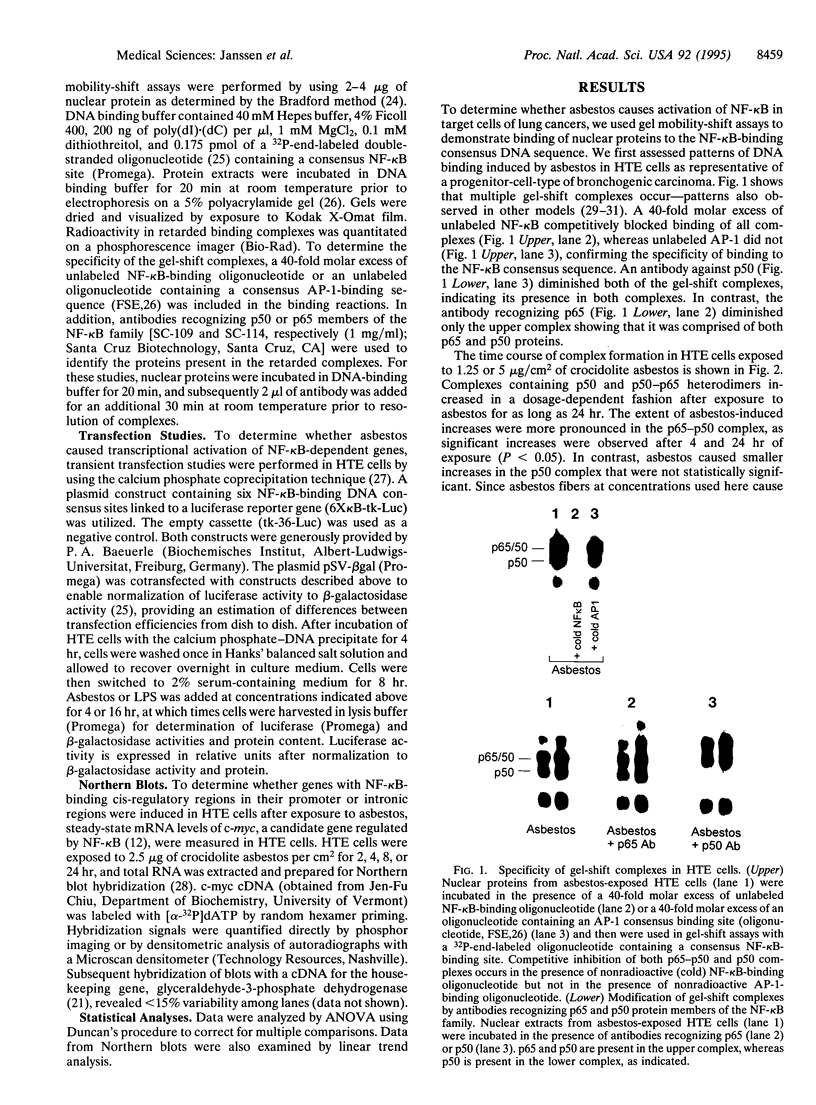
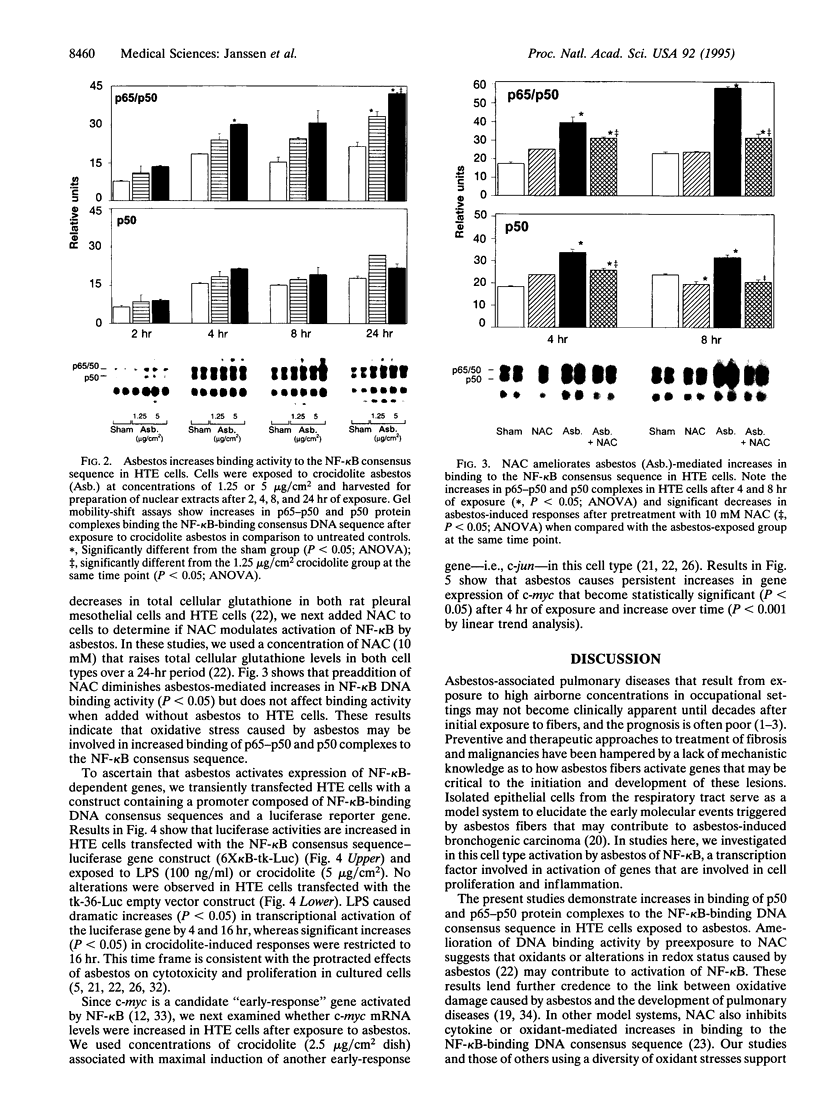
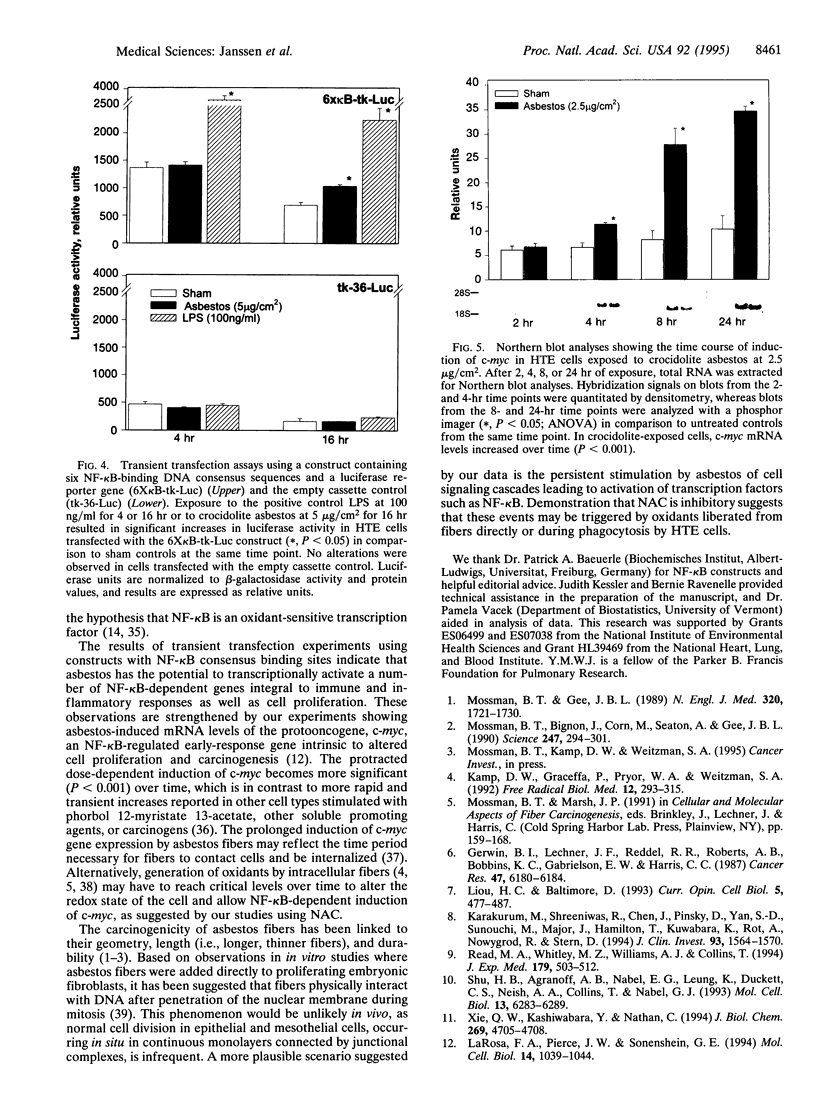
Abstract
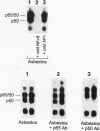
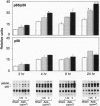
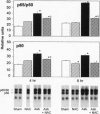
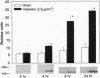
Nuclear factor kappa B (NF-kappa B) is a transcription factor regulating expression of genes intrinsic to inflammation and cell proliferation--features of asbestos-associated diseases. In studies here, crocidolite asbestos caused protracted and dose-responsive increases in proteins binding to nuclear NF-kappa B-binding DNA elements in hamster tracheal epithelial (HTE) cells. This binding was modulated by cellular glutathione levels. Antibodies recognizing p65 and p50 protein members of the NF-kappa B family revealed these proteins in two of the DNA complexes. Transient transfection assays with a construct containing six NF-kappa B-binding DNA consensus sites linked to a luciferase reporter gene indicated that asbestos induced transcriptional activation of NF-kappa B-dependent genes, an observation that was confirmed by northern blot analyses for c-myc mRNA levels in HTE cells. Studies suggest that NF-kappa B induction by asbestos is a key event in regulation of multiple genes involved in the pathogenesis of asbestos-related lung cancers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. T., Staal F. J., Gitler C., Herzenberg L. A., Herzenberg L. A. Separation of oxidant-initiated and redox-regulated steps in the NF-kappa B signal transduction pathway. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11527–11531. doi: 10.1073/pnas.91.24.11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Baldwin A. S., Jr Activation of multiple NF-kappa B/Rel DNA-binding complexes by tumor necrosis factor. Oncogene. 1994 May;9(5):1487–1492. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. D. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- Devary Y., Rosette C., DiDonato J. A., Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993 Sep 10;261(5127):1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- Ganchi P. A., Sun S. C., Greene W. C., Ballard D. W. A novel NF-kappa B complex containing p65 homodimers: implications for transcriptional control at the level of subunit dimerization. Mol Cell Biol. 1993 Dec;13(12):7826–7835. doi: 10.1128/mcb.13.12.