Abstract
Stimulus-response compatibility (SRC)—the fact that some stimulus-response pairs are faster than others—is attributed in part to automatic activation of the stimulus-compatible response representation. Cognitive models of SRC propose that automatic response activation can be strategically suppressed if the automatic response is likely to interfere with behavior; in particular, suppression is thought to occur in preparation for incompatible responses and when the required stimulus-response mapping is unknown before stimulus presentation. We test this preparatory suppression hypothesis in the context of imitation, a special form of SRC particularly relevant to human social behavior. Using TMS, we measured muscle-specific corticospinal excitability during action observation (motor resonance) while human participants prepared to perform imitative and counterimitative responses to action videos. Motor resonance was suppressed during preparation to counterimitate and for unknown mappings, compared to preparation to imitate and a baseline measure of motor resonance. These results provide novel neurophysiological evidence that automatic activation of stimulus-compatible responses can be strategically suppressed when it is likely to interfere with task goals. Insofar as motor resonance measures mirror neuron system activity, these results also suggest that preparatory control of automatic imitative tendencies occurs through modulation of mirror neuron system activity.
Keywords: stimulus response compatibility, imitation, motor resonance, transcranial magnetic stimulation, motor preparation
INTRODUCTION
Stimulus-response compatibility (SRC) describes the observation that reaction times are faster when a stimulus and its required response share some property (for example, they have similar spatial location), as compared to when they do not share any properties (Shaffer 1965; Kornblum 1990). Automatic imitation describes a special case of stimulus-response compatibility (SRC) in which the stimuli represent human actions; participants either imitate the stimulus by performing the same action (imitative/compatible response) or do not imitate the stimulus and instead perform a different action (non-imitative/incompatible response). In these tasks, “imitation” is defined as matching spatial and kinetic properties of the stimulus and response. As would be expected from the SRC literature using symbolic stimuli, reaction times are faster for imitative responses (which by definition share many properties with the action stimulus) than for non-imitative responses (Brass et al., 2000; Stürmer et al., 2000). For example, participants are faster to perform a grasping action while simultaneously observing a grasping action than while observing a hand opening (Stürmer et al., 2000). This reaction time benefit (henceforth, imitative compatibility effect) occurs even when the observed action is not relevant to successfully perform the task, indicating that the influence of the observed action on the motor response is unintentional, or automatic.
Like many other forms of SRC in which participants respond to static symbolic stimuli (De Jong et al., 1994; Eimer et al., 1995), imitative compatibility effects are attributed to automatic activation of the stimulus-compatible motor representation. In the case of imitation, the mirror neuron system (MNS) has been hypothesized to underlie automatic response activation (Ferrari et al., 2009), since it responds during the observation and execution of similar actions and provides input to primary motor cortex (Di Pellegrino et al., 1992; Iacoboni et al., 1999; Rizzolatti and Craighero, 2004).
Some cognitive models of SRC suggest that it is possible to strategically suppress the automatic activation of a stimulus-compatible response when this response is likely to interfere with task goals (Shaffer, 1965; De Jong, 1995; Vu and Proctor, 2004). In particular, suppression occurs in preparation for incompatible responses (when the stimulus-compatible response is incorrect) and in preparation for trials in which the required stimulus-response mapping is unknown in advance of the stimulus (when the stimulus-compatible response is incorrect half the time). This preparatory suppression manifests behaviorally as reduced compatibility effects in the unknown mapping trials: the compatible response no longer benefits from automatic response activation making compatible and incompatible reaction times similar. In the alternative, more common scenario—when the required mapping is known before the stimulus—the automatic response route is suppressed selectively for incompatible trials, so that compatible trials have a speed advantage due to automatic response activation (Shaffer, 1965; Heister and Schroeder-Heister, 1994; De Jong, 1995; Vu and Proctor, 2004).
When extended to imitation, this model of SRC suggests that the MNS may be suppressed in order to avoid imitation when it is likely to interfere with motor responses. This is in line with previous fMRI studies examining control of imitative tendencies, which have proposed mechanisms involving MNS modulation (Spengler et al., 2009; Cross et al., 2013). While there is accumulating evidence that both mirror neuron system activity (Newman Norlund 2007; Catmur 2007; Chong 2008; Molenberghs 2012) and imitative compatibility effects (Van Baaren 2003; Likowski 2008; Chong 2009; Liepelt 2009; Leighton 2010) can be modulated by attention and contextual factors, to date there is no neurophysiological evidence demonstrating that controlling imitative tendencies (i.e. avoiding unwanted imitation) occurs through mirror neuron system modulation. To test this hypothesis, we used transcranial magnetic stimulation (TMS) to measure corticospinal excitability during action observation in the setting of an imitative compatibility task. Facilitation of corticospinal excitability specifically in the muscles involved in performing an observed action (motor resonance) is a putative measure of MNS activity (Fadiga et al., 1995; Avenanti et al., 2007). Therefore, we measured motor resonance as a measure of MNS-mediated imitative response activation while participants prepared to imitate or counterimitate a simple finger movement.
In line with preparatory supression models, we predicted lower motor resonance during preparation to counterimitate and during preparation for an unknown stimulus-response mapping, as compared to preparation to imitate. In addition, since such a pattern could be explained by facilitation of motor resonance during preparation to imitate rather than suppression for incompatible and unknown conditions, we obtained a baseline measure of motor resonance during a control task with a similar design, except that participants prepared to perform an arbitrary stimulus-response mapping. This controlled for basic motor preparation effects, but removed any potential effects of compatibility between stimulus and response.
MATERIALS AND METHODS
In Experiment 1, we first ran a group of participants without applying TMS to ensure that our novel paradigm reproduced behavioral effects associated with preparatory suppression models (Experiment 1), because twitches from supra-threshold TMS are likely to interfere with reaction time measures. Specifically, we were looking for a reduction in the RT benefit for compatible compared to incompatible trials when the stimulus-response mapping is not known before the imperative stimulus. After replicating previous behavioral results that justify motor resonance predictions based on preparatory suppression models, in Experiment 2 we ran a second group of participants with TMS to test our hypothesis that motor resonance is suppressed in preparation for trials in which imitation may interfere with task goals. RT was not considered in this experiment due to interference caused by TMS-induced muscle twitches.
