Abstract
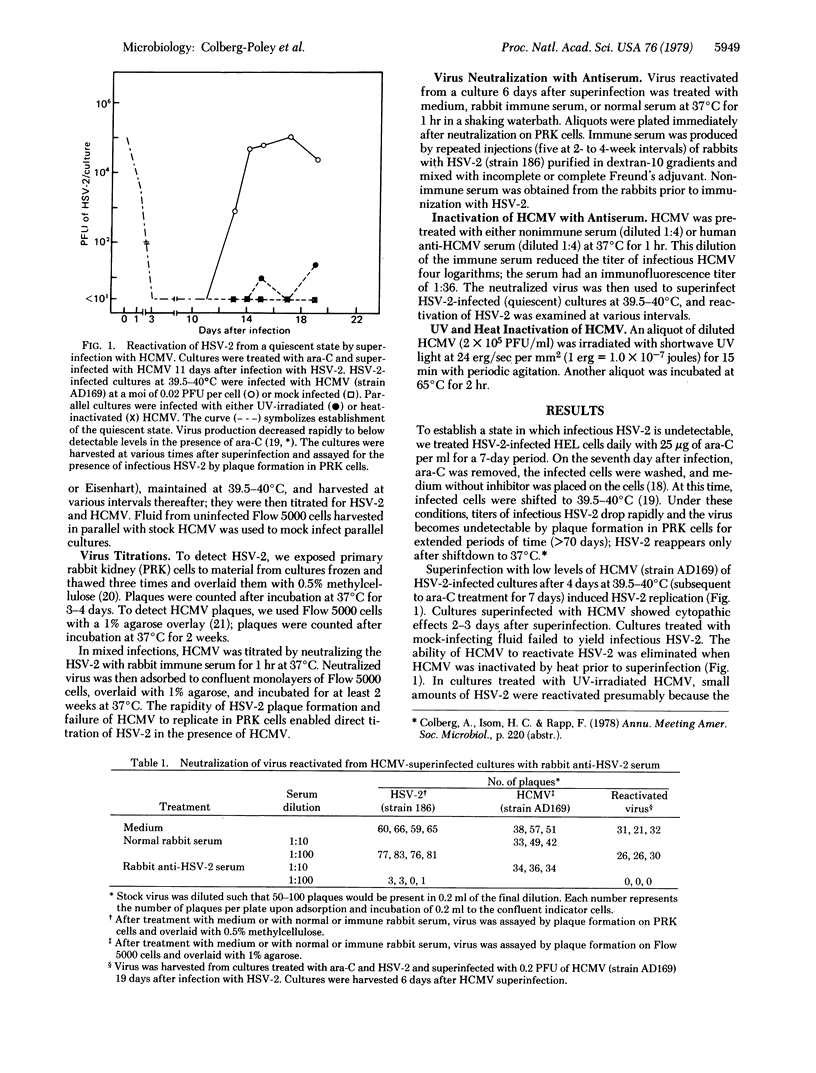
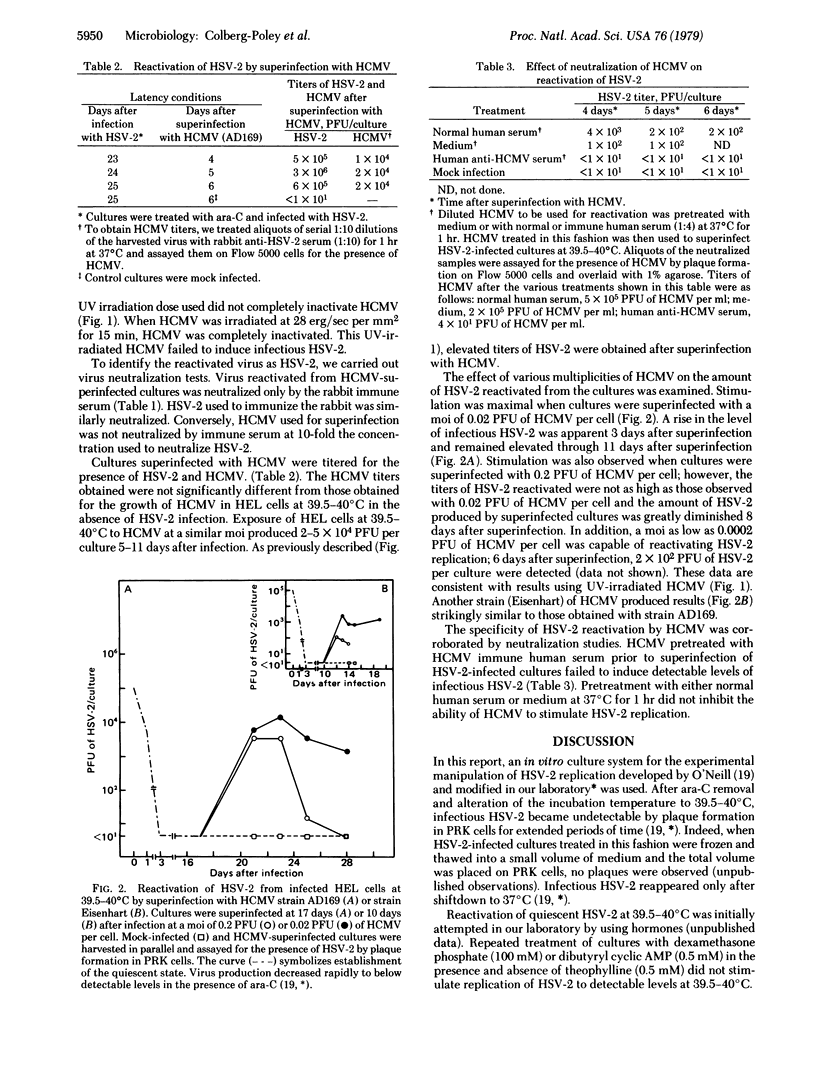
The ability of human cytomegalovirus to stimulate replication of herpes simplex virus type 2 (HSV-2) was examined. The system used involved HSV-2-infected human embryonic lung cells under conditions (39.5-40 degrees C) in which HSV-2 remains undetectable. Reactivation of HSV-2 was maximal and persisted for the longest duration when cultures were superinfected with 0.02 plaque-forming unit of human cytomegalovirus per cell. Infectious HSV-2 appeared 2 days after superinfection with human cytomegalovirus and ranged from 10(2) to 10(6) plaque-forming units per culture. Virus reactivated from these cultures was neutralized by rabbit immune serum produced against HSV-2. The specificity of this interaction was demonstrated by various criteria: production of HSV-2 was not observed in cultures treated with mock infecting fluid, and inactivation of human cytomegalovirus by heat, ultraviolet irradiation, or immune serum prior to superinfection eliminated its ability to induce HSV-2 replication. These results sugges that interaction between these two human herpesviruses may be of importance in herpesvirus latency in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R. Recovery of herpes simplex virus from human sacral ganglions. N Engl J Med. 1974 Oct 17;291(16):828–830. doi: 10.1056/NEJM197410172911606. [DOI] [PubMed] [Google Scholar]
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Bastian F. O., Rabson A. S., Yee C. L., Tralka T. S. Herpesvirus hominis: isolation from human trigeminal ganglion. Science. 1972 Oct 20;178(4058):306–307. doi: 10.1126/science.178.4058.306. [DOI] [PubMed] [Google Scholar]
- Boyd A. L., Derge J. G., Hampar B. Activation of endogenous type C virus in BALB/c mouse cells by herpesvirus DNA. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4558–4562. doi: 10.1073/pnas.75.9.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. M., Subak-Sharpe J. H., Warren K. G., Wroblewska Z., Koprowski H. Detection by complementation of defective or uninducible (herpes simplex type 1) virus genomes latent in human ganglia. Proc Natl Acad Sci U S A. 1979 May;76(5):2364–2368. doi: 10.1073/pnas.76.5.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M., Kaplan A. S. The role of defective cytomegalovirus particles in the induction of host cell DNA synthesis. Virology. 1977 Oct 1;82(1):93–99. doi: 10.1016/0042-6822(77)90035-6. [DOI] [PubMed] [Google Scholar]