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin B. I., Lechner J. F., Reddel R. R., Roberts A. B., Robbins K. C., Gabrielson E. W., Harris C. C. Comparison of production of transforming growth factor-beta and platelet-derived growth factor by normal human mesothelial cells and mesothelioma cell lines. Cancer Res. 1987 Dec 1;47(23):6180–6184. [PubMed] [Google Scholar]
- Heintz N. H., Janssen Y. M., Mossman B. T. Persistent induction of c-fos and c-jun expression by asbestos. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3299–3303. doi: 10.1073/pnas.90.8.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg T. W., Barrett J. C. Induction by asbestos fibers of anaphase abnormalities: mechanism for aneuploidy induction and possibly carcinogenesis. Carcinogenesis. 1985 Mar;6(3):473–475. doi: 10.1093/carcin/6.3.473. [DOI] [PubMed] [Google Scholar]
- Janssen Y. M., Heintz N. H., Marsh J. P., Borm P. J., Mossman B. T. Induction of c-fos and c-jun proto-oncogenes in target cells of the lung and pleura by carcinogenic fibers. Am J Respir Cell Mol Biol. 1994 Nov;11(5):522–530. doi: 10.1165/ajrcmb.11.5.7946382. [DOI] [PubMed] [Google Scholar]
- Janssen Y. M., Heintz N. H., Mossman B. T. Induction of c-fos and c-jun proto-oncogene expression by asbestos is ameliorated by N-acetyl-L-cysteine in mesothelial cells. Cancer Res. 1995 May 15;55(10):2085–2089. [PubMed] [Google Scholar]
- Janssen Y. M., Van Houten B., Borm P. J., Mossman B. T. Cell and tissue responses to oxidative damage. Lab Invest. 1993 Sep;69(3):261–274. [PubMed] [Google Scholar]
- Kamp D. W., Graceffa P., Pryor W. A., Weitzman S. A. The role of free radicals in asbestos-induced diseases. Free Radic Biol Med. 1992;12(4):293–315. doi: 10.1016/0891-5849(92)90117-y. [DOI] [PubMed] [Google Scholar]