Task Design
Imitation Task
Participants performed imitative or counter-imitative actions (flexion or extension of the right index finger) in response to video stimuli. They were asked to rest their index finger on the bottom right key of a keyboard (number pad “Enter”) so that the finger was completely relaxed between responses. Flexion and extension responses involved pressing the key and lifting the finger off the key, respectively.
In the first frame of each stimulus video, a left hand rested palm-down with fingers facing the subject and the index finger in a half-raised position (i.e. a mirror image of the starting position of the participant's response hand). This static frame was presented for 2.4 or 3.2 seconds and represented the preparatory period. Then, the target video (1.25 s) depicted the index finger either extending further (lifting upward) or flexing (tapping downward) from the starting position. The color of a thick border surrounding the video indicated whether subjects should imitate (green border; half of trials) or counter-imitate (red border; half of trials) the target video (Figure 1A, left).
Figure 1.
Task design. (A) Schemata of imitation task preparatory and target conditions are depicted at left. The border color defines 3 preparatory period conditions (labeled at left) and 4 target period conditions (labeled at right). The target video action is depicted by the last frame of the video stimulus. Only one of the two possible videos—lift or tap—is shown for each preparatory condition, and the correct response is indicated below the frame. At right, trial timing is shown for example PrepIm trials with (top) and without (middle) action observation (AO) videos (the image for the AO video depicts the final frame of a squeeze video). (B) Control task preparatory and target stimuli are shown at left (both target stimuli are shown). Trial timing for the control task (right) is identical to the imitation task.
On 2/3 of trials (Prep trials) the border color was presented during the preparatory period, so that subjects could prepare to imitate (PrepIm; 1/3 of trials) or counter-imitate (PrepCI; 1/3 of trials) before the target video. On the remaining 1/3 of trials (NoPrep trials), the border remained black throughout the preparatory period and changed to green or red at the onset of the target video. Therefore, on these trials participants did not know the appropriate stimulus-response mapping until the target video onset. The result is 3 different preparatory conditions, the crucial conditions of interest in the TMS experiment (prepare to imitate, PrepIm, prepare to counterimitate, PrepCI and prepare for unknown mapping, NoPrep; Figure 1A, left column), but 4 different target conditions (PrepCI, PrepIm, NoPrep-CI, NoPrep-Im; Figure1A, right column) because NoPrep trials are split into imitate and counter-imitate conditions upon presentation of the target video.
In order to measure motor resonance during the 3 different preparatory conditions, half of preparatory periods were interrupted by an action video (Figure 1A, right; this is when TMS was applied and MEPs were measured in Experiment 2). These action observation (AO) videos depicted a right hand either squeezing or releasing a ball held between the index finger and thumb. There were 32 different AO videos (16 squeeze, 16 release), which varied in hand orientation (index finger and thumb pointing left, as shown, or pointing down, not shown) and ball color (blue, orange, yellow, white) to reduce habituation. The inclusion of two different actions (squeeze and release) allowed us to measure from a single muscle (reducing the required TMS intensity) but still examine the specificity of MEP facilitation that is necessary to demonstrate motor resonance. Specifically, facilitation of the first dorsal interosseus (FDI) muscle during observation of an action that uses the muscle (squeeze) compared to an action that does not use the muscle (release) provides evidence of muscle specific facilitation and motor resonance.
AO videos were constructed of 20 frames presented at 60 Hz, with the last frame remaining on the screen for 834ms (total video length=1.15 s). AO videos were included on only half of trials to discourage participants from waiting until after the AO video to begin preparation. To maximize the likelihood that participants were preparing during the video, it was presented 2.4 or 3.2s after preparatory period onset—the same time as target videos appeared in trials without an AO video. After the AO video the preparatory period continued for 0.4 or 1.2 s before the target video was presented. The resulting trials were 3.65-6.8 seconds long, depending on Prep-Target, Prep-AO video and AO video-Target intervals; trials were separated by a 1.5 s intertrial interval.
A total of 192 trials were presented in a constrained random order. Because the goal of the study was to demonstrate modulation of MEPs obtained during the preparatory period, we balanced the number of each of the three preparatory conditions: There were 64 PrepIm, 64 PrepCI and 64 NoPrep trials and 32 trials in each preparatory condition included an AO video (16 squeeze, 16 release; each AO video presented once in each preparatory condition). This created a balanced 3 (PrepIm/PrepCI/NoPrep) × 2 (Squeeze/Release) design with 16 MEPs per preparatory condition and observed action in Experiment 2. It should be noted, however, that since NoPrep trials are split into NoPrep-Im and NoPrep-CI conditions upon presentation of the target video, target conditions relevant to reaction time analysis (Experiment 1) comprise a 2 (Prep/NoPrep) × 2 (Im/CI) design with 64 PrepIm, 64 PrepCI, 32 NoPrep-Im and 32 NoPrep-CI trials. There were not a sufficient number of trials to examine the effect of the AO video (squeeze vs. release) on reaction times, but this factor was counterbalanced and therefore should not affect results with respect to preparatory modulation of compatibility effects.
Each of the three preparatory conditions (PrepIm, PrepCI, NoPrep) followed each other condition with equal probability, as did imitate and counterimitate target conditions, and AO and no AO trials. There were an equal number of flexion and extension responses for each condition, with squeeze and release AO videos split evenly between responses. Following these constraints, a new order was generated for each participant.
