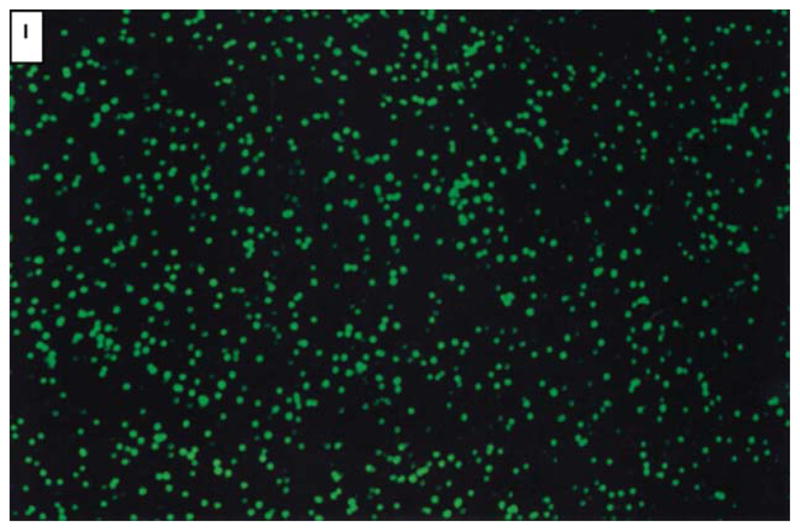
Microscope view of GloGerm™ particles (positive control).
Appendix B: Example Containment Test Record Sheet

Appendix C: Example Sample Information Form
Appendix D: Sample Standard Operating Procedures
BSL2 SOP–FACS Aria II™
Wear lab coat and gloves and safety glasses.
-
Turn on Aerosol Management System (biohazard vacuum) and operate at 20% or as recommended by instrument manufacturer.
Check vacuum reading. If vacuum is >2.4 inches of H2O, change HEPA filter. Note: HEPA filter must be changed every 6 months, regardless of vacuum reading.
-
Procedure for changing HEPA filter on AMS unit:
While wearing gloves, lab coat, N-95 rated face mask (respirator) or PAPR and goggles/safety glasses, place the Buffalo unit HEPA filter inside an orange biohazard plastic bag. Disconnect hose from the Aria and also place within the bag. Seal the bag and place within a Medical Pathological Waste (MPW) box. Install a new HEPA filter and hose.
Make sure collection chamber door and sort chamber door are closed during sorting procedures.
Do not eat or drink in laboratory.
Remove gloves before answering phone.
Remove lab coat and gloves and wash hands before leaving lab.
BSL2 with Enhanced Precautions SOP–FACS Aria II™
-
Preparation before the sort
If not using a sealed keyboard and mouse, cover keyboard, mouse and other instrument control surfaces with plastic wrap; clear surfaces of clutter, use absorbent pads for samples.
Using a damp paper towel(s), wipe up dried bleach residue from instrument areas, paying particular attention to the sample uptake area, O-rings, charge plates, and the side stream viewing window. Warning: Failure to remove salt residue from the sample uptake system may cause the pressurized seal to fail and release potential aerosols!
Prepare sort collection chamber as necessary. Install the correct collection tube holder. Close sort collection chamber door.
If the Aria is contained within a Biosafety Cabinet (BSC), turn the BSC blower fan on and turn the evacuation vacuum on low.
-
If not using a BSC, turn biohazard vacuum (Buffalo Filter Whisper Unit) on and operate at 20%. Check vacuum reading. If vacuum is >2.4 inches of H2O, change HEPA filter. Note: HEPA filter must be changed every 6 months, regardless of vacuum reading.
-
Procedure for changing HEPA filter on AMS unit:
While wearing gloves, lab coat, N-95 rated face mask (respirator) or PAPR and goggles/safety glasses, place the Buffalo unit HEPA filter inside an orange biohazard plastic bag. Disconnect hose from the Aria and also place within the bag. Seal the bag and place within a Medical Pathological Waste container for disposal according to Institutional guidelines. Install a new HEPA filter and hose.
-
Make sure sheath tank is filled and standard waste tank contains enough bleach to give a final 10% (1:10 dilution of household bleach) solution when filled. Fill a spray bottle with a freshly made 10% (1:10 dilution) bleach solution for work area decontamination.
