Abstract
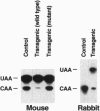
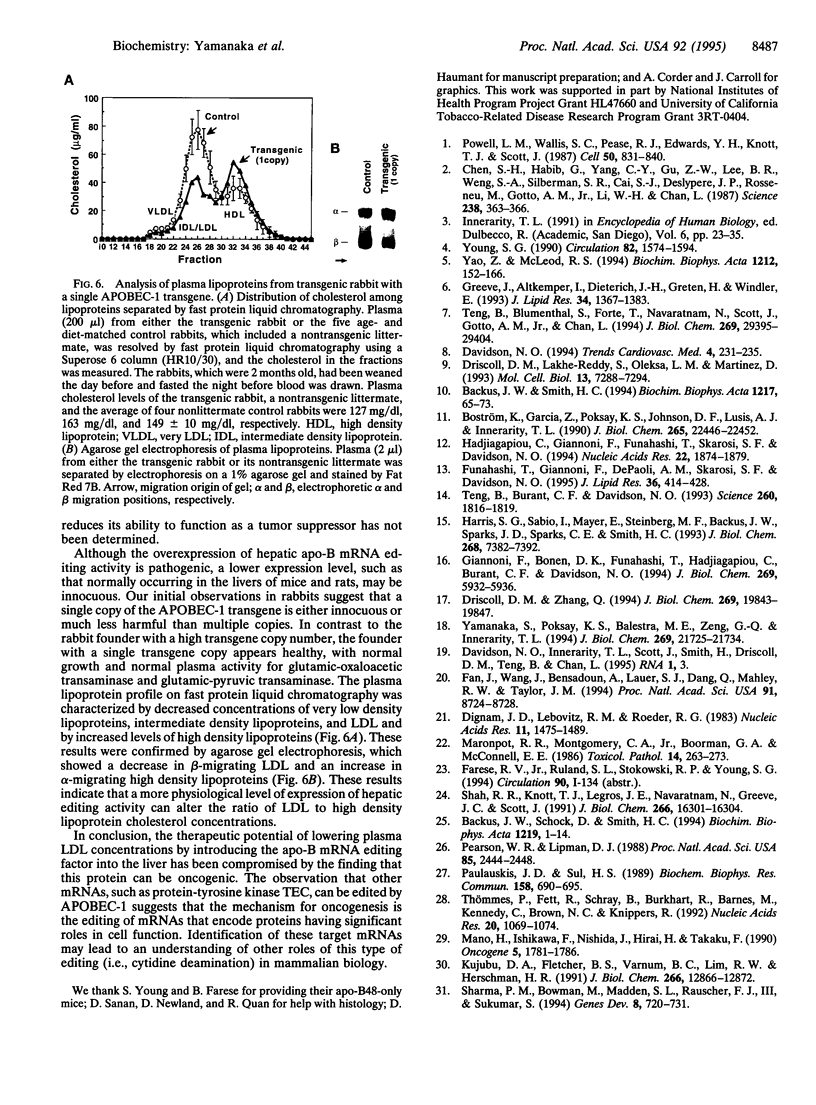
Apolipoprotein (apo-) B mRNA editing is the deamination of cytidine that creates a new termination codon and produces a truncated version of apo-B (apo-B48). The cytidine deaminase catalytic subunit [apo-B mRNA-editing enzyme catalytic polypeptide 1 (APOBEC-1)] of the multiprotein editing complex has been identified. We generated transgenic rabbits and mice expressing rabbit APOBEC-1 in their livers to determine whether hepatic expression would lower low density lipoprotein cholesterol concentrations. The apo-B mRNA from the livers of the transgenic mice and rabbit was extensively edited, and the transgenic animals had reduced concentrations of apo-B100 and low density lipoproteins compared with control animals. Unexpectedly, all of the transgenic mice and a transgenic rabbit had liver dysplasia, and many transgenic mice developed hepatocellular carcinomas. Many of the mouse livers were hyperplastic and filled with lipid. Other hepatic mRNAs with sequence motifs similar to apo-B mRNA were examined for this type of editing (i.e., cytidine deamination). One of these, tyrosine kinase, was edited in livers of transgenic mice but not of controls. This result demonstrates that other mRNAs can be edited by the overexpressed editing enzyme and suggests that aberrant editing of hepatic mRNAs involved in cell growth and regulation is the cause of the tumorigenesis. Finally, these findings compromise the potential use of APOBEC-1 for gene therapy to lower plasma levels of low density lipoproteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abachi S, Baringer P, Bylsma BG, DeBonte R, Koltick D, Loeffler FJ, Low EH, McIlwain RL, Miller DH, Ng CR. Observation of tensor and scalar mesons produced in e+e- annihilation at 29 GeV. Phys Rev Lett. 1986 Oct 20;57(16):1990–1993. doi: 10.1103/PhysRevLett.57.1990. [DOI] [PubMed] [Google Scholar]
- Backus J. W., Schock D., Smith H. C. Only cytidines 5' of the apolipoprotein B mRNA mooring sequence are edited. Biochim Biophys Acta. 1994 Sep 13;1219(1):1–14. doi: 10.1016/0167-4781(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Boström K., Garcia Z., Poksay K. S., Johnson D. F., Lusis A. J., Innerarity T. L. Apolipoprotein B mRNA editing. Direct determination of the edited base and occurrence in non-apolipoprotein B-producing cell lines. J Biol Chem. 1990 Dec 25;265(36):22446–22452. [PubMed] [Google Scholar]
- Chen S. H., Habib G., Yang C. Y., Gu Z. W., Lee B. R., Weng S. A., Silberman S. R., Cai S. J., Deslypere J. P., Rosseneu M. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987 Oct 16;238(4825):363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll D. M., Lakhe-Reddy S., Oleksa L. M., Martinez D. Induction of RNA editing at heterologous sites by sequences in apolipoprotein B mRNA. Mol Cell Biol. 1993 Dec;13(12):7288–7294. doi: 10.1128/mcb.13.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll D. M., Zhang Q. Expression and characterization of p27, the catalytic subunit of the apolipoprotein B mRNA editing enzyme. J Biol Chem. 1994 Aug 5;269(31):19843–19847. [PubMed] [Google Scholar]
- Fan J., Wang J., Bensadoun A., Lauer S. J., Dang Q., Mahley R. W., Taylor J. M. Overexpression of hepatic lipase in transgenic rabbits leads to a marked reduction of plasma high density lipoproteins and intermediate density lipoproteins. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8724–8728. doi: 10.1073/pnas.91.18.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi T., Giannoni F., DePaoli A. M., Skarosi S. F., Davidson N. O. Tissue-specific, developmental and nutritional regulation of the gene encoding the catalytic subunit of the rat apolipoprotein B mRNA editing enzyme: functional role in the modulation of apoB mRNA editing. J Lipid Res. 1995 Mar;36(3):414–428. [PubMed] [Google Scholar]