Control Task
A second control task was included as a baseline condition in which similar two-forced choice motor preparation was required, but in the absence of any stimulus-response compatibility. Participants performed the same flexion/extension responses depending on the color (cyan or magenta) of a square patch (Figure 1B, left). Trials began with an open black square (preparatory period) that was then filled in with either cyan or magenta (target). The color-response mapping was counterbalanced across participants: half of subjects performed finger flexion for cyan squares and extension for magenta squares and the other half performed the opposite mapping. An AO video interrupted the preparatory period in half of trials and timing was identical to the imitation task (Figure 1B, right). Although ideally the baseline condition would be randomized with the imitation task conditions, pilot studies made it clear that this would not be possible due to the difficulty remembering and switching between the different stimulus-response mapping rules associated with the two tasks. As such, the control task was performed in a separate 7-minute run comprising 64 trials (32 AO videos: 16 squeeze, 16 release).
Experiment 1: Reaction Time
Participants
10 participants (2/8 M/F, 18-24 years old) were recruited from an undergraduate subject pool and received course credit for participating. Participants were right-handed, neurologically healthy and were not taking psychoactive medications. The study was approved by the UCLA Institutional Review Board and written informed consent was obtained from all participants.
Procedure
Participants were familiarized with the imitation task first with no AO trials for 5 minutes. They were instructed to “prepare as much as possible while waiting for the finger movement so you can respond quickly and accurately.” AO trials were then added for an additional minute of practice. At this time, subjects were told an additional video might occur while they were preparing. They were instructed that the video was not relevant to the task, and therefore, to try to maintain preparation for the upcoming response throughout the preparatory period even if an AO video occurred. The imitation task was separated into three consecutive runs lasting about 7 minutes each, with a short break between runs. The order of imitation and control tasks was counterbalanced across subjects.
EMG Recording and Analysis
To measure reaction time, EMG activity was recorded from surface electrodes placed over the first dorsal interosseus (FDI) and extensor digitorum communis (EDC) muscles of the right hand and forearm (button presses could not be used for reaction time since they occurred on only half of trials—those requiring a flexion response). In each trial, data were recorded for 4.8 seconds starting 2 seconds after the onset of the preparatory period so that recordings included 0.4 or 1.2 seconds of preparation, the AO (when present) and target videos, and at least 1.2 seconds after the target video onset (response window). EMG signals were amplified (×1000), bandpass filtered online (50-450 Hz; Delsys, Inc., Boston, MA) and digitized at 5000 Hz for offline analysis.
The time of muscle activation was determined for flexion (FDI) and extension (EDC) responses using custom MATLAB software implementing a double threshold procedure (Lidierth, 1986) and verified visually for each trial while blind to condition. Although the FDI was often active during finger extension as well as during flexion, activity in the EDC was selective for extension, making it possible to distinguish flexion and extension responses on EMG (see Figure 2). When EMG onset or response action could not be determined due to excessive background activity or other noise, the trial was discarded (only 1.5% of trials).
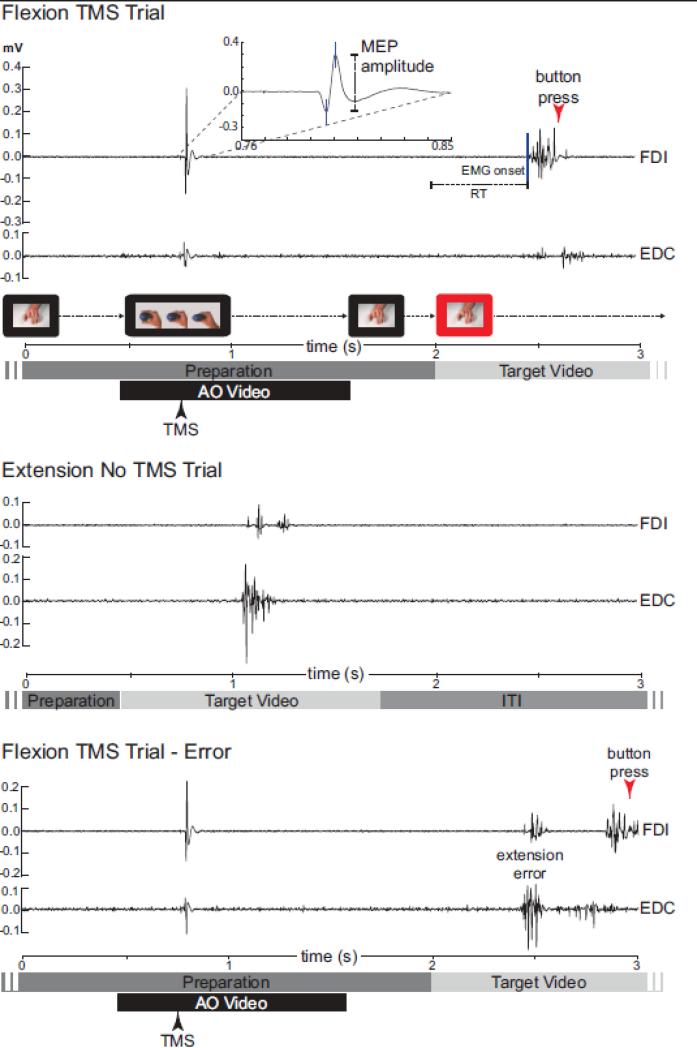
Figure 2.
EMG (Exp. 1 & 2) and MEP (Exp. 2) analysis. The first 3 seconds of representative FDI and EDC EMG traces are shown for 3 trials from Experiment 2, Subject 4. Top trial depicts activity in FDI indicative of a flexion response in a TMS trial. Inset illustrates MEP amplitude measurement and blue line indicates automated EMG onset identification for RT calculation. The middle trace shows activity indicative of an extension response for comparison (primarily in EDC). The bottom trace shows an error trial, which was excluded from analysis. Bars below each trace indicate the preparatory period and onset and offset of the action observation (AO) and target videos. Arrows indicate time of TMS pulse and time of button responses as recorded by stimulus presentation software.