Wear gloves, lab coat, N-95 rated face mask (respirator) or PAPR and goggles/safety glasses (or N-95 mask with face shield) before handling samples. Lab door must be closed and investigators are to remain outside of the lab until data files of the experimental controls and samples have been collected and tubes are no longer being manipulated. Note that investigators may remain in the lab if wearing N-95 or better respiratory protection, but must have been fit-tested and cleared by Occupational Medicine.
Respirators must remain on during all procedures associated with sample manipulation, including sample tube cap removal and loading of sample on instrument, or when removing collection tubes or other procedures where the sort or collection chamber is opened as outlined below in Section 3. Note that respirator protection may otherwise be removed during the sorting process except during procedures as outlined above.
Aria within a Class II BSC: Operator must wear gloves and closed front lab coat and goggles/safety glasses. N-95 rated face mask (respirator) or PAPR must be worn during instrument/sample manipulation. Lab door may remain open, but notification of a potential biohazard must be posted outside the lab entrance. Investigators may remain in the room during sorting operations and respirators are not required for personnel other than the operator.
Have a spare nozzle, with new O-ring installed (or spare integrated nozzle), available in case of a clog.
-
Procedures during sorting/analysis
Filter samples before sort to avoid clogs.
Fill sample tube with as much sample as possible to minimize loading and unloading sample. DO NOT fill higher than ¼ inch from the top of the tube.
Make sure the “Sweet Spot” is enabled.
Close sort collection chamber door before starting sample.
-
When changing collection tubes:
Stop the sample flow and close the aspirator drawer by clicking the ‘Acquire’ button.
Wait at least 60 s before opening sort collection chamber door.
When removing collection tubes, be aware that the outside of the tube is potentially contaminated, use alcohol swab or bleach to wipe outside of tubes.
-
Procedures in the event of a nozzle obstruction
-
If during the sort the stream is deflected (due in part to a clogged nozzle), the sort is designed to stop automatically and block the sort tubes. The sort will not restart until the operator has cleared the clog. In the event of a nozzle clog, DO NOT open sort collection chamber door or sort block door before following this procedure:
If the system has not already shut down automatically, turn off the stream using the button labeled with an “✓” on the Breakoff window. This will shut off the stream, unload the sample and close the aspirator door.
Open aspirator drawer using software controls.
Increase the air evacuation rate on the AMS unit to 100%, or if using a BSC, push the high evacuation button (low button must also remain on).
Wait at least 60 s. This procedure will clear aerosols from the sort chamber. (Note that this step assumes that a modification to tube holder(s) (universal top component on Aria II) involving the drilling of three holes and the sort chamber door involving the drilling of 1 hole with attachment of 0.22 μm filter, has been previously performed. see Ref. 3).
Close the aspirator drawer.
With the sort block chamber door, aspirator drawer and collection chamber door all closed, turn the stream on and off several times or perform the “Clean flow Cell” procedure with DI H20 followed by turning the stream on to see if the clog will clear itself.
Turn stream off.
Open the aspirator drawer and evacuate for at least 60 s before closing the aspirator drawer again.
The sort block chamber door and sort collection chamber door can now be opened.
If it is necessary to change nozzles, remove nozzle and O-ring and place in tube with 10% (1:10 dilution) bleach for 30 min. Thoroughly rinse nozzle in water and let air-dry. Discard O-ring if not using nozzles with integrated O-rings. Spare integrated nozzle or spare nozzle with O-ring may be installed while obstructed nozzle is soaking in bleach.
With stream turned off, open the sort block chamber door and dry plates and surfaces as needed.
When removing collection tubes, be aware that the outside of the tube is potentially contaminated, use alcohol swab or bleach to wipe outside of tubes.
Set AMS unit to 20% vacuum or if enclosed within a BSC, toggle the high evacuation button off.
Make sure that all chamber doors are closed and restart the stream.
-
-
Decontamination Procedures:
Disengage “Sweet Spot” and turn the stream off.
-
Disinfect sample lines using a freshly made 10% bleach solution as follows:
Fill a tube with a volume of 10% bleach equal to or greater than the volume of sample that was sorted and place on the sample stage.
Select from the menu—Instrument >Cleaning Modes >Clean Flow Cell. Perform this step three times or until a bleach drop is visible in the stream camera view.
Wait 30 or more minutes with 10% bleach in flow cell.
Fill a tube with DI water, Select from the menu—Instrument >Cleaning Modes >Clean Flow Cell.
