Abstract
Background
Phosphodiesterase (PDE) inhibitors in the central nervous system have been shown to stimulate neuronal functions and increase neurogenesis in Alzheimer disease (AD) patients.
Material/Methods
The aim of this study was to investigate the effects of zaprinast, a PDE5 inhibitor, and rolipram, a PDE4 inhibitor, on learning and memory in elevated plus maze (EPM) and passive avoidance (PA) tests in naive mice. Male Balb-c mice received short-term treatment with zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.1 mg/kg) before the acquisition trial of the EPM and PA tests. The exploratory activity of the animals was also investigated in the Hughes box test.
Results
Both zaprinast (10 mg/kg) and rolipram (0.1 mg/kg) significantly decreased second-day latency compared to the control group in the EPM test, while only rolipram (0.1 mg/kg) significantly increased second-day latency in the PA test. Both zaprinast (10 mg/kg) and rolipram (0.1 mg/kg) significantly decreased the number of entries to new areas and time spent in new areas in the Hughes box test.
Conclusions
Our study revealed that both zaprinast and rolipram enhanced spatial memory in EPM, while rolipram seemed to have more emotional memory-enhancing effects in the PA test compared to zaprinast. Both zaprinast and rolipram diminished exploratory activity in the Hughes box test, which can be attributed to the drugs’ anxiogenic effects.
MeSH Keywords: Behavior, Animal, Memory, Mice
Background
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic AMP (cAMP) and/or cyclic GMP (cGMP) throughout the body, including the brain. Accumulating evidence indicates that the inhibition of phosphodiesterase (PDE) activity may be a particularly interesting mechanism for memory enhancement [1,2]. PDE inhibitors present a novel therapeutic approach with which to arrest cognitive decline [3,4] or to possibly reverse this decline with cognitive enhancement [5,6].
Eleven subclasses of PDEs have been identified thus far, but only PDE4, 5 and, recently, PDE2 inhibitors have been demonstrated to be effective in memory enhancement [7]. The underlying mechanism for the cognition-enhancing effects of PDE4 inhibitors may involve the modulation of activity within the cAMP/protein kinase A/cAMP response element binding protein (cAMP/PKA/CREB) pathway [8]. The prototypical PDE4 inhibitor most widely used in cognition studies is rolipram. Rolipram attenuates deficits in spatial and non-spatial short-term memory and working memory in several behavioral tasks [9,10]. Rolipram reverses the disruption of reference memory and/or working memory caused by the glutamate antagonist MK-801 during the radial-arm maze task and reverses the effects of MK-801 in the passive avoidance task [11].
Treating mice with rolipram or HT0712 20 min before training in the object recognition task improves memory retention 24 h later [12]. Similarly, the retention performance 24 h after contextual fear learning is improved in mice treated with rolipram 30 min before training [13].
PDE5 inhibitors (PDE5-I), such as sildenafil and vardenafil, are effective in the treatment of erectile dysfunction and are candidate drugs for cognitive enhancement. For instance, sildenafil and vardenafil have been shown to improve object recognition memory when injected immediately following the first trial [14]. PDE5-Is are assumed to improve early processes of memory consolidation via either a presynaptic or postsynaptic mechanism. The presynaptic mechanism acts through the nitric oxide (NO) – cGMP signaling pathway, and the postsynaptic mechanism acts through the cGMP/protein kinase G/cAMP response element-binding protein (cCMP/PKG/CREB) signaling pathway [15].
Zaprinast has been used to inhibit PDE5, and when given immediately after training at a dose of 10 mg/kg (i.p.), zaprinast improved the long-term memory performance of rats in the object recognition task [15]. Previous studies have also shown that zaprinast reversed the object memory deficits induced by the NOS inhibitor 7-nitroindazole in rats in the object recognition task [16]. However, zaprinast was unable to reverse memory deficits in aged rats in this task [15]. Animal studies indicate that PDE5 inhibitors have the potential to improve the early consolidation processes of long-term memory, although PDE5 inhibitors may not affect spatial information. This memory improvement might be mediated by elevations in central cGMP levels.
Our literature search found a few studies investigating the effects of zaprinast and rolipram on memory in the passive avoidance test (PA), although there were no studies investigating the effects of zaprinast and rolipram on memory in the elevated plus maze test (EPM) or on exploratory activity in the Hughes box test. The aim of this study was to investigate the effects of the phosphodiesterase-5 inhibitor, zaprinast, and the phosphodiesterase-4 inhibitor, rolipram, on spatial memory in the EPM, on emotional memory in the PA [17], and also on exploratory activity in the Hughes box test in naive mice.