Reaction time (RT) for each trial was calculated as the time of muscle activation relative to the target video onset. Mean percent error and reaction times (errors and outliers greater than 3 SD from the mean excluded) for each condition and subject were calculated and analyzed with 3-way repeated measures ANOVAs [2 (Prep, NoPrep) × 2 (Imitate, Counterimitate) × 2 (AO video, No AO video)]. Because we had clear directional predictions from previous compatibility studies, the significant 2-way interaction (Prep/NoPrep × Imitate/Counterimitate) was explored with planned paired t-tests to determine whether the compatibility effects (difference between counterimitation and imitation) were reduced in NoPrep compared to Prep trials as proposed by the suppression hypothesis. The control task was used for comparison of motor resonance in Experiment 2, and was included in Experiment 1 only to ensure that behavioral data were collected under identical procedures as Experiment 2 (aside from the absence of TMS). Therefore, behavioral data were not analyzed for the control task.
Experiment 2: TMS/MEPs
Participants
21 participants recruited through a campus newspaper and posted fliers completed Experiment 2 (8/13 M/F, 18-34 years old). Participants were right-handed, neurologically healthy, not taking psychoactive medications and had no seizure risk factors. The study was approved by the UCLA Institutional Review Board and written informed consent was obtained from all participants. Data from 1 subject were lost due to data collection error. In addition, 4 participants were unable to relax the FDI muscle consistently despite repeated reminders and were therefore excluded (11-43% of trials with >50μV root mean squared EMG activity during 100ms pre-TMS window vs. 0-5% in relaxed subjects). Data from the remaining 16 participants (4/12 M/F) were analyzed.
Procedures
Task procedures were identical to Experiment 1 with the addition of TMS stimulation during AO videos to measure motor resonance. The imitation task was also divided into 4 runs instead of 3. In addition, at the end of the session participants performed 7-10 trials in which they squeezed and released a ball, as done in the AO videos, to provide a measure of FDI activity during execution of the same actions.
Transcranial Magnetic Stimulation
TMS was applied through a figure-of-eight coil (70mm diameter) connected to a Magstim 2002 magnetic stimulator (Magstim, Whitland, Dyfed, UK). The coil was placed tangential to the scalp over left M1 with the handle pointing backward and angled 45° from the midsagittal line. At the beginning of the session, the optimal site to evoke an MEP from the FDI (“hotspot”) was located. A frameless stereotaxy system (Brainsight, RogueResearch, Montreal, Canada) was used to record the location of the hotspot and then to monitor coil placement throughout the experiment. After locating the hotspot, the resting motor threshold (MT) was determined as the lowest intensity required to evoke an MEP of at least 50μV peak-to-peak amplitude in at least 5 out of 10 trials while the participant was relaxed. The intensity was then raised to 120% of MT for the duration of the task.
During task performance, single TMS pulses were administered over left primary motor cortex (M1) during each AO video to evoke an MEP in the right FDI. The FDI muscle was chosen for MEPs because it is selectively active for the squeeze compared to release actions depicted in the AO videos (shown below) and because it is easier to obtain stable single-muscle MEPs from the FDI compared to the EDC. The fact that the EMG activity of the FDI was not as selective as the EDC for the flexion/extension task responses (Experiment 1) is irrelevant to muscle selection for measuring motor resonance. Indeed, the flexion/extension task responses are different actions than those depicted in AO videos.
TMS stimulation occurred at the onset of the last AO video frame (317ms after video onset) when the hand was in the fully squeezed or fully released position. This very short period between AO video onset and TMS stimulation was chosen to increase the likelihood that participants were in the appropriate preparatory state during stimulation, since the presence of the AO video signals that a response is not required immediately and therefore may reduce preparation. Although short periods of action observation have only rarely been used in the motor resonance literature (Barchiesi and Cattaneo, 2013), a pilot study confirmed that the stimuli and timing did in fact evoke motor resonance.
EMG/MEP Recording and Analysis
EMG acquisition was the same as Experiment 1. MEPs recorded from the FDI were analyzed offline. Since even very small muscle contractions increase MEP size (Rösler et al., 2002), MEPs were excluded when activity was identified in the 100ms prior to TMS stimulation upon visual inspection while blind to condition (only 2.7% of trials). MEPs from error trials were also excluded from analysis. As a result of these procedures, an average of 14.3 ± 0.4 MEPs per condition were analyzed for each subject (total possible = 16). The size of each MEP (peak-to-peak amplitude) was used as a measure of corticospinal excitability of the FDI muscle. Peak-to-peak amplitude was determined in a 40ms window starting 10 ms after stimulation (Figure 2, inset). MEP amplitudes were normalized to reduce the impact of between-subject and between-run variability (Baldissera et al., 2001). This was accomplished by dividing each MEP measurement by the mean of all MEPs from the same run. Therefore, normalized MEP amplitudes represent proportion of the run mean amplitude.
Means of the normalized MEPs were calculated for each condition (Imitation task, 6 conditions: PrepIm/PrepCI/NoPrep × Squeeze/Release; Control task, 2 conditions: Squeeze/Release) and analyzed with repeated measures ANOVA and planned t-tests in two stages. First, we analyzed the imitation task with a 3 (Preparatory condition: PrepCI, PrepIm, NoPrep) × 2 (Action observed: Squeeze, Release) repeated measures ANOVA to determine whether there was a preparatory effect on motor resonance consistent with suppression models. We explored the interaction with one-tailed t-tests based on clear a-priori directional predictions. We identified (1) which preparatory periods showed significant motor resonance (greater MEP amplitude during observation of an action involving the FDI, squeeze, compared to an action not involving the FDI, release; see Figure 3) and (2) whether the magnitude of motor resonance (the difference between squeeze and release MEPs) was greater for PrepIm than NoPrep and PrepCI. This allowed us to determine whether the magnitude of motor resonance is modulated by preparatory state in accordance with the preparatory suppression hypothesis for imitation. However, it does not make clear whether the pattern of motor resonance magnitudes is due to facilitation in the PrepIm condition, or suppression in the NoPrep and Prep CI conditions, relative to the baseline level of motor resonance.