Fill a tube with 70% ETOH, Select from the menu—Instrument >Cleaning Modes >Clean Flow Cell. Perform this step three times or until an ETOH drop is visible in the stream camera view. Shutdown instrument.
Clean all surfaces around optical bench, sort block chamber and charge plates, sort collection chamber, sample introduction area and sample tube holder(s) with a prepackaged 10% bleach towel and/or 10% (1:10 dilution) bleach from a spray bottle. Clean keyboard cover, remove any plastic wrap and discard in Medical Pathological Waste.
-
When leaving the lab:
Make sure all samples are capped.
Remove gloves, respirator, and lab coat (remember outside of gloves are contaminated!).
WASH HANDS!
Footnotes
See Section 3.1.1.2.
This article is a US government work and, as such, is in the public domain in the United States of America.
Literature Cited
- 1.Schmid I, Nicholson JK, Giorgi JV, Janossy G, Kunkl A, Lopez PA, Perfetto S, Seamer LC, Dean PN. Biosafety guidelines for sorting of unfixed cells. Cytometry. 1997;28:99–117. doi: 10.1002/(sici)1097-0320(19970601)28:2<99::aid-cyto2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Schmid I, Lambert C, Ambrozak D, Marti GE, Moss DM, Perfetto SP. International Society for Analytical Cytology biosafety standard for sorting of unfixed cells. Cytometry Part A J Int Soc Anal Cytol. 2007;71A:414–437. doi: 10.1002/cyto.a.20390. [DOI] [PubMed] [Google Scholar]
- 3.Holmes KL. Characterization of aerosols produced by cell sorters and evaluation of containment. Cytometry A. 2011;79A:1000–1008. doi: 10.1002/cyto.a.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding AL, Byers KB. Epidemiology of Laboratory-Associated Infections. In: Fleming DO, Hunt DL, editors. Biological Safety: Principles and Practices. 4. Washington, D.C: ASM Press; 2006. pp. 53–77. [Google Scholar]
- 5.Sewell DL. Laboratory-associated infections and biosafety. Clin Microbiol Rev. 1995;8:389–405. doi: 10.1128/cmr.8.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins CH, Kennedy DA. Laboratory-Acquired Infections: History, Incidence, Causes, and Prevention. 4. Oxford: Butterworth-Heinemann; 1999. Exposure, Sources and Routes of Infection; pp. 38–53. [Google Scholar]
- 7.Singh K. Laboratory-acquired infections. Clin Infect Dis. 2009;49:142–147. doi: 10.1086/599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pike RM. Laboratory-associated infections: incidence, fatalities, causes, and prevention. Annu Rev Microbiol. 1979;33:41–66. doi: 10.1146/annurev.mi.33.100179.000353. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institutes of Health. Biosafety in Microbiological and Biomedical Laboritories. Vol. 5. Washington, DC: US Govt. Printing Office; 2009. [Google Scholar]
- 10.Sulkin SE. Laboratory-acquired infections. Bacteriol Rev. 1961;25:203–209. doi: 10.1128/br.25.3.203-209.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler WG, Fashinell TR, Leffingwell L, Howard P, Conomy P. Airborne rabies transmission in a laboratory worker. JAMA. 1973;226:1219–1221. [PubMed] [Google Scholar]
- 12.Oh M, Kim N, Huh M, Choi C, Lee E, Kim I, Choe K. Scrub typhus pneumonitis acquired through the respiratory tract in a laboratory worker. Infection. 2001;29:54–56. doi: 10.1007/s15010-001-0139-5. [DOI] [PubMed] [Google Scholar]
- 13.Schlesinger RB. Comparative deposition of inhaled aerosols in experimental animals and humans: a review. J Toxicol Environ Health. 1985;15:197–214. doi: 10.1080/15287398509530647. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho TC, Peters JI, Williams RO., III Influence of particle size on regional lung deposition–What evidence is there? Int J Pharm. 2011;406:1–10. doi: 10.1016/j.ijpharm.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Couch RB, Douglas RG, Jr, Lindgren KM, Gerone PJ, Knight V. Airborne transmission of respiratory infection with coxsackievirus A type 21. Am J Epidemiol. 1970;91:78–86. doi: 10.1093/oxfordjournals.aje.a121115. [DOI] [PubMed] [Google Scholar]