Material and Methods
Animals
Ninety-five male inbred BALB/c ByJ mice (MAM TUBİTAK, Gebze, Kocaeli, Turkey) aged 7 weeks were used in this study upon arrival to the laboratory. Animals (4–5 per cage) were kept in the laboratory at 21±1.5°C with 60% relative humidity under a 12 h light/dark cycle (lights on at 8:00 p.m.) for 2 weeks before experimentation. Tap water and food pellets were available ad libitum. All procedures involving animals were in compliance with the European Community Council Directive of 24 November 1986, and ethics approval was granted by the Kocaeli University Ethics Committee (Number: AEK 9/4-2010, Kocaeli, Turkey). All animals were naive to the experimental apparatus, and different animals were used for each test.
Modified elevated plus-maze test
Cognitive behavior was evaluated using the mEPM learning task, which measures spatial long-term memory [18]. The maze was made of wood and consisted of 2 open arms (29×5 cm) surrounded by a short (1 cm) plexiglas edge to prevent falls and 2 enclosed arms (29×5×15 cm) arranged such that the 2 open arms were opposite to each other. The arms were connected by a central platform (5×5 cm). The maze was elevated 40 cm above the floor. This arrangement is based upon the aversion of rodents to open spaces and heights. The animals prefer the enclosed, protected areas of the maze.
The procedure was performed as described previously [18–21]. During the acquisition session (Day 1), each mouse was gently placed at the distal end of an open arm facing away from central platform. The time it took for the mice to move from the open arm to either of the enclosed arms (transfer latency) was recorded. Training (repeated exposure of animals to the open arms) shortened this parameter, possibly as a consequence of learning acquisition and retention. If the mouse did not enter the enclosed arm within 90 s, it was excluded from further experimentation. Animal entry into the enclosed arm required the animal to cross an imaginary line separating the enclosed arm from the central space with all four 4 legs. After entering the enclosed arm, mice were allowed to move freely in the maze, regardless of open and enclosed arms, for 10 s. Mice were then returned to their home cage. The retention session followed 24 h after the acquisition session (on Day 2). Mice were placed into the open arm, and the transfer latency was recorded again. Experiments were conducted between the hours of 10:00 and 14:00 in a dimly lit, semi-soundproof room under a natural light.
Passive avoidance test
Animals were trained in a one-trial, step-through, PA apparatus for evaluating memory, based on contextual fear conditioning and instrumental learning [22]. A decrease in retention latency indicates impairment in memory in the PA task. The apparatus consisted of a box with an illuminated part (L 7×12.5×h 14 cm) and a dark part (L 24×12.5×h 14 cm), both equipped with a grid floor composed of steel bars (0.3-cm diameter) spaced 0.9 cm apart. The inhibitory avoidance task consisted of 2 trials. On the first day of training, mice were placed individually into the light compartment and allowed to explore the boxes. The intercompartment door was opened after a 60-s acclimation period. In the acquisition trial, each mouse was placed in the illuminated compartment, which was lighted by a bright bulb (2000 lux). The animals received drugs prior to acquisition training. If the mouse stepped into the dark compartment (2/3 of the tail in the dark compartment), the door was closed by the experimenter, and an inescapable foot shock (0.25 mA/1 s) was delivered through the grid floor of the dark compartment. A cutoff time of 5 min was selected. The time taken to enter the dark compartment (training latency) was recorded. Immediately after the shock, the mouse was returned back to the home cage. The retention trial started 24 h after the end of the acquisition trial. Each mouse was placed in the illuminated compartment as in the training trial. The door was opened after a 30-s acclimation period. The step-through latency in the retention trial (with a maximum 300-s cutoff time) was used as the index of retention of the learned experience. Shock was not applied during the retention trial.
Free exploratory paradigm (Hughes box)
The apparatus consisted of a polyvinyl chloride box (30×20×20 cm) covered with Plexiglas and subdivided into 6 identical square exploration units, all interconnected by small doors [23]. A temporary partition divided the apparatus in half lengthwise. To familiarize the animals with the apparatus, each subject was placed in one half of the apparatus with the temporary partition in place approximately 24 h before testing. The floor was covered with sawdust and the animal was given unlimited access to food and water. The next day, the same mouse was exposed to both the familiar and novel environments after the temporary partition was removed without the animal being removed from the box. The subject was then observed under red light for 10 min. Parameters recorded were the number of units entered to the novel side and the time spent in the novel side.