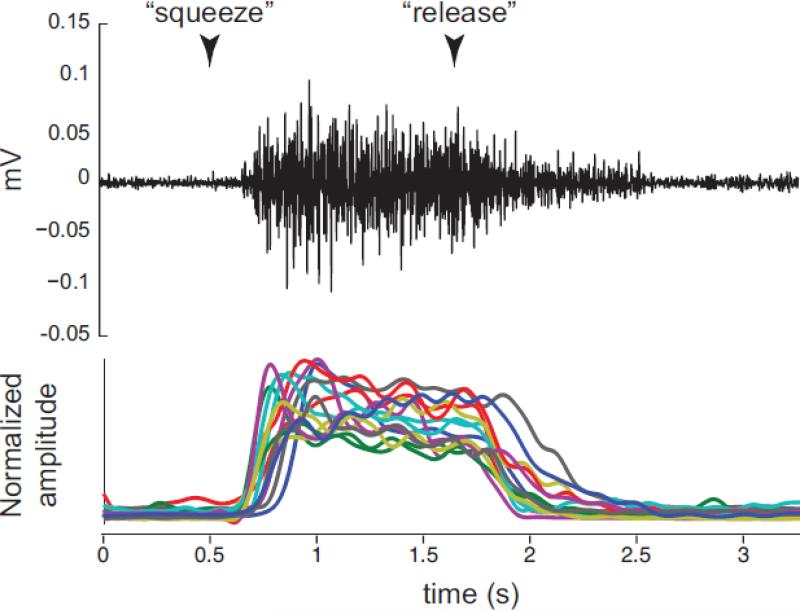
Figure 3.
EMG during action execution. EMG activity from FDI measured while subjects squeezed and released a ball between the index finger and thumb (as in AO videos) in response to visual cue (cue onset indicated with arrows). Raw EMG activity averaged across subjects (top); and processed EMG for each subject averaged across 7-10 trials (bottom) demonstrates that the FDI is active during squeeze and not during release.
To explore this issue, we introduced the control task in the second stage of the analysis. We compared the magnitude of motor resonance (the difference between squeeze and release MEPs) in each imitation task preparatory condition to the magnitude of baseline motor resonance obtained during preparation to perform an arbitrary stimulus-response mapping (the control task).
We felt this two-stage method was the best approach, given the psychological constraints requiring that the control condition be collected in a separate task run from the imitation conditions. The alternative approach of analyzing imitation and control tasks in a single ANOVA was considered suboptimal because it would involve comparison of absolute MEP magnitudes for 3 conditions that were collected together (randomized within the same runs) and 1 condition that was obtained in a separate run. MEPs are known to drift significantly over time, leading to the strategy of normalizing MEPs within task blocks (Baldissera et al., 2001). Using the two stage method allowed us to compared difference scores, which are less susceptible to variations in absolute MEP size, when examining conditions collected in different task runs.
Finally, we analyzed EMG signals obtained during execution of the squeeze and release actions to demonstrate muscle selectivity during performance. Raw EMG signals were averaged across trials and subjects to illustrate muscle activity during action execution at the group level (Figure 3, top). In addition, to facilitate display of individual subject muscle activity on a single axis (Figure 3, bottom), EMG signal was rectified, divided by the subject maximum to normalize across subjects, and lowpass filtered with a 4th order butterworth filter at 5Hz.
RESULTS
Experiment 1: Reaction Time
The 3-way ANOVA (Prep/NoPrep × Imitate/Counterimitate × AO/NoAO) on reaction times showed significant effects of preparation (F(1,9)=102.6, p<0.0001), response mapping (F(1,9)=55.6, p<0.0001) and AO (F(1,9)=70.0, p<0.0001; AO=434±17; No AO=495±16) (Figure 4). The main effect of AO is likely due to the increased preparatory time available for AO trials. Most importantly, there was an interaction between preparatory condition and response mapping (F(1,9)=4.57, p=0.036). Although imitation was faster than counterimitation for both Prep (t(9)=6.06, p=0.0001) and NoPrep trials (t(9)=3.43, p=0.004), the difference between imitation and counterimitation was greater when preparatory information was provided than when it was not (t(9)=2.09, p=0.033; Figure 4). For accuracy, only the main effect of response mapping was significant (F(1,9)=5.1, p=0.027) with greater accuracy for imitation (95.8%±0.5%) compared to counterimitation trials (93.3%±0.1%), precluding a speed-accuracy tradeoff for the compatibility effects. Thus, Experiment 1 replicates previous behavioral results supporting the suppression hypothesis in this more complex task, and validates the predictions based on this model for the MEPs in Experiment 2.
Figure 4.
Experiment 1 results. Mean reaction time for each condition. Error bars reflect standard error of the mean.
Experiment 2: MEPs
The 3×2 ANOVA (PrepCI/PrepIm/NoPrep × Squeeze/Release) on normalized MEPs from the imitation task revealed main effects of preparatory condition (F(2,15)=5.49, p=0.006) and an interaction between preparatory condition and observed action (F(2,15)=3.27, p=0.044), indicating that motor resonance in the imitation task was modulated depending on the preparatory state (Figure 5A). Planned t-tests demonstrate that motor resonance (greater excitability in the FDI during observation of squeeze actions than release actions) occurred only during preparation to imitate (PrepIm; t(15)=2.02, p=0.031). In contrast, and as predicted by the direct route suppression hypothesis, there was no difference between MEPs for observation of squeeze and release actions when subjects prepared to counterimitate (PrepCI; t(15)=-0.59, p=0.719) or when the required response mapping was unknown (NoPrep; t(15)=0.39, p=0.351). Importantly, direct comparison between motor resonance magnitudes (difference between squeeze and release MEPs) confirms that motor resonance is significantly greater during PrepIm than during PrepCI (t(15)=2.71, p=0.008) and NoPrep (t(15)=1.82, p=0.044; Figure 5B). Thus, motor resonance is modulated in accordance with the preparatory suppression model. Post-hoc t-tests to explore the main effect of preparation indicate that overall excitability was greater for NoPrep trials than for both PrepIm (t(15)=3.79, p=0.002) and PrepCI (t(15)=3.17, p=0.006), but there was no difference between PrepIm and PrepCI corticospinal excitability (t(15)=0.72, p=0.48).
