Abstract
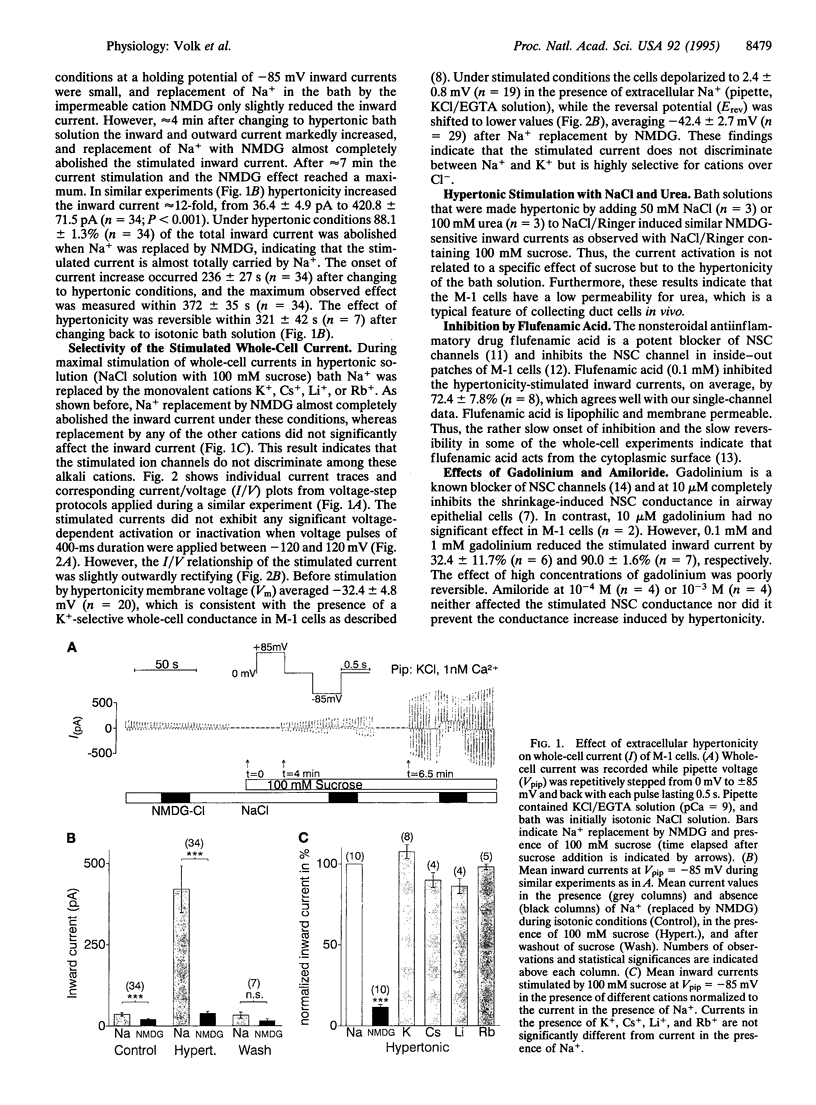
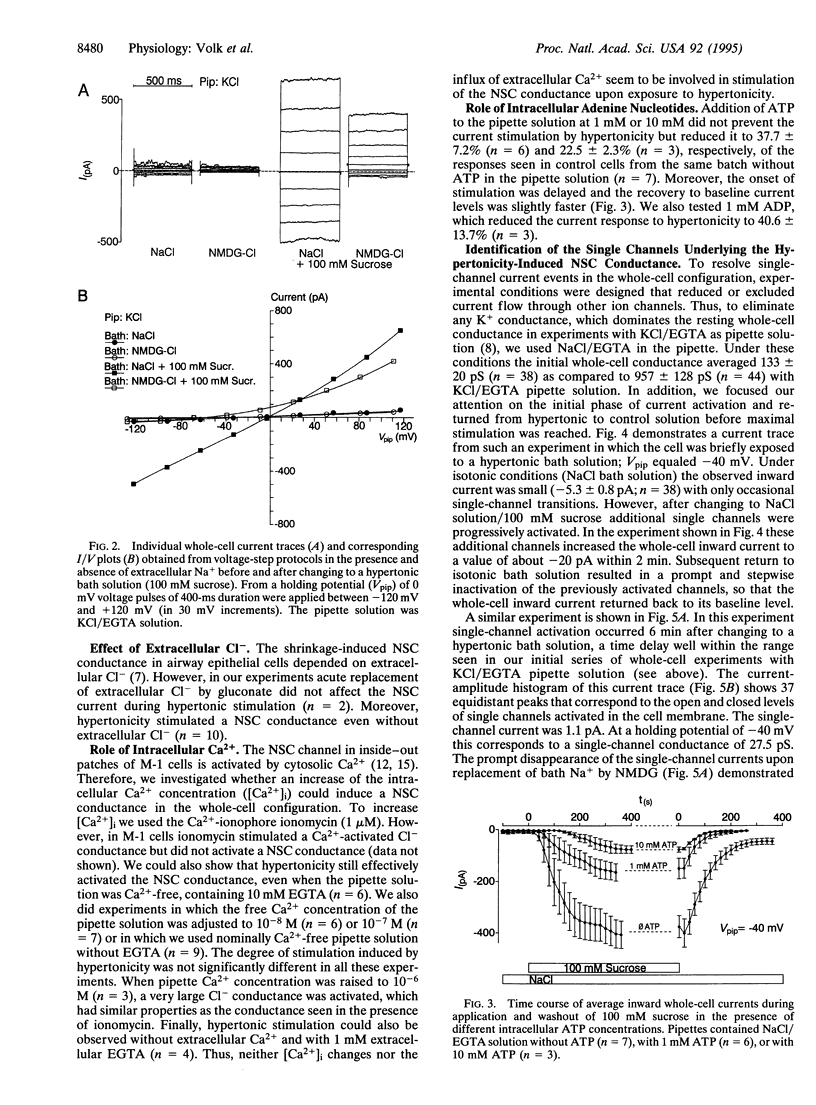
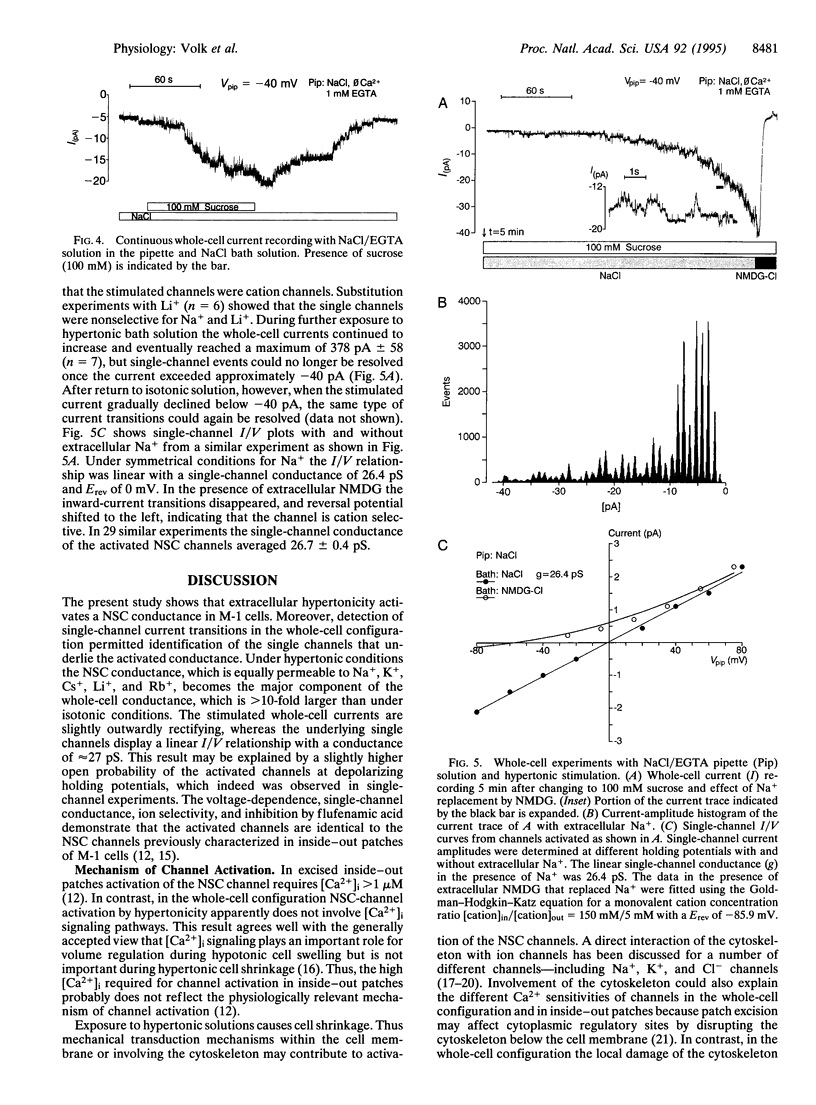
We investigated the effect of cell shrinkage on whole-cell currents of M-1 mouse cortical collecting duct cells. Addition of 100 mM sucrose to an isotonic NaCl bath solution induced cell shrinkage and increased whole-cell currents within 5-10 min by approximately 12-fold. The effect was reversible upon return to isotonic solution and could also be elicited by adding 100 mM urea or 50 mM NaCl. Replacement of bath Na+ by K+, Cs+, Li+, or Rb+ did not significantly affect the stimulated inward current, but replacement by N-methyl-D-glucamine reduced it by 88.1 +/- 1.3% (n = 34); this demonstrates that hypertonicity activates a nonselective alkali cation conductance. The activation was independent of extra- and intracellular Ca2+, but 1 or 10 mM ATP in the pipette suppressed it in a concentration-dependent manner, indicating that intracellular ATP levels may modulate the degree of channel activation. Flufenamic acid (0.1 mM) and gadolinium (0.1 mM) inhibited the stimulated current by 68.7 +/- 5.9% (n = 9) and 32.4 +/- 11.7% (n = 6), respectively, whereas 0.1 mM amiloride had no significant effect. During the early phase of hypertonic stimulation single-channel transitions could be detected in whole-cell current recordings, and a gradual activation of 30 and more individual channels with a single-channel conductance of 26.7 +/- 0.4 pS (n = 29) could be resolved. Thus, we identified the nonselective cation channel underlying the shrinkage-induced whole-cell conductance that may play a role in volume regulation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Korbmacher C., Segal A. S., Cheung P., Boulpaep E. L., Barnstable C. J. Mouse cortical collecting duct cells show nonselective cation channel activity and express a gene related to the cGMP-gated rod photoreceptor channel. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10262–10266. doi: 10.1073/pnas.89.21.10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993 Mar 19;259(5102):1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Strange K. Anisosmotic cell volume regulation: a comparative view. Am J Physiol. 1989 Aug;257(2 Pt 1):C159–C173. doi: 10.1152/ajpcell.1989.257.2.C159. [DOI] [PubMed] [Google Scholar]