Drug administration
Zaprinast and rolipram were purchased from Sigma Chemical Company (Sigma, St. Louis, MO) and were dissolved in saline supplemented with small amounts of DMSO. All drugs were freshly prepared and administered in a volume of 0.1 ml per 10 g body weight. The control groups received the same volume of vehicle. Zaprinast (3 and 10 mg/kg), rolipram (0.05 and 0.1 mg/kg), or vehicle was administered intraperitoneally (i.p.) 60 and 30 min, respectively, before the first session (acquisition session, Day 1) of the mEPM (n=6) and PA tests (n=7). Zaprinast (3 and 10 mg/kg), rolipram (0.05 and 0.1 mg/kg), or vehicle was administered intraperitoneally (i.p.) 60 and 30 min, respectively, before the exploratory activity test (n=6). The animals were administered a single injection before the start of the behavioral tests. The number of animals per group ranged from 6 to 7. Different animals were used for each test. The effective dose of each drug was selected according to previous behavioral and neurochemical studies [24].
Statistics
One-way analysis of variance (ANOVA) with a post-hoc Tukey test was used to analyze PA and exploratory activity tests data. To evaluate the differences among drug treatment groups during the first and second transfer latencies in the mEPM test, the Kruskal-Wallis non-parametric test was used, followed by Dunn’s post-hoc test. The Wilcoxon signed rank test was used to compare the differences between the first and the second day latencies in the mEPM and passive avoidance tests. Data are expressed as the mean values ±SEM. P<0.05 was accepted as statistically significant.
Results
Effects of zaprinast and rolipram on learning and memory in the mEPM test
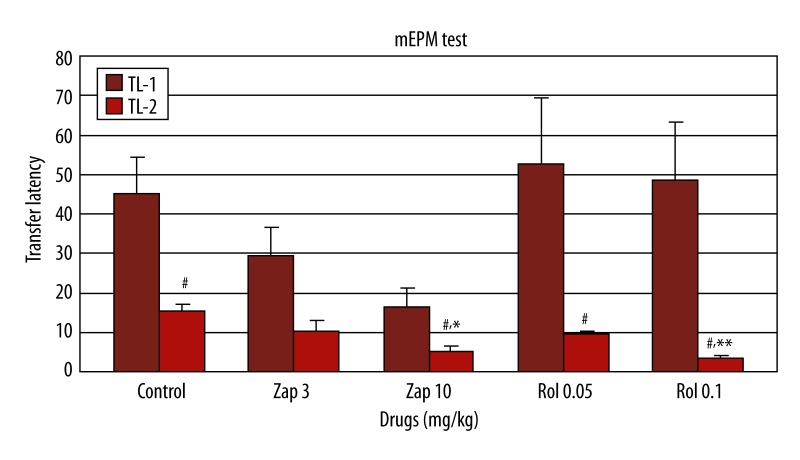
When zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.01 mg/kg) were administered before the acquisition session (training; Day 1), there was no significant difference in first-day latency (TL1) among the groups (H=7.12; p=0.12, Figure 1). Zaprinast (10 mg/kg) and rolipram (0.1 mg/kg) significantly shortened latency (TL2) on the second day compared to the control group when the drug was administered before the acquisition session (Kruskal-Wallis H=16.36; p<0.05 and p<0.01, respectively) (Figure 1). In the comparison of TL1 and TL2 for each drug-treated group, TL2 was significantly decreased in the control, zaprinast 10 mg/kg and rolipram (0.05 and 0.1 mg/kg) groups (p<0.05), but this measure was not significantly different between the zaprinast 3 mg/kg groups (p>0.05) (Figure 1).
Figure 1.
Effect of zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.1 mg/kg) on learning and memory (n=6) (in which zaprinast and rolipram was administered 60 and 30 min; respectively before the training) in the elevated plus maze test in mice. The data are expressed as mean ±SEM values of animals. # p<0.05, when the first and second sessions of groups were compared; * p<0.05, ** p<0.01 compared to second session of the control group.