Figure 5.
Experiment 2 Results. (A) Normalized MEP amplitudes for each condition are shown for the imitation and control tasks. Stars indicate significant (p<0.05) motor resonance. (B) Comparison of magnitude of motor resonance across conditions and tasks. Stars indicate significant differences (p<0.05) between magnitudes of motor resonance. The dotted line indicates a trend (p=0.06).
To determine whether the difference in motor resonance magnitude for the 3 preparatory states can indeed be attributed to suppression on PrepCI and NoPrep trials, rather than facilitation on PrepIm trials, we performed comparisons with the baseline motor resonance measure in the control task. Significant motor resonance occurred in the control task (t(15)=2.27, p=0.019), when general motor preparation demands were similar to the imitation task but the stimulus-response mappings were arbitrary (Figure 5A, right). The magnitude of motor resonance (difference between squeeze and release MEPs) during the PrepIm condition was similar to that observed for the control task (t(15)=0.23, p=0.409). In contrast, motor resonance was significantly decreased compared to the control task during PrepCI trials (t(15)=2.35, p=0.017) and showed a similar trend for NoPrep trials (t(15)=1.67, p=0.058; Figure 5B).
DISCUSSION
Cognitive models of stimulus-response compatibility suggest that although stimuli often activate a compatible response, this “automatic” response activation can be suppressed when it is likely to interfere with task goals (Shaffer, 1965; De Jong, 1995; Vu and Proctor, 2004). Imitation—the copying of others actions—is a form of SRC involving human actions, where responses are stimulus-compatible with respect to spatial and kinetic features (Brass et al., 2000; Stürmer et al., 2000). In Experiment 1 we extend behavioral SRC effects that are typically attributed to suppression of automatic response activation to imitation. In line with previous studies using non-imitative stimuli (Stoffels, 1996; Ehrenstein and Proctor, 1998; De Jong, 1995; Vu and Proctor, 2004), the compatibility effect (faster imitative than counterimitative responses) was reduced when stimulus-response mapping information was not provided in advance of the imperative stimulus (NoPrep trials).
Data from Experiment 2 provide novel neurophysiologic evidence that these behavioral effects are related to preparatory suppression of specific stimulus-response links. Motor resonance—defined as facilitation of primary motor cortex during action observation that is muscle-to-action specific—was greater during preparation to imitate than during preparation to counterimitate, or when the required stimulus-response mapping was unknown. In fact, motor resonance occurred only when imitative response activation would be helpful, and was absent altogether during preparation for the two conditions in which the imitative response might interfere with behavior. While this pattern is exactly as predicted by preparatory suppression models, without a baseline comparison these differences could be attributable to facilitation of motor resonance when it would aid responding (e.g. in the case of imitation), rather than suppression of motor resonance when it would interfere (as proposed by cognitive models). Therefore, we obtained a baseline measure of motor resonance in a task with similar two-forced choice task demands but without any influence of stimulus-response compatibility. Comparison with this control task supports the suppression account: Motor resonance was similar to baseline during preparation to imitate, and lower than baseline during the counterimitation and unknown mapping conditions. Thus, is seems that resonance in the motor system during action observation occurs by default, and that this default state is modulated depending on task demands.
The data are not consistent with the alternative possibility that preparatory suppression occurs through changes in general motor preparation, as opposed to suppression of specific stimulus-response links. If suppression were accomplished by changes in motor preparation (i.e. greater endogenous motor activation when preparing to imitate), we would expect to see higher average MEPs during PrepIm trials compared to PrepCI and NoPrep trials, irrespective of the action observation video. We did not observe this pattern; instead the NoPrep condition had the highest excitability overall, and excitability did not differ between preparation to imitate and counterimitate. Thus, although there are some detectable differences that may be attributable to general motor preparation for the different conditions, a pattern consistent with cognitive models of preparatory suppression is observed only when examining MEP size as a function of the specific action being observed (motor resonance).
This observation of greater MEPs in the NoPrep condition brings up a second issue relevant to motor resonance. As described above, when motor resonance is defined as facilitation of FDI MEPs during observation of squeeze relative to release actions (i.e. Figure 5B), the data are entirely consistent with the motor resonance suppression account. However, an examination of absolute MEP magnitudes during observation of squeeze actions (Figure 5A, imitation task grey bars) appears to contradict a pure suppression account because squeeze MEPs are actually larger for the NoPrep condition, in which we argue for suppression, compared to the PrepIm condition. This finding is easily explained by a non-specific increase in MEP magnitude for the NoPrep condition, perhaps due to the increased difficulty. Indeed, non-specific factors such as attention and task difficulty are known to modulate corticospinal excitability and plasticity (Beck and Hallett, 2010; Conte et al., 2007; Pearce and Kidgell, 2009; Stefan et al., 2004). According to this view, the motor resonance suppression effect is superimposed on an increase of baseline corticospinal excitability. However, we cannot entirely rule out the alternative possibility that the lack of motor resonance observed in the NoPrep condition is caused by a ceiling effect on corticospinal excitability, rather than suppression of motor resonance. Nonetheless, given the concordance of motor resonance effects with the predictions of the cognitive model, we find this explanation to be less compelling.
What are the implications of motor resonance modulation? Since its discovery, motor resonance has been attributed to MNS activity and recent work has bolstered this claim. Ventral premotor and parietal regions that are homologous to macaque regions containing mirror neurons have been shown to be causally involved in motor resonance (Avenanti et al., 2007; Koch et al., 2010). Thus, the present data indicate that preparatory processes inhibit the influence of MNS activity on the motor system when it is likely to activate responses that conflict with task goals.