- Chan H. C., Nelson D. J. Chloride-dependent cation conductance activated during cellular shrinkage. Science. 1992 Jul 31;257(5070):669–671. doi: 10.1126/science.1379742. [DOI] [PubMed] [Google Scholar]
- Chen S., Inoue R., Ito Y. Pharmacological characterization of muscarinic receptor-activated cation channels in guinea-pig ileum. Br J Pharmacol. 1993 Jul;109(3):793–801. doi: 10.1111/j.1476-5381.1993.tb13644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chraïbi A., Van den Abbeele T., Guinamard R., Teulon J. A ubiquitous non-selective cation channel in the mouse renal tubule with variable sensitivity to calcium. Pflugers Arch. 1994 Nov;429(1):90–97. doi: 10.1007/BF02584034. [DOI] [PubMed] [Google Scholar]
- Farrugia G., Rae J. Effect of volume changes on a potassium current in rabbit corneal epithelial cells. Am J Physiol. 1993 May;264(5 Pt 1):C1238–C1245. doi: 10.1152/ajpcell.1993.264.5.C1238. [DOI] [PubMed] [Google Scholar]
- Filipovic D., Sackin H. A calcium-permeable stretch-activated cation channel in renal proximal tubule. Am J Physiol. 1991 Jan;260(1 Pt 2):F119–F129. doi: 10.1152/ajprenal.1991.260.1.F119. [DOI] [PubMed] [Google Scholar]
- Galcheva-Gargova Z., Dérijard B., Wu I. H., Davis R. J. An osmosensing signal transduction pathway in mammalian cells. Science. 1994 Aug 5;265(5173):806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Dahlem D., Englert H. C., Lang H. J. Flufenamic acid, mefenamic acid and niflumic acid inhibit single nonselective cation channels in the rat exocrine pancreas. FEBS Lett. 1990 Jul 30;268(1):79–82. doi: 10.1016/0014-5793(90)80977-q. