Abstract
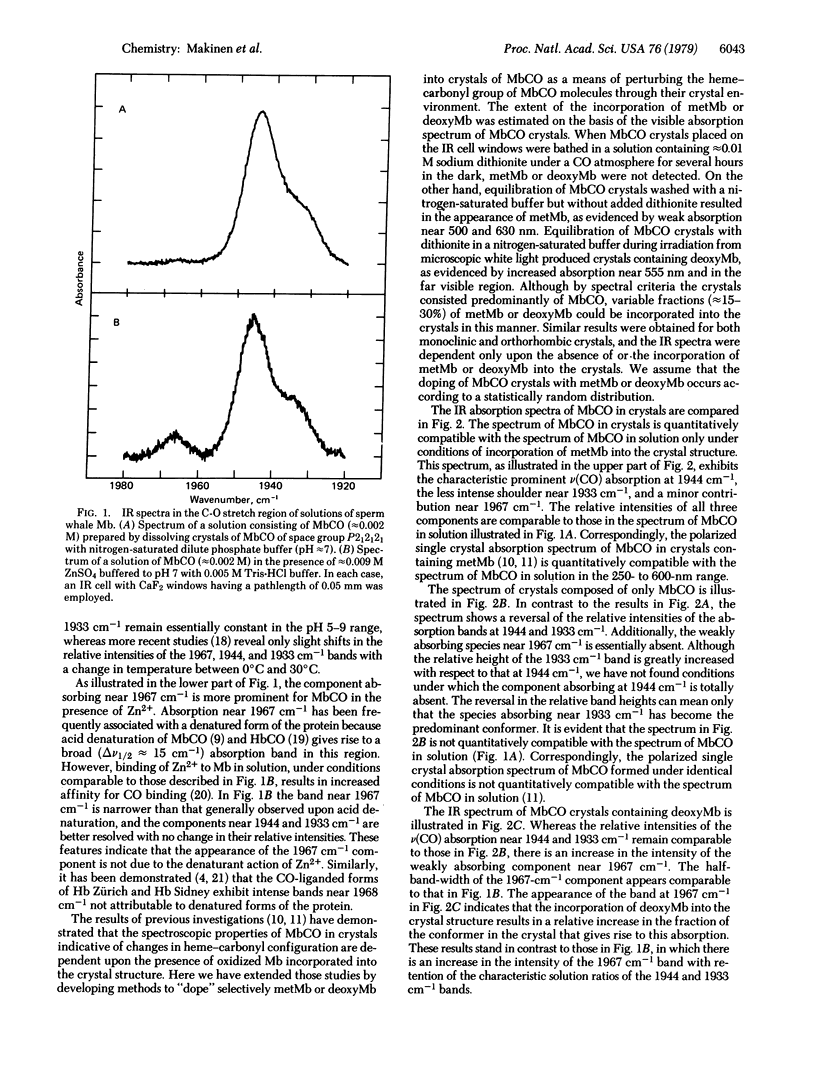
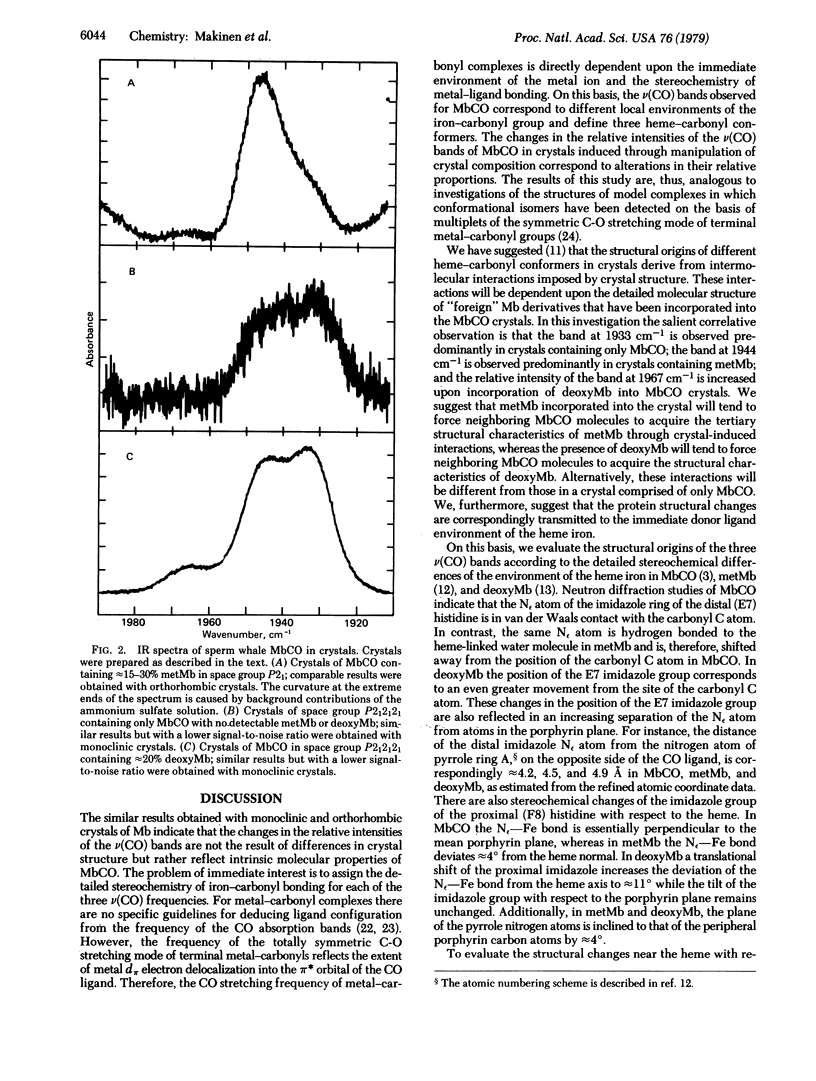
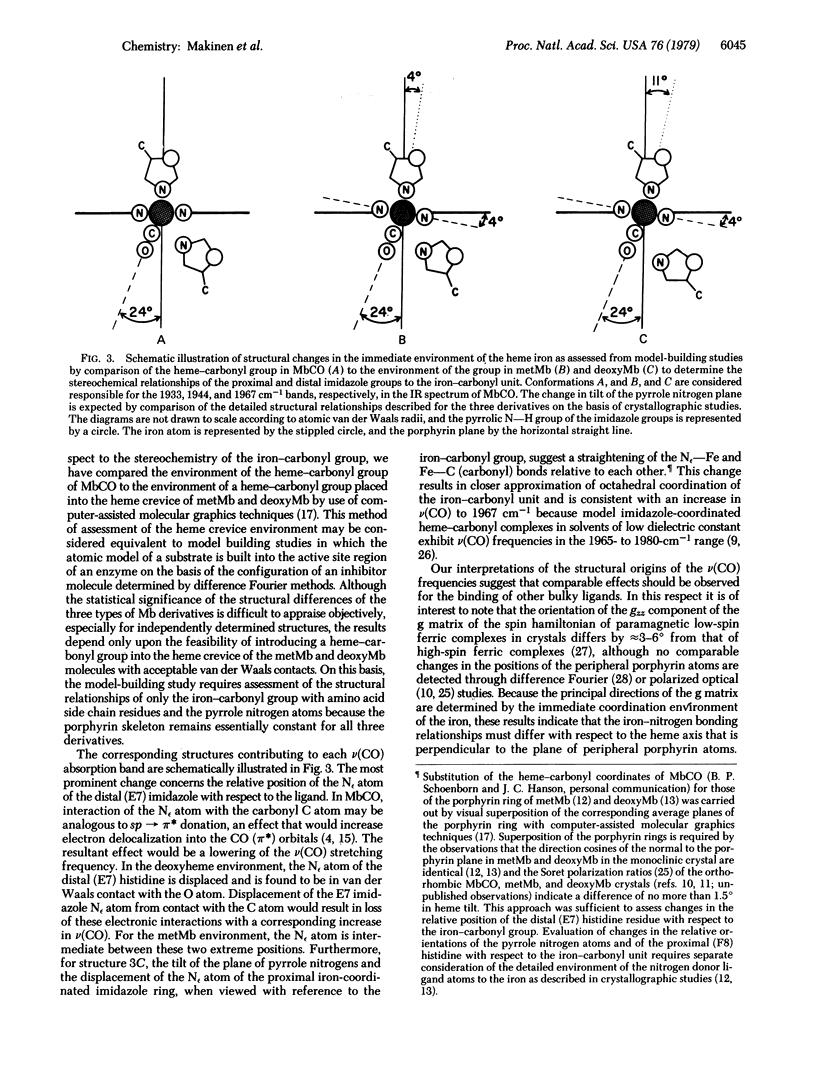
The configuration of the heme-carbonyl group upon binding of carbon monoxide to sperm whale myoglobin (Mb) in crystals is evaluated on the basis of infrared spectroscopic methods. Multiplets of the totally symmetric C-O stretching mode are observed for the heme-bound ligand near 1933, 1944, and 1967 cm-1, corresponding to three different heme-carbonyl conformers. Variations in the relative proportions of these conformers can be induced by incorporation of small fractions of metMb or deoxyMb into MbCO crystals. The configuration of the iron-carbonyl with respect to the immediate coordination environment of the heme iron is assigned for each v(CO) stretching frequency on the basis of a detailed comparison of the three-dimensional structures of the heme environments of MbCO, metMb, and deoxyMb defined by crystallographic methods. The structures of the three heme-carbonyl conformers account for the v(CO) infrared absorption bands that can be observed for MbCO in solution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alben J. O., Caughey W. S. An infrared study of bound carbon monoxide in the human red blood cell, isolated hemoglobin, and heme carbonyls. Biochemistry. 1968 Jan;7(1):175–183. doi: 10.1021/bi00841a022. [DOI] [PubMed] [Google Scholar]
- Case D. A., Karplus M. Stereochemistry of carbon monoxide binding to myoglobin and hemoglobin. J Mol Biol. 1978 Aug 25;123(4):697–701. doi: 10.1016/0022-2836(78)90214-0. [DOI] [PubMed] [Google Scholar]