- Forghani B., Klassen T., Baringer J. R. Radioimmunoassay of herpes simplex virus antibody: correlation with ganglionic infection. J Gen Virol. 1977 Sep;36(3):371–375. doi: 10.1099/0022-1317-36-3-371. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C., Shevchuk M., McDougall J. K. Detection of herpes simplex RNA in human sensory ganglia. Virology. 1979 May;95(1):265–268. doi: 10.1016/0042-6822(79)90429-x. [DOI] [PubMed] [Google Scholar]
- Hampar B., Aaronson S. A., Derge J. G., Chakrabarty M., Showalter S. D., Dunn C. Y. Activation of an endogenous mouse type C virus by ultraviolet-irradiated herpes simplex virus types 1 and 2. Proc Natl Acad Sci U S A. 1976 Feb;73(2):646–650. doi: 10.1073/pnas.73.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Pagano J. S. Human cytomegalovirus. II. Lack of relatedness to DNA of herpes simples I and II, Epstein-Barr virus, and nonhuman strains of cytomegalovirus. J Virol. 1974 Mar;13(3):642–645. doi: 10.1128/jvi.13.3.642-645.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom H., Colberg A., Reed C., Rapp F. Conditions required for induction of murine p30 by herpes simplex virus. Int J Cancer. 1978 Jul 15;22(1):22–27. doi: 10.1002/ijc.2910220106. [DOI] [PubMed] [Google Scholar]
- Jeor S. C., Albrecht T. B., Funk F. D., Rapp F. Stimulation of cellular DNA synthesis by human cytomegalovirus. J Virol. 1974 Feb;13(2):353–362. doi: 10.1128/jvi.13.2.353-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Zeigel R., Buchsbaum R., Paoletti E. Biochemical transformation of L-cells with ultraviolet-irradiated herpes simplex virus. Fed Proc. 1972 Nov-Dec;31(6):1669–1673. [PubMed] [Google Scholar]
- Naegele R. F., Rapp F. Enhancement of the replication of human adenoviruses in simian cells by simian adenovirus SV15. J Virol. 1967 Aug;1(4):838–840. doi: 10.1128/jvi.1.4.838-840.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill F. J., Goldberg R. J., Rapp F. Herpes simplex virus latency in cultured human cells following treatment with cytosine arabinoside. J Gen Virol. 1972 Feb;14(2):189–197. doi: 10.1099/0022-1317-14-2-189. [DOI] [PubMed] [Google Scholar]