Effects of zaprinast and rolipram on learning and memory in the passive avoidance test
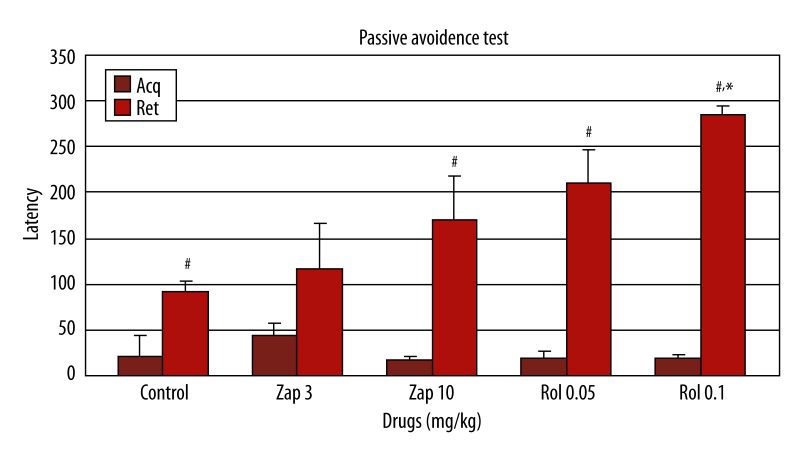
When zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.1 mg/kg) were administered before the acquisition session of passive avoidance test, there was no significant difference in first-day latency among the groups [F(4.34)=2.12, p>0.05, Figure 2]. Rolipram (0.1 mg/kg) significantly prolonged retention latency compared to the control group and zaprinast had a partial effect; however, this effect did not reach significance when the drugs were administered before the acquisition session [F(4.34)=4.64; p<0.01 Figure 2]. In the comparison of first- and second-day latencies for each drug-treated group, retention latency was significantly prolonged in the control, zaprinast 10 mg/kg, and rolipram (0.05 and 0.1 mg/kg) groups (p<0.05), but this measure was not significantly different between the zaprinast 3 mg/kg groups (p>0.05; Figure 2).
Figure 2.
Effect of zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.1 mg/kg) on learning and memory (n=7) (in which zaprinast and rolipram was administered 60 and 30 min; respectively before the acquisition) in the passive avoidance test in mice. The data are expressed as mean ±SEM values of animals. # p<0.05, when the first and second sessions of groups were compared; * p<0.01 compared to second session of the control group.
Effects of zaprinast and rolipram on exploratory activity in the Hughes box
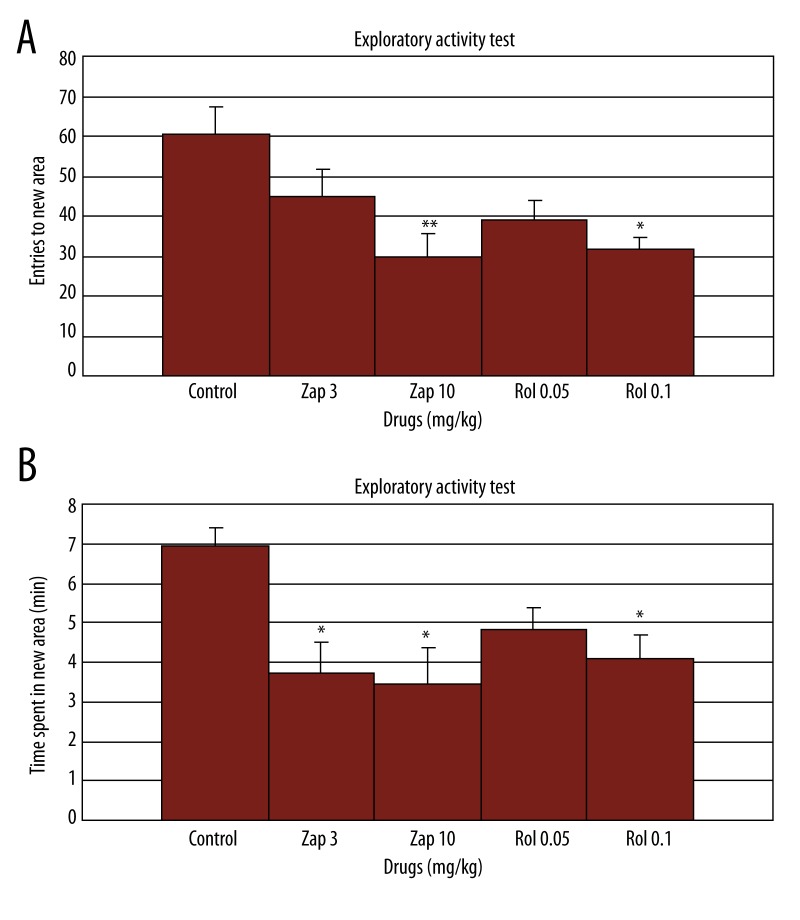
There was a significant difference between groups after evaluating the total number of entries to the novel side [F(4.29)=4.86, p=0.0049; Figure 3A] and total time spent in the novel side [F(4.29)=4.25, p=0.009, Figure 3B] in the Hughes box. Both zaprinast (10 mg/kg) and rolipram (0.1 mg/kg) significantly decreased entries to the novel side compared to the control group in the Hughes box when administered before the test (p<0.01 and p<0.05; respectively; Figure 3A). Zaprinast (3 and 10 mg/kg) and rolipram (0.1 mg/kg) also significantly shortened the time spent in the novel side in the Hughes box (p<0.05; Figure 3B).