- Karakurum M., Shreeniwas R., Chen J., Pinsky D., Yan S. D., Anderson M., Sunouchi K., Major J., Hamilton T., Kuwabara K. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J Clin Invest. 1994 Apr;93(4):1564–1570. doi: 10.1172/JCI117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koong A. C., Chen E. Y., Giaccia A. J. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994 Mar 15;54(6):1425–1430. [PubMed] [Google Scholar]
- La Rosa F. A., Pierce J. W., Sonenshein G. E. Differential regulation of the c-myc oncogene promoter by the NF-kappa B rel family of transcription factors. Mol Cell Biol. 1994 Feb;14(2):1039–1044. doi: 10.1128/mcb.14.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bail O., Schmidt-Ullrich R., Israël A. Promoter analysis of the gene encoding the I kappa B-alpha/MAD3 inhibitor of NF-kappa B: positive regulation by members of the rel/NF-kappa B family. EMBO J. 1993 Dec 15;12(13):5043–5049. doi: 10.1002/j.1460-2075.1993.tb06197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou H. C., Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol. 1993 Jun;5(3):477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- Lund L. G., Aust A. E. Iron-catalyzed reactions may be responsible for the biochemical and biological effects of asbestos. Biofactors. 1991 Jun;3(2):83–89. [PubMed] [Google Scholar]
- Matsusaka T., Fujikawa K., Nishio Y., Mukaida N., Matsushima K., Kishimoto T., Akira S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan N., Meltz M. L. Induction of nuclear factor kappa B after low-dose ionizing radiation involves a reactive oxygen intermediate signaling pathway. Radiat Res. 1994 Oct;140(1):97–104. [PubMed] [Google Scholar]
- Mossman B. T., Bignon J., Corn M., Seaton A., Gee J. B. Asbestos: scientific developments and implications for public policy. Science. 1990 Jan 19;247(4940):294–301. doi: 10.1126/science.2153315. [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Ezerman E. B., Adler K. B., Craighead J. E. Isolation and spontaneous transformation of cloned lines of hamster tracheal epithelial cells. Cancer Res. 1980 Dec;40(12):4403–4409. [PubMed] [Google Scholar]