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Han J., Lee J. D., Bibbs L., Ulevitch R. J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994 Aug 5;265(5173):808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Korbmacher C., Segal A. S., Fejes-Tóth G., Giebisch G., Boulpaep E. L. Whole-cell currents in single and confluent M-1 mouse cortical collecting duct cells. J Gen Physiol. 1993 Oct;102(4):761–793. doi: 10.1085/jgp.102.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbmacher C., Volk T., Segal A. S., Boulpaep E. L., Frömter E. A calcium-activated and nucleotide-sensitive nonselective cation channel in M-1 mouse cortical collecting duct cells. J Membr Biol. 1995 Jul;146(1):29–45. doi: 10.1007/BF00232678. [DOI] [PubMed] [Google Scholar]
- Laskowski F. H., Christine C. W., Gitter A. H., Beyenbach K. W., Gross P., Frömter E. Cation channels in the apical membrane of collecting duct principal cell epithelium in culture. Ren Physiol Biochem. 1990 Jan-Apr;13(1-2):70–81. doi: 10.1159/000173349. [DOI] [PubMed] [Google Scholar]
- Light D. B., McCann F. V., Keller T. M., Stanton B. A. Amiloride-sensitive cation channel in apical membrane of inner medullary collecting duct. Am J Physiol. 1988 Aug;255(2 Pt 2):F278–F286. doi: 10.1152/ajprenal.1988.255.2.F278. [DOI] [PubMed] [Google Scholar]
- Ling B. N., Hinton C. F., Eaton D. C. Potassium permeable channels in primary cultures of rabbit cortical collecting tubule. Kidney Int. 1991 Sep;40(3):441–452. doi: 10.1038/ki.1991.231. [DOI] [PubMed] [Google Scholar]
- McCarty N. A., O'Neil R. G. Calcium signaling in cell volume regulation. Physiol Rev. 1992 Oct;72(4):1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- Milton R. L., Caldwell J. H. How do patch clamp seals form? A lipid bleb model. Pflugers Arch. 1990 Aug;416(6):758–762. doi: 10.1007/BF00370626. [DOI] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C., Guggino W. B. Cell volume regulation in the nephron. Annu Rev Physiol. 1990;52:761–772. doi: 10.1146/annurev.ph.52.030190.003553. [DOI] [PubMed] [Google Scholar]
- Natke E., Jr Cell volume regulation of rabbit cortical collecting tubule in anisotonic media. Am J Physiol. 1990 Jun;258(6 Pt 2):F1657–F1665. doi: 10.1152/ajprenal.1990.258.6.F1657. [DOI] [PubMed] [Google Scholar]