Figure 3.
Effect of zaprinast (3 and 10 mg/kg) and rolipram (0.05 and 0.1 mg/kg) administration on exploratory activity in free exploratory paradigm (Hughes Box) (n=6). Drugs were injected 60 and 30 min, respectively, prior to testing. The data are expressed as mean ±SEM values. a: total number of entries to novel side, b: total time spent in the novel side. * p<0.05, ** p<0.01 compared to the control group.
Discussion
This study revealed that the PDE5 inhibitor, zaprinast (10 mg/kg), and the PDE4 inhibitor, rolipram (0.1 mg/kg), significantly decreased second-day latency in the EPM test, but only rolipram (0.1 mg/kg) significantly increased second-day latency in the PA test compared to the control mice. Both zaprinast (10 mg/kg) and rolipram (0.1 mg/kg) significantly decreased entry to new areas and time spent in new areas in the Hughes box test.
Phosphodiesterase enzymes may be involved in the etiology of a number of CNS diseases, including Alzheimer disease, schizophrenia, and affective disorders, and have recently been proposed as potential targets for therapeutic intervention [25,26]. In addition, PDEs may be targeted for cognitive enhancement, and inhibitors of PDEs have proven to be useful experimental tools for exploring the mechanisms of learning and memory [1,27].
These memory enhancements may be related to the subsequent increases in intracellular cGMP and/or cAMP levels after PDE inhibition, as both cGMP and cAMP are important intracellular second messenger molecules that have been observed in consolidation processes [28]. Interestingly, selective PDE inhibitor treatments are involved in a sequence of molecular changes that take place in the hippocampus during memory consolidation, as recently described by Izquierdo et al. [29]. Possible underlying mechanisms for memory enhancement after PDE inhibition are closely related to electrophysiological theories of learning and memory. Thus, the cAMP/PKA/CREB pathway and the cGMP/PKG/CREB pathway are key candidates for providing a biochemical substrate of long-term memory enhancement. The activation of both pathways may lead to CREB phosphorylation and, consequently, de novo protein synthesis.
There is ample evidence that cGMP and cAMP are differentially involved in memory consolidation processes [1]. Several studies have observed the memory-enhancing effects of PDE5-Is when injected before or immediately after training [30,31]. Initially, cGMP was thought to act mainly presynaptically in the early phase of long-term potentiation via the NO/sGC/cGMP pathway when affected by PDE5-Is. Alternatively, the cGMP/PKG pathway has been repeatedly proposed as the underlying mechanism of early memory consolidation [32,33]. Presently, it is unclear which pathway underlies the memory-enhancing effects of selective PDE5 inhibition. Studies are underway to locate the PDE5 enzyme at the subcellular level. The data for PDE4 inhibitors are assumed to be related to elevated cAMP levels [34]. cAMP is involved in late-phase long-term potentiation via the cAMP/PKA/CREB pathway [35,36]. Furthermore, it has been suggested that late consolidation processes are particularly affected by cAMP 3 h after acquisition [37].
The prototypical PDE4 inhibitor most widely used in cognition studies is rolipram. It possesses good brain penetration and a half-life of 1–3 h [38], and in vitro studies have shown that cAMP levels are increased in hippocampal slices treated with rolipram [39]. Studies have shown that rolipram produces memory-enhancing effects in a number of models and has antidepressant-like activity in both preclinical [40] and clinical models [41]. In 1997, it was first described that PDE5 inhibition improves memory processes [14]. Zaprinast was used to inhibit PDE5, and when given immediately after training at a dose of 10 mg/kg (i.p.), zaprinast improved the LTM performance of rats in an object recognition task [14]. However, zaprinast also inhibits PDE1, 9, 10, and 11.
The memory-improving effects of PDE5 inhibitors may also, or alternatively, be related to increased blood flow and, consequently, increased glucose metabolism, because PDE5 inhibitors are known to cause vasodilatation, most likely via cGMP [42,43]. A decrease in blood flow generally results in a decrease in blood pressure. It was observed that a 10 mg/kg dose of zaprinast administration (i.p.) slightly increased the mean arterial blood pressure in conscious rats from 1 to 4 h, after which recovery occurred [16]. This increase had been observed before, and the mechanism by which zaprinast elevates mean arterial blood pressure is not clear [42]. However, a depressor response after systemic administration of zaprinast has been observed at doses above 10 mg/kg [42,43]. It has been demonstrated that 10 mg/kg zaprinast (i.p.) clearly improves the early consolidation of object information [16]. Because the same dose of zaprinast did not affect mean arterial blood pressure, it is unlikely that effects on peripheral blood pressure after zaprinast treatment contributed to memory improvement.