- 16.Cate TR, Couch RB, Fleet WF, Griffith WR, Gerone EPJ, Knight V. Production of tracheobronchitis in volunteers with rhinovirus in a small-particle aerosol. Am J Epidemiol. 1965;81:95–105. doi: 10.1093/oxfordjournals.aje.a120501. [DOI] [PubMed] [Google Scholar]
- 17.Alford RH, Kasel JA, Gerone PJ, Knight V. Human influenza resulting from aerosol inhalation. Proc Soc Exp Biol Med. 1966;122:800. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- 18.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim SF, van den Engh G. High-speed cell sorting: Fundamentals and recent advances. Curr Opin Biotechnol. 2003;14:5–12. doi: 10.1016/s0958-1669(02)00009-5. [DOI] [PubMed] [Google Scholar]
- 20.Leary JF. Ultra high-speed sorting. Part A J Int Soc Anal Cytol. 2005;67A:76–85. doi: 10.1002/cyto.a.20160. [DOI] [PubMed] [Google Scholar]
- 21.U S. Department of Labor. Occupational Exposure to Bloodborne Pathogens. Final Rule, 29 CFR 1910. 1030: Occupational Safety and Health Administration. Fed Regist. 1991;56:64175–64182. [Google Scholar]
- 22.U. S. Department of Health and Human Services. Select Agents and Toxins. Final Rule, 42 CFR 73. Fed Regist. 2012;77:61083–61115. [PubMed] [Google Scholar]
- 23.U S. Department of Agriculture. Possession, Use, and Transfer of Select Agents and Toxins. 7 CFR 331. Fed Regist. 2005;70:13242–13291. [Google Scholar]
- 24.Office of Biotechnology Activities. NIH Guidelines for Research Involving Recombinant and Synthetic Nucleic Acid Molecules. Office of Biotechnology Activities; 2013. [Online: the most current version can be found at http://osp.od.nih.gov/office-biotechnology-activities/biosafety/nih-guidelines] [Google Scholar]
- 25.Clinical and Laboratory Standards Insitute (CLSI) CSLI document M29-A3. 3. Wayne, PA: CLSI; 2005. Protection of Laboratory Workers From Occupationally Acquired Infections; Approved Guideline. [Google Scholar]
- 26.Miller JM, Astles R, Baszler T, Chapin K, Carey R, Garcia L, Gray L, Larone D, Pentella M, Pollock A, et al. Guidelines for safe work practices in human and animal medical diagnostic laboratories. Recommendations of a CDC-convened, Biosafety Blue Ribbon Panel. MMWR Morb Mortal Wkly Rep Surveill Summ (Washington, DC: 2002) 2012;61(Suppl):1–102. [PubMed] [Google Scholar]
- 27.Schmid I, Kunkl A, Nicholson JK. Biosafety considerations for flow cytometric analysis of human immunodeficiency virus-infected samples. Cytometry. 1999;38:195–200. doi: 10.1002/(sici)1097-0320(19991015)38:5<195::aid-cyto1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 28.Schmid I, Merlin S, Perfetto SP. Biosafety concerns for shared flow cytometry core facilities. Part A J Int Soc Anal Cytol. 2003;56A:113–119. doi: 10.1002/cyto.a.10085. [DOI] [PubMed] [Google Scholar]
- 29.Pentella MA, Kostle PA, Desjardin L, Gilchrist MJR. Biosafety for airborne pathogens. In: Fleming DO, Hunt DL, editors. Biological Safety: Principles and Practices. 4. Washington, D.C: ASM Press; 2006. pp. 209–220. [Google Scholar]
- 30.Hunt DL. Standard (Universal) precautions for handling human specimens. In: Fleming DO, Hunt DL, editors. Biological Safety: Principles and Practices. 4. Washington, DC: ASM Press; 2006. pp. 341–359. [Google Scholar]
- 31.Aloisio CH, Nicholson JK. Recovery of infectious human immunodeficiency virus from cells treated with 1% paraformaldehyde. J Immunol Methods. 1990;128:281–285. doi: 10.1016/0022-1759(90)90221-g. [DOI] [PubMed] [Google Scholar]