- Giannoni F., Bonen D. K., Funahashi T., Hadjiagapiou C., Burant C. F., Davidson N. O. Complementation of apolipoprotein B mRNA editing by human liver accompanied by secretion of apolipoprotein B48. J Biol Chem. 1994 Feb 25;269(8):5932–5936. [PubMed] [Google Scholar]
- Greeve J., Altkemper I., Dieterich J. H., Greten H., Windler E. Apolipoprotein B mRNA editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J Lipid Res. 1993 Aug;34(8):1367–1383. [PubMed] [Google Scholar]
- Hadjiagapiou C., Giannoni F., Funahashi T., Skarosi S. F., Davidson N. O. Molecular cloning of a human small intestinal apolipoprotein B mRNA editing protein. Nucleic Acids Res. 1994 May 25;22(10):1874–1879. doi: 10.1093/nar/22.10.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. G., Sabio I., Mayer E., Steinberg M. F., Backus J. W., Sparks J. D., Sparks C. E., Smith H. C. Extract-specific heterogeneity in high-order complexes containing apolipoprotein B mRNA editing activity and RNA-binding proteins. J Biol Chem. 1993 Apr 5;268(10):7382–7392. [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Mano H., Ishikawa F., Nishida J., Hirai H., Takaku F. A novel protein-tyrosine kinase, tec, is preferentially expressed in liver. Oncogene. 1990 Dec;5(12):1781–1786. [PubMed] [Google Scholar]
- Maronpot R. R., Montgomery C. A., Jr, Boorman G. A., McConnell E. E. National Toxicology Program nomenclature for hepatoproliferative lesions of rats. Toxicol Pathol. 1986;14(2):263–273. doi: 10.1177/019262338601400217. [DOI] [PubMed] [Google Scholar]
- Paulauskis J. D., Sul H. S. Structure of mouse fatty acid synthase mRNA. Identification of the two NADPH binding sites. Biochem Biophys Res Commun. 1989 Feb 15;158(3):690–695. doi: 10.1016/0006-291x(89)92776-9. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L. M., Wallis S. C., Pease R. J., Edwards Y. H., Knott T. J., Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987 Sep 11;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Shah R. R., Knott T. J., Legros J. E., Navaratnam N., Greeve J. C., Scott J. Sequence requirements for the editing of apolipoprotein B mRNA. J Biol Chem. 1991 Sep 5;266(25):16301–16304. [PubMed] [Google Scholar]
- Sharma P. M., Bowman M., Madden S. L., Rauscher F. J., 3rd, Sukumar S. RNA editing in the Wilms' tumor susceptibility gene, WT1. Genes Dev. 1994 Mar 15;8(6):720–731. doi: 10.1101/gad.8.6.720. [DOI] [PubMed] [Google Scholar]
- Teng B., Blumenthal S., Forte T., Navaratnam N., Scott J., Gotto A. M., Jr, Chan L. Adenovirus-mediated gene transfer of rat apolipoprotein B mRNA-editing protein in mice virtually eliminates apolipoprotein B-100 and normal low density lipoprotein production. J Biol Chem. 1994 Nov 25;269(47):29395–29404. [PubMed] [Google Scholar]
- Teng B., Burant C. F., Davidson N. O. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science. 1993 Jun 18;260(5115):1816–1819. doi: 10.1126/science.8511591. [DOI] [PubMed] [Google Scholar]
- Thömmes P., Fett R., Schray B., Burkhart R., Barnes M., Kennedy C., Brown N. C., Knippers R. Properties of the nuclear P1 protein, a mammalian homologue of the yeast Mcm3 replication protein. Nucleic Acids Res. 1992 Mar 11;20(5):1069–1074. doi: 10.1093/nar/20.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S., Poksay K. S., Balestra M. E., Zeng G. Q., Innerarity T. L. Cloning and mutagenesis of the rabbit ApoB mRNA editing protein. A zinc motif is essential for catalytic activity, and noncatalytic auxiliary factor(s) of the editing complex are widely distributed. J Biol Chem. 1994 Aug 26;269(34):21725–21734. [PubMed] [Google Scholar]
- Yao Z., McLeod R. S. Synthesis and secretion of hepatic apolipoprotein B-containing lipoproteins. Biochim Biophys Acta. 1994 May 13;1212(2):152–166. doi: 10.1016/0005-2760(94)90249-6. [DOI] [PubMed] [Google Scholar]
- Young S. G. Recent progress in understanding apolipoprotein B. Circulation. 1990 Nov;82(5):1574–1594. doi: 10.1161/01.cir.82.5.1574. [DOI] [PubMed] [Google Scholar]