The mEPM test is a simple method that evaluates spatial memory. Shortened transfer latency in the second trial is used as a parameter to measure the retention or consolidation of memory, and drug treatment prior to the first day may be utilized to determine the effects on memory acquisition [44]. In our study, drugs were administered before the first session to evaluate the effects on memory acquisition. In addition, drugs can be administered just after the first session to evaluate the effects on memory consolidation and/or just before the second session to evaluate the effects on memory retention. The evaluation of the effects of the drug in the first trial may be confounded by nonspecific effects, such as effects on anxiety, locomotion, and motility [45]. Both zaprinast and rolipram increased second-day latency in the EPM test, supporting their spatial memory enhancing effects in the EPM test.
Passive avoidance is an adaptive response to a stressful experience, which serves as a measure of learning and memory [17]. In our study, drugs were injected just before an electrical shock so that we could study the drugs’ effects on memory acquisition and retrieval. Administering the drug just before the electrical shock can cause nonspecific effects (e.g., analgesic effect, pain perception, and motility). Only rolipram significantly increased second-day latency in the PA test, and zaprinast had no significant effect. Higher doses of zaprinast should be investigated in further studies. In our study, both zaprinast and rolipram exerted dose-dependent effects of both drugs on memory, correlating with previous findings [46,47].
In the free exploratory test (Hughes box), mice that spend more time in the novel side are considered less anxious. The effect of NO on anxiety remains controversial. In some studies, NOS inhibitors displayed anxiolytic effects [48], whereas NO donors had anxiogenic effects [49]. In our study, both zaprinast and rolipram decreased exploratory activity in the Hughes box test, a result that indicated an anxiogenic effect.
Conclusions
The present study demonstrates that both the PDE 5 inhibitor zaprinast and the PDE 4 inhibitor rolipram enhanced spatial memory in the EPM test, and rolipram enhanced emotional memory in the PA test compared to zaprinast. Both zaprinast and rolipram diminished exploratory activity in the Hughes box test, which can be attributed to their anxiogenic effects. Our results confirm that the effects of zaprinast and rolipram on learning and memory seem to be test-dependent, and future studies using different PDE inhibitors with different cognition methods should be performed to verify our findings.
Footnotes
Source of support: Departmental sources
References
- 1.Prickaerts J, Sik A, van Staveren WC, et al. Phosphodiesterase type 5 inhibition improves early memory consolidation of object information. Neurochem Int. 2004;45:915–28. doi: 10.1016/j.neuint.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Fedele E, Raiteri M. In vivo studies of the cerebral glutamate receptor/NO/cGMP pathway. Prog Neurobiol. 1999;58:89–120. doi: 10.1016/s0301-0082(98)00077-x. [DOI] [PubMed] [Google Scholar]
- 3.Gong B, Vitolo OV, Trinchese F, et al. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–34. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitolo OV, Sant’Angelo A, Costanzo V, et al. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci USA. 2002;99:13217–21. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halene TB, Siegel SJ. PDE inhibitors in psychiatry – future options for dementia, depression and schizophrenia? Drug Discov Today. 2007;12:870–78. doi: 10.1016/j.drudis.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Rutten K, Basile JL, Prickaerts J, et al. Selective PDE inhibitors rolipram and sildenafil improve object retrieval performance in adult cynomolgus macaques. Psychopharmacology (Berl) 2008;196:643–48. doi: 10.1007/s00213-007-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blokland A, Schreiber R, Prickaerts J. Improving memory: a role for phosphodiesterases. Curr Pharmacol Design. 2006;12:2511–23. doi: 10.2174/138161206777698855. [DOI] [PubMed] [Google Scholar]