- 32.Ericson JG, Trevino AV, Toedter GP, Mathes LE, Newbound GC, Lairmore MD. Effects of whole blood lysis and fixation on the infectivity of human T-lymphotropic virus type 1 (HTLV-I) Cytometry. 1994;18:49–54. doi: 10.1002/cyto.990180110. [DOI] [PubMed] [Google Scholar]
- 33.Kost TA, Condreay JP, Mickelson CA. Biosafety and viral gene transfer vectors. In: Fleming DO, Hunt DL, editors. Biological Safety: Principles and Practices. 4. Washington, DC: ASM Press; 2006. pp. 509–530. [Google Scholar]
- 34.Pauwels K, Gijsbers R, Toelen J, Schambach A, Willard-Gallo K, Verheust C, Debyser Z, Herman P. State-of-the-art lentiviral vectors for research use: Risk assessment and biosafety recommendations. Curr Gene Ther. 2009;9:459–474. doi: 10.2174/156652309790031120. [DOI] [PubMed] [Google Scholar]
- 35.Stovel RT. The influence of particles on jet breakoff. J Histochem Cytochem. 1977;25:813–820. doi: 10.1177/25.7.894007. [DOI] [PubMed] [Google Scholar]
- 36.Rutala WA, Weber DJ HICPAC. Guideline for disinfection and sterilization in healthcare facilities. CDC; 2008. [Online: see: http://www.cdc.gov/hicpac/Disinfection_Sterilization/acknowledg.html] [Google Scholar]
- 37.Perfetto SP, Ambrozak DR, Nguyen R, Roederer M, Koup RA, Holmes KL. Standard practice for cell sorting in a BSL-3 facility. Methods Mol Biol. 2011;699:449–469. doi: 10.1007/978-1-61737-950-5_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid I, Roederer M, Koup RA, Ambrozak D, Perfetto SP, Holmes KL. Biohazard sorting. In: Darzynkiewicz Z, Robinson JP, Roederer M, editors. Essential Cytometry Methods: Reliable Lab Solutions. 1. London: Academic Press; 2009. pp. 183–204. [Google Scholar]
- 39.Arnold LW, Lannigan J. Curr Protoc Cytom. Vol. 1. New York: John Wiley and Sons, Inc; 2010. Practical issues in high-speed cell sorting; pp. 1.24.1–1.24.30. [DOI] [PubMed] [Google Scholar]
- 40.Oberyszyn AS. Curr Protoc Cytom. Vol. 3. New York: John Wiley and Sons, Inc; 2002. Method for visualizing aerosol contamination in flow sorters; p. 3.5. [DOI] [PubMed] [Google Scholar]
- 41.Perfetto SP, Ambrozak DR, Koup RA, Roederer M. Measuring containment of viable infectious cell sorting in high-velocity cell sorters. Part A J Int Soc Anal Cytol. 2003;52A:122–130. doi: 10.1002/cyto.a.10033. [DOI] [PubMed] [Google Scholar]
- 42.NSF International Standard/American National Standard. NSF/ANSI. Vol. 49. Ann Arbor: NSF International; 2009. Biosafety Cabinetry: Design, Construction, Performance, and Field Certification. [Google Scholar]
- 43.European Committee for Standardization (CEN) Biotechnology—Performance criteria for microbiological safety cabinets. Brussels: CEN, EU; 2000. EN 12469: 2000. [Google Scholar]
- 44.Japanese Standards Assoication, Japan Air Cleaning Assoication. Class II Biological Safety Cabinets. Tokyo: Japanese Standards Association; 2009. JIS K 3800. [Google Scholar]
- 45.Standards Association of Australia, Standards Australia Limited. AS 2252: Biological Safety Cabinets. Standards Association of Australia; Homebush, Australia: 1981. [Google Scholar]
- 46.U S. Department of Labor. Personal Protective Equipment. 29 CFR 1910. 134: Occupational Safety and Health Administration. Fed Regist. 1998;63:1152–1300. [Google Scholar]
- 47.Canadian Standards Association (CSA) Seclection, Use, Care of Respirators. 4. Toronto: Canadian Standards Association; 2011. CSA Z94.4-11. [Google Scholar]
- 48.Australia Standards (AS) Selection, Use and Maintenance of Respiratory Protective Devices. Standards Association of Australia; Homebush, Australia: 1994. AS/NZS 1715–1994. [Google Scholar]
- 49.European Committee for Standardization (CEN) Guidance Document. Brussels: CEN, EU; 2005. Respiratory Protective Devices. Recommendations for Selection, Use, Care and Maintenance. BS EN 529:2005. [Google Scholar]