- Mossman B. T., Gee J. B. Asbestos-related diseases. N Engl J Med. 1989 Jun 29;320(26):1721–1730. doi: 10.1056/NEJM198906293202604. [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Kessler J. B., Ley B. W., Craighead J. E. Interaction of crocidolite asbestos with hamster respiratory mucosa in organ culture. Lab Invest. 1977 Feb;36(2):131–139. [PubMed] [Google Scholar]
- Mossman B. T., Marsh J. P., Sesko A., Hill S., Shatos M. A., Doherty J., Petruska J., Adler K. B., Hemenway D., Mickey R. Inhibition of lung injury, inflammation, and interstitial pulmonary fibrosis by polyethylene glycol-conjugated catalase in a rapid inhalation model of asbestosis. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1266–1271. doi: 10.1164/ajrccm/141.5_Pt_1.1266. [DOI] [PubMed] [Google Scholar]
- Palombella V. J., Rando O. J., Goldberg A. L., Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994 Sep 9;78(5):773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Read M. A., Whitley M. Z., Williams A. J., Collins T. NF-kappa B and I kappa B alpha: an inducible regulatory system in endothelial activation. J Exp Med. 1994 Feb 1;179(2):503–512. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Albermann K., Baeuerle P. A. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun. 1992;17(4):221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- Shu H. B., Agranoff A. B., Nabel E. G., Leung K., Duckett C. S., Neish A. S., Collins T., Nabel G. J. Differential regulation of vascular cell adhesion molecule 1 gene expression by specific NF-kappa B subunits in endothelial and epithelial cells. Mol Cell Biol. 1993 Oct;13(10):6283–6289. doi: 10.1128/mcb.13.10.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull S., Heintz N. H., Periasamy M., Manohar M., Janssen Y. M., Marsh J. P., Mossman B. T. Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem. 1991 Dec 25;266(36):24398–24403. [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Mossman B. T., Craighead J. E. Induction of squamous metaplasia in organ cultures of hamster trachea by naturally occurring and synthetic fibers. Cancer Res. 1983 Oct;43(10):4906–4912. [PubMed] [Google Scholar]
- Xie Q. W., Kashiwabara Y., Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994 Feb 18;269(7):4705–4708. [PubMed] [Google Scholar]