Abstract
The results of electron-nuclear double resonance and electron paramagnetic resonance (EPR) studies on the hydrogen peroxide compound of yeast cytochrome c peroxidase are inconsistent with previous proposals for the source of the EPR signal in this compound, in particular with its identification with an aromatic amino acid radical such as would arise by oxidation of a tryptophanyl side chain. The present observations lead us to propose that the EPR signal is associated with a cluster containing at least one methionine and in which proximate side chains share the charge created by loss of one electron.
Full text
PDF
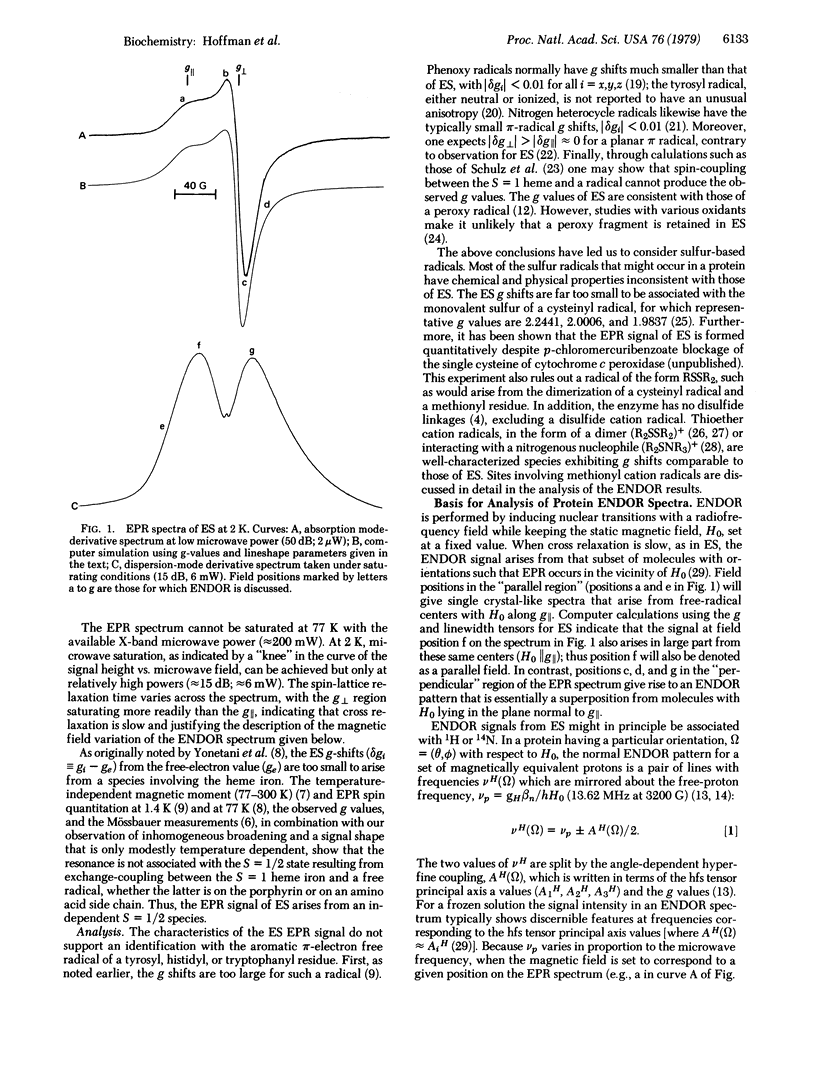
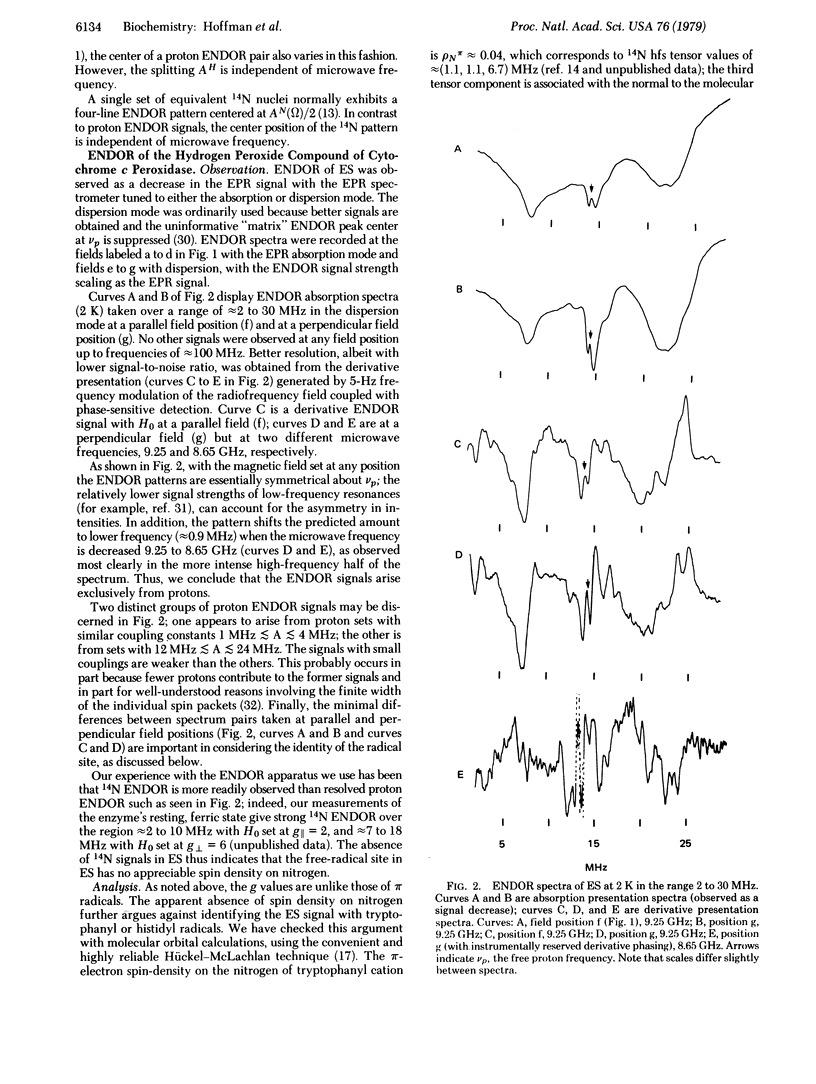


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B. Enzyme-substrate compounds. Adv Enzymol Relat Subj Biochem. 1951;12:153–190. doi: 10.1002/9780470122570.ch3. [DOI] [PubMed] [Google Scholar]
- Coulson A. F., Erman J. E., Yonetani T. Studies on cytochrome c peroxidase. XVII. Stoichiometry and mechanism of the reaction of compound ES with donors. J Biol Chem. 1971 Feb 25;246(4):917–924. [PubMed] [Google Scholar]
- Coulson A. F., Yonetani T. Mechanism of cytochrome c peroxidase. O-benzoylhydroxylamine as an analog of hydrogen peroxide. Biochemistry. 1975 Jun 3;14(11):2389–2396. doi: 10.1021/bi00682a019. [DOI] [PubMed] [Google Scholar]
- Coulson A. F., Yonetani T. Oxidation of cytochrome c peroxidase with hydrogen peroxide: identification of the "endogenous donor". Biochem Biophys Res Commun. 1972 Oct 17;49(2):391–398. doi: 10.1016/0006-291x(72)90423-8. [DOI] [PubMed] [Google Scholar]
- Iizuka T., Kotani M., Yonetani T. A thermal equilibrium between high- and low-spin states in ferric cytochrome c peroxidase and some discussion on the enzyme-substrate complex. Biochim Biophys Acta. 1968 Oct 8;167(2):257–267. doi: 10.1016/0005-2744(68)90204-0. [DOI] [PubMed] [Google Scholar]
- Kominami S. A radical cation produced in a -irradiated single crystal of DL-methionine as studied by electron spin resonance and optical absorption spectroscopy. J Phys Chem. 1972 Jun 8;76(12):1729–1733. doi: 10.1021/j100656a010. [DOI] [PubMed] [Google Scholar]