- O'Neill F. J. Prolongation of herpes simplex virus latency in cultured human cells by temperature elevation. J Virol. 1977 Oct;24(1):41–46. doi: 10.1128/jvi.24.1.41-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. P., Kufe D., Schlom J., Frankel J. W., Prickett C. O., Groupé V., Spiegelman S. Biological and biochemical evidence for an interaction between Marek's disease herpesvirus and avian leukosis virus in vivo. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3175–3178. doi: 10.1073/pnas.70.11.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A., Rosenthal J. D., Openshaw H., Notkins A. L. Herpes simplex virus DNA and mRNA sequences in acutely and chronically infected trigeminal ganglia of mice. Virology. 1978 Aug;89(1):102–111. doi: 10.1016/0042-6822(78)90044-2. [DOI] [PubMed] [Google Scholar]
- RAPP F., MELNICK J. L., BUTEL J. S., KITAHARA T. THE INCORPORATION OF SV40 MATERIAL INTO ADENOVIRUS 7 AS MEASURED BY INTRANUCLEAR SYNTHESIS OF SV40 TUMOR ANTIGEN. Proc Natl Acad Sci U S A. 1964 Dec;52:1348–1352. doi: 10.1073/pnas.52.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F. VARIANTS OF HERPES SIMPLEX VIRUS: ISOLATION, CHARACTERIZATION, AND FACTORS INFLUENCING PLAQUE FORMATION. J Bacteriol. 1963 Nov;86:985–991. doi: 10.1128/jb.86.5.985-991.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., BAUM S. G. EVIDENCE FOR A POSSIBLE GENETIC HYBRID BETWEEN ADENOVIRUS TYPE 7 AND SV40 VIRUSES. Proc Natl Acad Sci U S A. 1964 Dec;52:1340–1347. doi: 10.1073/pnas.52.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed C. L., Rapp F. Induction of murine p30 by superinfecting herpesviruses. J Virol. 1976 Sep;19(3):1028–1033. doi: 10.1128/jvi.19.3.1028-1033.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jeor S., Rapp F. Cytomegalovirus replication in cells pretreated with 5-iodo-2'-deoxyuridine. J Virol. 1973 Jun;11(6):986–990. doi: 10.1128/jvi.11.6.986-990.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Brown S. M., Wroblewska Z., Gilden D., Koprowski H., Subak-Sharpe J. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med. 1978 May 11;298(19):1068–1069. doi: 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Walz M. A., Notkins A. L. Viral-specific thymidine kinase in sensory ganglia of mice infected with herpes simplex virus. Virology. 1977 Feb;76(2):866–869. doi: 10.1016/0042-6822(77)90267-7. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Rapp F. Induction of host DNA synthesis and DNA polymerase by DNA-negative temperature-sensitive mutants of human cytomegalovirus. Virology. 1979 Apr 15;94(1):237–241. doi: 10.1016/0042-6822(79)90457-4. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Rapp F. Temperature-sensitive mutants of human cytomegalovirus. J Virol. 1977 Oct;24(1):416–418. doi: 10.1128/jvi.24.1.416-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