These findings are consistent with theories proposing MNS modulation as a way to control unwanted imitation (Spengler et al., 2009). An automatic (unintended or unconscious) tendency to imitate observed actions has been demonstrated in both laboratory and naturalistic settings (Chartrand and Bargh, 1999; Brass et al., 2000), and the existence of patients who imitate uncontrollably after brain damage (Lhermitte et al., 1986; De Renzi et al., 1996) suggests that some active inhibitory mechanism is required to control automatic imitation. Consistent with this view, the motor resonance modulation observed here suggests that MNS influence on the motor system is suppressed when imitation would interfere with behavior. Thus, our data add to accumulating evidence that one mechanism used to suppress automatic imitative tendencies may be through modulation of the mirror neuron system, and this suppression can occur in a preparatory manner.
It is important to note, however, that the specific locus of this preparatory modulation of motor resonance requires further study; since TMS gives access only to the primary motor cortex readout of MNS activity, it is impossible to say whether the preparatory suppression observed here occurs through inhibition of input to the MNS, the MNS itself, or output from the MNS to M1. For example, it is possible that the mirror neuron system responds to observed actions regardless of task demands, and that preparatory suppression mechanisms inhibit this MNS activity or prevent it from affecting primary motor cortex. Alternatively, it is possible that preparatory suppression occurs through inhibition of visual input to the MNS preventing MNS responses to action observation altogether. A top-down visual suppression mechanism could be either specific, suppressing visual action processing in particular (e.g. in the superior temporal sulcus), or more general, suppressing visual processing at earlier stages. A general mechanism would mean that both the stimulus-response link relevant to the task (motor resonance, in the case of imitation), but also all other automatic stimulus-response links, would have reduced influence on motor responses. In this case, one would predict similar patterns of motor resonance modulation in a task requiring compatible and incompatible responses to non-imitative stimuli (e.g. in a spatial compatibility task). Thus, further work to test this hypothesis by addressing whether motor resonance modulation is specific to imitation tasks is warranted.
Several additional limitations should be addressed with future work. In the present study the imitative actions were very simple in order to replicate previous SRC literature and accommodate the methodological requirements of TMS. This raises questions about external validity of the task with regard to real world imitation; however, recent work demonstrates that contextual modulation of imitative behaviors is similar when they are in the laboratory with simplistic tasks such as this one and in more naturalistic environments as social mimicry (VanBaaren 2003; Leighton 2010). This provides some support for the assumption that imitative interference tasks are relevant to real-world mimicry. Another potential problem is that one of the response actions used in the study was object-directed (press key) and the other was not (lift finger off key). While it would be ideal for responses to be similar on this dimension, this seems less likely to effect the overall results, given that the human mirror neuron system responds to both transitive and intransitive actions (Rizzolatti and Craighero, 2004) and that automatic imitation has also been demonstrated for both transitive and intransitive actions (Press et al., 2008; Bertenthal et al. 2006). Another interesting issue to explore is the timing of the effects observed here. It is conceivable that the pattern of motor resonance modulation across conditions could differ depending on the precise time of stimulation during the action video. If this were the case, reaction time effects may be related to differences in the time course of motor resonance suppression depending on the condition, rather the complete presence or absence of suppression as suggested above.
In summary, the present study provides novel evidence in line with cognitive models that relatively automatic stimulus-response links can be inhibited in a preparatory manner when they are likely to interfere with behavior. Suppression may occur either through suppression of visual input to sensory-motor links or suppression of the stimulus-response link itself. In the case of imitation, this preparatory suppression of the MNS provides a mechanism by which the automatic tendency to imitate can be reduced when it would interfere with current goals.
HIGHLIGHTS.
Automatic imitation is a socially-relevant form of stimulus-response compatibility
Automatic imitation is strategically suppressed when imitation is undesirable.
Suppression is associated with modulation of motor resonance.
Avoiding unwanted imitation involves mirror neuron system modulation.
ACKNOWLEDGEMENTS
For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund. This work was supported by the National Center for Research Resources (NCRR) (grant numbers RR12169, RR13642 and RR00865) and the National Institute of Mental Health (grant number 5F30MH091808-02), components of the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- Avenanti A, Bolognini N, Maravita A, Aglioti SM. Somatic and motor components of action simulation. Curr Biol. 2007;17:2129–2135. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Barchiesi G, Cattaneo L. Early and late motor responses to action observation. Soc Cogn Affect Neurosci. 2013;8:711–714. doi: 10.1093/scan/nss049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Hallett M. Surround inhibition is modulated by task difficulty. Clinical Neurophys. 2010;121:98–103. doi: 10.1016/j.clinph.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertenthal BI, Longo MR, Kosobud A. Imitative response tendencies following observation of intransitive actions. J Exp Psychol Hum Percept Perform. 2006;32:210–225. doi: 10.1037/0096-1523.32.2.210. [DOI] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Wohlschläger A, Prinz W. Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain Cogn. 2000;44:124–143. doi: 10.1006/brcg.2000.1225. [DOI] [PubMed] [Google Scholar]