- Lang G., Spartalian K., Yonetani T. Mössbauer spectroscopic study of compound ES of cytochrome c peroxidase. Biochim Biophys Acta. 1976 Nov 18;451(1):250–258. doi: 10.1016/0304-4165(76)90275-0. [DOI] [PubMed] [Google Scholar]
- Nelson C. E., Sitzman E. V., Kang C. H., Margoliash E. Preparation of cytochrome c peroxidase from baker's yeast. Anal Biochem. 1977 Dec;83(2):622–631. doi: 10.1016/0003-2697(77)90066-5. [DOI] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Wittenberg B. A., Wittenberg J. B. The electronic structure of protoheme proteins. 3. Configuration of the heme and its ligands. J Biol Chem. 1968 Apr 25;243(8):1871–1880. [PubMed] [Google Scholar]
- Poulos T. L., Freer S. T., Alden R. A., Xuong N. H., Edwards S. L., Hamlin R. C., Kraut J. Crystallographic determination of the heme orientation and location of the cyanide binding site in yeast cytochrome c peroxidase. J Biol Chem. 1978 May 25;253(10):3730–3735. [PubMed] [Google Scholar]
- Schulz C. E., Devaney P. W., Winkler H., Debrunner P. G., Doan N., Chiang R., Rutter R., Hager L. P. Horseradish peroxidase compound I: evidence for spin coupling between the heme iron and a 'free' radical. FEBS Lett. 1979 Jul 1;103(1):102–105. doi: 10.1016/0014-5793(79)81259-4. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Kampa L., Wittenberg J. B., Blumberg W. E., Peisach J. The electronic structure of protoheme proteins. II. An electron paramagnetic resonance and optical study of cytochrome c peroxidase and its derivatives. J Biol Chem. 1968 Apr 25;243(8):1863–1870. [PubMed] [Google Scholar]
- Yonetani T. Cytochrome c peroxidase. Adv Enzymol Relat Areas Mol Biol. 1970;33:309–335. doi: 10.1002/9780470122785.ch6. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Schleyer H. Studies on cytochrome c peroxidase. VII. Electron paramagnetic resonance absorptions of the enzyme and complex ES in dissolved and crystalline forms. J Biol Chem. 1966 Jul 10;241(13):3240–3243. [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. II. Stoichiometry between enzyme, H2O2, and ferrocytochrome c and enzymic determination of extinction coefficients of cytochrome c. J Biol Chem. 1965 Nov;240(11):4509–4514. [PubMed] [Google Scholar]