- Deatherage J. F., Loe R. S., Anderson C. M., Moffat K. Structure of cyanide methemoglobin. J Mol Biol. 1976 Jul 5;104(3):687–706. doi: 10.1016/0022-2836(76)90129-7. [DOI] [PubMed] [Google Scholar]
- Feldmann R. J., Bing D. H., Furie B. C., Furie B. Interactive computer surface graphics approach to study of the active site of bovine trypsin. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5409–5412. doi: 10.1073/pnas.75.11.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidner E. J., Ladner R. C., Perutz M. F. Structure of horse carbonmonoxyhaemoglobin. J Mol Biol. 1976 Jul 5;104(3):707–722. doi: 10.1016/0022-2836(76)90130-3. [DOI] [PubMed] [Google Scholar]
- Hori H. Analysis of the principal g-tensors in single crystals of ferrimyoglobin complexes. Biochim Biophys Acta. 1971 Nov 19;251(2):227–235. doi: 10.1016/0005-2795(71)90106-1. [DOI] [PubMed] [Google Scholar]
- Huber R., Epp O., Formanek H. Structures of deoxy- and carbonmonoxy-erythrocruorin. J Mol Biol. 1970 Sep 14;52(2):349–354. doi: 10.1016/0022-2836(70)90035-5. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Eaton W. A. Optically detected conformational changes in haemoglobin single crystals. Nature. 1974 Jan 4;247(5435):62–64. doi: 10.1038/247062a0. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Fink A. L. Reactivity and cryoenzymology of enzymes in the crystalline state. Annu Rev Biophys Bioeng. 1977;6:301–343. doi: 10.1146/annurev.bb.06.060177.001505. [DOI] [PubMed] [Google Scholar]