- Palmer L. G. Epithelial Na channels: function and diversity. Annu Rev Physiol. 1992;54:51–66. doi: 10.1146/annurev.ph.54.030192.000411. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Frindt G. Conductance and gating of epithelial Na channels from rat cortical collecting tubule. Effects of luminal Na and Li. J Gen Physiol. 1988 Jul;92(1):121–138. doi: 10.1085/jgp.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat A. G., Bertorello A. M., Ausiello D. A., Cantiello H. F. Activation of epithelial Na+ channels by protein kinase A requires actin filaments. Am J Physiol. 1993 Jul;265(1 Pt 1):C224–C233. doi: 10.1152/ajpcell.1993.265.1.C224. [DOI] [PubMed] [Google Scholar]
- Schwiebert E. M., Mills J. W., Stanton B. A. Actin-based cytoskeleton regulates a chloride channel and cell volume in a renal cortical collecting duct cell line. J Biol Chem. 1994 Mar 11;269(10):7081–7089. [PubMed] [Google Scholar]
- Smith P. R., Saccomani G., Joe E. H., Angelides K. J., Benos D. J. Amiloride-sensitive sodium channel is linked to the cytoskeleton in renal epithelial cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6971–6975. doi: 10.1073/pnas.88.16.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoos B. A., Náray-Fejes-Tóth A., Carretero O. A., Ito S., Fejes-Tóth G. Characterization of a mouse cortical collecting duct cell line. Kidney Int. 1991 Jun;39(6):1168–1175. doi: 10.1038/ki.1991.148. [DOI] [PubMed] [Google Scholar]
- Sun A., Hebert S. C. Rapid hypertonic cell volume regulation in the perfused inner medullary collecting duct. Kidney Int. 1989 Nov;36(5):831–842. doi: 10.1038/ki.1989.269. [DOI] [PubMed] [Google Scholar]
- Tohda H., Foskett J. K., O'Brodovich H., Marunaka Y. Cl- regulation of a Ca(2+)-activated nonselective cation channel in beta-agonist-treated fetal distal lung epithelium. Am J Physiol. 1994 Jan;266(1 Pt 1):C104–C109. doi: 10.1152/ajpcell.1994.266.1.C104. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]