- 8.Nagakura A, Niimura M, Takeo S. Effects of a phosphodiesterase IV inhibitor rolipram on microsphere embolism-induced defects in memory function and cerebral cyclic AMP signal transduction system in rats. Br J Pharmacol. 2002;135:1783–93. doi: 10.1038/sj.bjp.0704629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang HT, O’Donnell JM. Effects of rolipram on scopolamine induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacology. 2000;150:311–16. doi: 10.1007/s002130000414. [DOI] [PubMed] [Google Scholar]
- 10.Imanishi T, Sawa A, Ichimaru Y, et al. Ameliorating effects of rolipram on experimentally induced impairments of learning and memory in rodents. Eur J Pharmacol. 1997;321:273–78. doi: 10.1016/s0014-2999(96)00969-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhang HT, Huang Y, Suvarna NU, et al. Effects of the novel PDE4 inhibitors MEM1018 and MEM1091 in models of memory and antidepressant sensitivity. Soc Neurosci Abstr. 2002;684:12. [Google Scholar]
- 12.Bourtchouladze R, Lidge R, Catapano R, et al. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci USA. 2003;100:10518–22. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barad M, Bourtchouladze R, Winder DG, et al. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA. 1998;95:15020–25. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prickaerts J, van Staveren WC, Şık A, et al. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience. 2002;113:351–61. doi: 10.1016/s0306-4522(02)00199-9. [DOI] [PubMed] [Google Scholar]
- 15.Domek-Lopacinska K, Strosznajder JB. The effect of selective inhibition of cyclic GMP hydrolyzing phosphodiesterases 2 and 5 on learning and memory processes and nitric oxide synthase activity in brain during aging. Brain Res. 2008;1216:68–77. doi: 10.1016/j.brainres.2008.02.108. [DOI] [PubMed] [Google Scholar]
- 16.Prickaerts J, Steinbusch HWM, Smits JFM, De Vente J. Possible role of nitric oxide-cyclic GMP pathway in object recognition memory: effects of 7-nitroindazole and zaprinast. Eur J Pharmacol. 1997;337:125–36. doi: 10.1016/s0014-2999(97)01301-0. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji M, Takeda H, Matsumiya T. Modulation of passive avoidance in mice by the 5-HT1A receptor agonist flesinoxan: comparison with the benzodiazepine receptor agonist diazepam. Neuropsychopharmacol. 2003;28:664–74. doi: 10.1038/sj.npp.1300080. [DOI] [PubMed] [Google Scholar]
- 18.Reddy DS, Kulkarni SK. Possible role of nitric oxide in the nootropic and antiamnesic effects of neurosteroids on aging- and dizocilpine-induced learning impairment. Brain Res. 1998;799:215–29. doi: 10.1016/s0006-8993(98)00419-3. [DOI] [PubMed] [Google Scholar]
- 19.Hlinak Z, Krejci I. Concurrent administration of subeffective doses of scopolamine and MK-801 produces a short-term amnesia for the elevated plus-maze in mice. Behav Brain Res. 1998;91:83–89. doi: 10.1016/s0166-4328(97)00107-1. [DOI] [PubMed] [Google Scholar]
- 20.Hlinak Z, Krejci I. MK-801 induced amnesia for the elevated plus-maze in mice. Behav Brain Res. 2002;131:221–25. doi: 10.1016/s0166-4328(01)00347-3. [DOI] [PubMed] [Google Scholar]
- 21.Hlinak Z, Krejci I. Oxiracetam prevents the MK-801 induced amnesia for the elevated plus-maze in mice. Behav Brain Res. 2000;117:147–51. doi: 10.1016/s0166-4328(00)00298-9. [DOI] [PubMed] [Google Scholar]
- 22.Ogren SO, Johansson C, Magnusson O. Forebrain serotonergic involvement in avoidance learning. Neurosci Lett. 1985;58:305–9. doi: 10.1016/0304-3940(85)90071-0. [DOI] [PubMed] [Google Scholar]
- 23.Hughes RN. Food deprivation and locomotor exploration in the white rat. Anim Behav. 1965;13:30–32. [Google Scholar]
- 24.Reneerkens OA, Rutten K, Steinbusch HW, et al. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl) 2009;202:419–43. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menniti FS, Chappie TA, Humphrey JM, Schmidt CJ. Phosphodiesterase 10A inhibitors: a novel approach to the treatment of the symptoms of schizophrenia. Curr Opin Invest Drugs. 2007;8:54–59. [PubMed] [Google Scholar]
- 26.Reyes-Irisarri E, Markerink-Van Ittersum M, et al. Expression of the cGMP-specific phosphodiesterases 2 and 9 in normal and Alzheimer’s disease human brains. Eur J Neurosci. 2007;25:3332–38. doi: 10.1111/j.1460-9568.2007.05589.x. [DOI] [PubMed] [Google Scholar]