- Maxwell J. C., Caughey W. S. An infrared study of NO bonding to heme B and hemoglobin A. Evidence for inositol hexaphosphate induced cleavage of proximal histidine to iron bonds. Biochemistry. 1976 Jan 27;15(2):388–396. doi: 10.1021/bi00647a023. [DOI] [PubMed] [Google Scholar]
- Maxwell J. C., Caughey W. S. Infrared spectroscopy of ligands, gases, and other groups in aqueous solutions and tissue. Methods Enzymol. 1978;54:302–323. doi: 10.1016/s0076-6879(78)54021-4. [DOI] [PubMed] [Google Scholar]
- Norvell J. C., Nunes A. C., Schoenborn B. P. Neutron diffraction analysis of myoglobin: structure of the carbon monoxide derivative. Science. 1975 Nov 7;190(4214):568–570. doi: 10.1126/science.1188354. [DOI] [PubMed] [Google Scholar]
- Peng S. M., Ibers J. A. Stereochemistry of carbonylmetalloporphyrins. The structure of (pyridine)(carbonyl)(5, 10, 15, 20-tetraphenylprophinato)iron(II). J Am Chem Soc. 1976 Dec 8;98(25):8032–8036. doi: 10.1021/ja00441a025. [DOI] [PubMed] [Google Scholar]
- Rifkind J. M., Keyes M. H., Lumry R. Linkage between the binding sites for zinc and oxygen on sperm whale myoglobin. Biochemistry. 1977 Dec 13;16(25):5564–5568. doi: 10.1021/bi00644a027. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]
- Tucker P. W., Phillips S. E., Perutz M. F., Houtchens R., Caughey W. S. Structure of hemoglobins Zürich [His E7(63)beta replaced by Arg] and Sydney [Val E11(67)beta replaced by Ala] and role of the distal residues in ligand binding. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1076–1080. doi: 10.1073/pnas.75.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]