- Catmur C, Walsh V, Heyes C. Sensorimotor learning configures the human mirror system. Curr Biol. 2007;17:1527–1531. doi: 10.1016/j.cub.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Chartrand TL, Bargh JA. The chameleon effect: the perception-behavior link and social interaction. J Pers Soc Psychol. 1999;76:893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- Chartrand TL, Bargh JA. The chameleon effect: the perception-behavior link and social interaction. J Pers Soc Psychol. 1999;76:893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- Chong TT, Cunnington R, Williams MA, Mattingley JB. The role of selective attention in matching observed and executed actions. Neuropsychologia. 2009;47:786–795. doi: 10.1016/j.neuropsychologia.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Chong TTJ, Williams MA, Cunnington R, Mattingley JB. Selective attention modulates inferior frontal gyrus activity during action observation. Neuroimage. 2008;40:298–307. doi: 10.1016/j.neuroimage.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Conte A, Gilio F, Iezzi E, Frasca V, Inghilleri M, Berardelli A. Attention influences the excitability of cortical motor areas in healthy humans. Exp Brain Res. 2007;182:109–17. doi: 10.1007/s00221-007-0975-3. [DOI] [PubMed] [Google Scholar]
- Cross KA, Torrisi S, Losin EA, Iacoboni M. Controlling automatic imitative tendencies: Interactions between mirror neuron and cognitive control systems. Neuroimage. 2013;83:493–504. doi: 10.1016/j.neuroimage.2013.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong R. Strategical determinants of compatibility effects with task uncertainty. Acta Psychol (Amst) 1995;88:187–207. [Google Scholar]
- De Jong R, Liang CC, Lauber E. Conditional and unconditional automaticity: a dual-process model of effects of spatial stimulus-response correspondence. J Exp Psychol Hum Percept Perform. 1994;20:731–750. doi: 10.1037//0096-1523.20.4.731. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Cavalleri F, Facchini S. Imitation and utilisation behaviour. J Neurol Neurosurg Psychiatry. 1996;61:396–400. doi: 10.1136/jnnp.61.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Ehrenstein A, Proctor RW. Selecting mapping rules and responses in mixed compatibility four-choice tasks. Psychol Res. 1998;61:231–248. [Google Scholar]
- Eimer Hommel, Prinz. S-R compatibility and response selection. Acta Psychol (Amst) 1995;90:301–313. [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Bonini L, Fogassi L. From monkey mirror neurons to primate behaviours: possible 'direct' and ‘indirect’ pathways. Philos Trans R Soc Lond B Biol Sci. 2009;364:2311–2323. doi: 10.1098/rstb.2009.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heister G, Schroeder-Heister P. Spatial S-R compatibility: positional instruction vs. compatibility instruction. Acta Psychol (Amst) 1994;85:15–24. doi: 10.1016/0001-6918(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Koch G, Versace V, Bonnì S, Lupo F, Lo Gerfo E, Oliveri M, Caltagirone C. Resonance of cortico-cortical connections of the motor system with the observation of goal directed grasping movements. Neuropsychologia. 2010;48:3513–3520. doi: 10.1016/j.neuropsychologia.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Leighton J, Bird G, Orsini C, Heyes C. Social attitudes modulate automatic imitation. J Exp Soc Psychol. 2010;46:905–910. [Google Scholar]
- Lhermitte F, Pillon B, Serdaru M. Human autonomy and the frontal lobes. Part I: Imitation and utilization behavior: a neuropsychological study of 75 patients. Ann Neurol. 1986;19:326–334. doi: 10.1002/ana.410190404. [DOI] [PubMed] [Google Scholar]
- Lidierth M. A computer based method for automated measurement of the periods of muscular activity from an EMG and its application to locomotor EMGs. Electroencephalogr Clin Neurophysiol. 1986;64:378–380. doi: 10.1016/0013-4694(86)90163-x. [DOI] [PubMed] [Google Scholar]
- Liepelt R, Ullsperger M, Obst K, Spengler S, von Cramon DY, Brass M. Contextual movement constraints of others modulate motor preparation in the observer. Neuropsychologia. 2009;47:268–275. doi: 10.1016/j.neuropsychologia.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Likowski KU, Mühlberger A, Seibt B, Pauli P, Weyers P. Modulation of facial mimicry by attitudes. J Exp Soc Psychol. 2008;44:1065–1072. [Google Scholar]
- Molenberghs P, Hayward L, Mattingley JB, Cunnington R. Activation patterns during action observation are modulated by context in mirror system areas. Neuroimage. 2012;59:608–615. doi: 10.1016/j.neuroimage.2011.07.080. [DOI] [PubMed] [Google Scholar]
- Newman-Norlund RD, van Schie HT, van Zuijlen AMJ, Bekkering H. The mirror neuron system is more active during complementary compared with imitative action. Nat Neurosci. 2007;10:817–818. doi: 10.1038/nn1911. [DOI] [PubMed] [Google Scholar]
- Pearce AJ, Kidgell DJ. Corticomotor excitability during precision motor tasks. J Sci Med Sport. 2009;12:280–3. doi: 10.1016/j.jsams.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Press C, Bird G, Walsh E, Heyes C. Automatic imitation of intransitive actions. Brain Cogn. 2008;67:44–50. doi: 10.1016/j.bandc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rösler KM, Petrow E, Mathis J, Arányi Z, Hess CW, Magistris MR. Effect of discharge desynchronization on the size of motor evoked potentials: an analysis. Clin Neurophysiol. 2002;113:1680–1687. doi: 10.1016/s1388-2457(02)00263-8. [DOI] [PubMed] [Google Scholar]
- Shaffer LH. Choice reaction with variable S-R mapping. J Exp Psychol. 1965;70:284–288. doi: 10.1037/h0022207. [DOI] [PubMed] [Google Scholar]
- Spengler S, von Cramon DY, Brass M. Control of shared representations relies on key processes involved in mental state attribution. Hum Brain Mapp. 2009;30:3704–3718. doi: 10.1002/hbm.20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophys. 2004;92:66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Stoffels EJ. Uncertainty and processing routes in the selection of a response: An SR compatibility study. Acta Psychol (Amst) 1996;94:227–252. doi: 10.1016/0001-6918(95)00063-1. [DOI] [PubMed] [Google Scholar]
- Stürmer B, Aschersleben G, Prinz W. Correspondence effects with manual gestures and postures: a study of imitation. J Exp Psychol Hum Percept Perform. 2000;26:1746–1759. doi: 10.1037//0096-1523.26.6.1746. [DOI] [PubMed] [Google Scholar]
- van Baaren RB, Maddux WW, Chartrand TL, de Bouter C, van Knippenberg A. It takes two to mimic: Behavioral consequences of self-construals. J Pers Soc Psychol. 2003;84:1093–1102. doi: 10.1037/0022-3514.84.5.1093. [DOI] [PubMed] [Google Scholar]
- Vu KP, Proctor RW. Mixing compatible and incompatible mappings: elimination, reduction, and enhancement of spatial compatibility effects. Q J Exp Psychol A. 2004;57:539–556. doi: 10.1080/02724980343000387. [DOI] [PubMed] [Google Scholar]