- 27.Izquierdo LA, Barros DM, Vianna MR, et al. Molecular pharmacological dissection of short- and long-term memory. Cell Mol Neurobiol. 2002;22:269–87. doi: 10.1023/A:1020715800956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–64. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 29.Izquierdo I, Bevilaqua LR, Rossato JI, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Prickaerts J, Şık A, van der Staay FJ, et al. Dissociable effects of acetylcholinesterase inhibitors and phosphodiesterase type 5 inhibitors on object recognition memory: acquisition versus consolidation. Psychopharmacology (Berl) 2005;177:381–90. doi: 10.1007/s00213-004-1967-7. [DOI] [PubMed] [Google Scholar]
- 31.Rutten K, Vente JD, Şık A, et al. The selective PDE5 inhibitor, sildenafil, improves object memory in Swiss mice and increases cGMP levels in hippocampal slices. Behav Brain Res. 2005;164:11–16. doi: 10.1016/j.bbr.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 33.Zhuo M, Hu Y, Schultz C, et al. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature. 1994;368:635–39. doi: 10.1038/368635a0. [DOI] [PubMed] [Google Scholar]
- 34.Barad M, Bourtchouladze R, Winder DG, et al. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA. 1998;95:15020–25. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93:13445–52. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–64. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 37.Prickaerts J, de Vente J, Honig W, et al. cGMP, but not cAMP, in rat hippocampus is involved in early stages of object memory consolidation. Eur J Pharmacol. 2002;436:83–87. doi: 10.1016/s0014-2999(01)01614-4. [DOI] [PubMed] [Google Scholar]
- 38.Krause W, Kuhne G. Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica. 1988;18:561–71. doi: 10.3109/00498258809041693. [DOI] [PubMed] [Google Scholar]
- 39.van Staveren WC, Markerink-van Ittersum M, Steinbusch HW, de Vente J. The effects of phosphodiesterase inhibition on cyclic GMP and cyclic AMP accumulation in the hippocampus of the rat. Brain Res. 2001;888:275–86. doi: 10.1016/s0006-8993(00)03081-x. [DOI] [PubMed] [Google Scholar]
- 40.Itoh T, Abe K, Tokumura M, et al. Different regulation of adenylyl cyclase and rolipram sensitive phosphodiesterase activity on the frontal cortex and hippocampus in learned helpnessness rats. Brain Res. 2003;991(1–2):142–49. doi: 10.1016/j.brainres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Scott AI, Perini AF, Shering PA, Whalley LJ. ln-patient major depression: is rolipram as effective as amitriptyline. Eur J Clin Pharmacol. 1991;40(2):127–29. doi: 10.1007/BF00280065. [DOI] [PubMed] [Google Scholar]
- 42.Dundore RL, Clas DM, Wheeler LT, et al. Zaprinast increases cyclic GMP levels in plasma and in aortic tissue of rats. Eur J Pharmacol. 1993;249:293–97. doi: 10.1016/0014-2999(93)90525-m. [DOI] [PubMed] [Google Scholar]
- 43.Dundore RL, Habeeb PG, Pratt PF, et al. Differential hemodynamic responses to selective inhibitors of cyclic nucleotide phosphodiesterases in conscious rats. J Cardiovasc Pharmacol. 1992;19:937–44. doi: 10.1097/00005344-199206000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Sharma AC, Kulkarni SK. Evaluation of learning and memory mechanisms employing elevated plus-maze in rats and mice. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:117–25. doi: 10.1016/0278-5846(92)90014-6. [DOI] [PubMed] [Google Scholar]
- 45.Hunter B, Zornetzer SF, Jarvik ME, McGaugh JL. Modulation of learning and memory: effects of drugs influencing neurotransmitters. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of Psychopharmacology. Vol. 19. Plenum Press; New York: 1988. pp. 531–77. [Google Scholar]
- 46.Akar F, Mutlu O, Celikyurt IK, et al. Effects of zaprinast and rolipram on olfactory and visual memory in the social transmission of food preference and novel object recognition tests in mice. Drug Target Insights. 2014;8:23–29. doi: 10.4137/DTI.S14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akar F, Mutlu O, Celikyurt IK, et al. Effects of Rolipram and Zaprinast on Learning and Memory in the Morris Water Maze and Radial Arm Maze Tests in Naive Mice. Drug Res (Stuttg) 2014;64:1–5. doi: 10.1055/s-0034-1372646. [DOI] [PubMed] [Google Scholar]
- 48.Yildiz F, Ulak G, Erden F, Gacar N. Anxiolytic- like effects of 7-nitroindazole in the rat plus-maze test. Pharmacol Biochem Behav. 2000;65:199–202. doi: 10.1016/s0091-3057(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 49.Caton PW, Tousman SA, Quock RM. Involvement of nitric oxide in nitrous oxide anxiolysis in the elevated plus-maze. Pharmacol Biochem Behav. 1994;48:689–92. doi: 10.1016/0091-3057(94)90333-6. [DOI] [PubMed] [Google Scholar]