Abstract
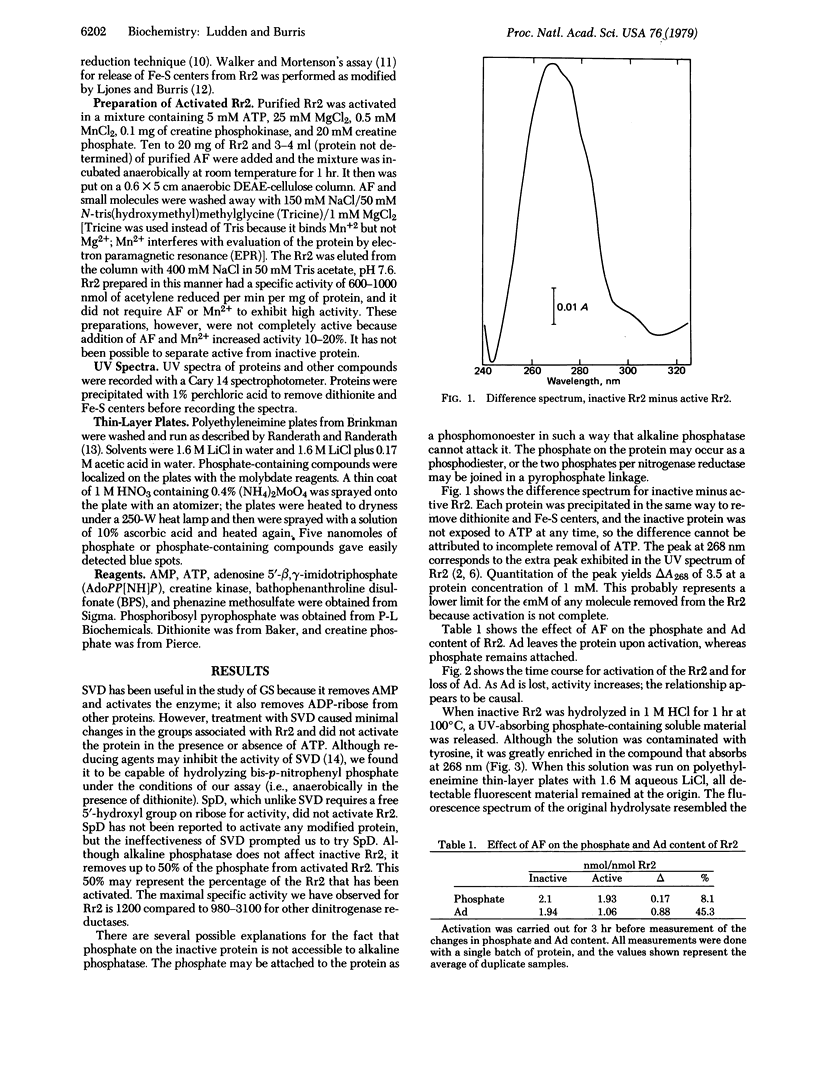
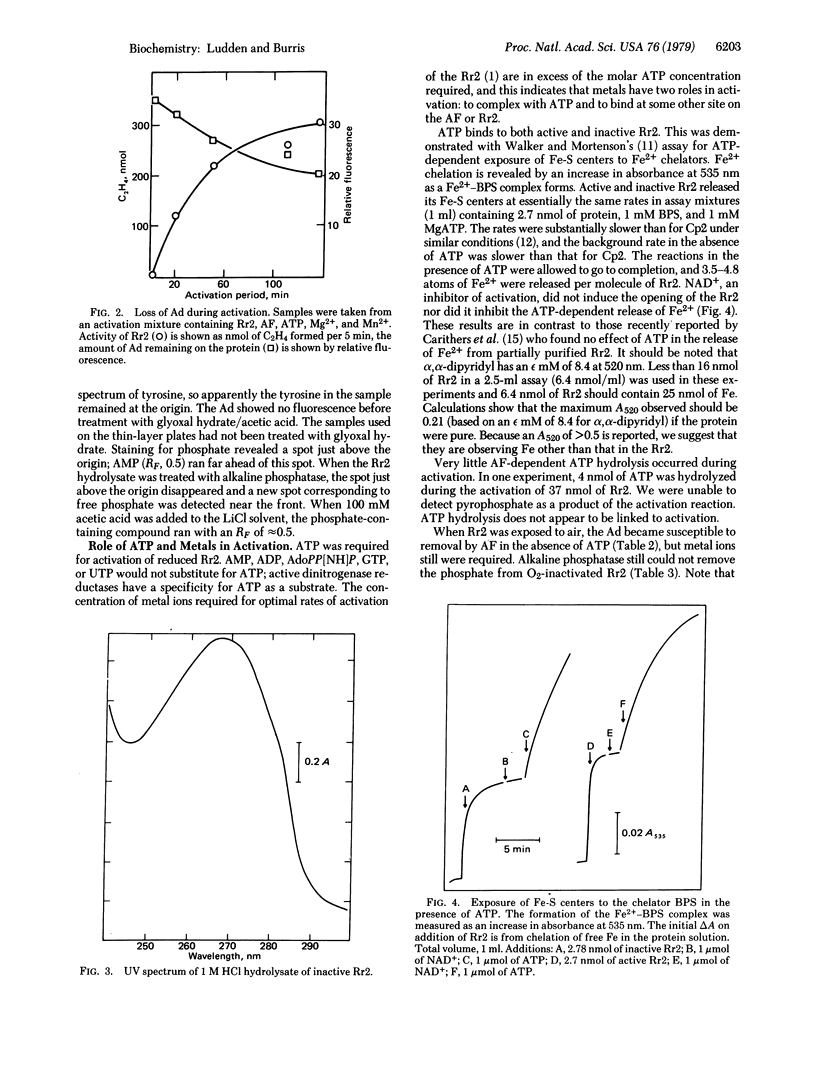
During the activation of the inactive dinitrogenase reductase from Rhodospirillum rubrum, an adenine-like molecules is lost and phosphate is found on both active and inactive forms of the protein. ATP and divalent metals are required for activation of the reduced protein, but ATP is not required for activation of phenazine methosulfate-oxidized dinitrogenase reductase. Snake venom diesterase and spleen diesterase have no effect on the inactive protein; alkaline phosphatase removes phosphate from the activated protein but not from the inactive protein. ATP binds to both active and inactive forms of the protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carithers R. P., Yoch D. C., Arnon D. I. Two forms of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol. 1979 Feb;137(2):779–789. doi: 10.1128/jb.137.2.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff C. G. Chemical structure of a modification of the Escherichia coli ribonucleic acid polymerase alpha polypeptides induced by bacteriophage T4 infection. J Biol Chem. 1974 Oct 10;249(19):6181–6190. [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Nitrogenase and nitrogenase reductase associate and dissociate with each catalytic cycle. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2699–2702. doi: 10.1073/pnas.75.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljones T., Burris R. H. Nitrogenase: the reaction between the Fe protein and bathophenanthrolinedisulfonate as a probe for interactions with MgATP. Biochemistry. 1978 May 16;17(10):1866–1872. doi: 10.1021/bi00603a010. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Activating factor for the iron protein of nitrogenase from Rhodospirillum rubrum. Science. 1976 Oct 22;194(4263):424–426. doi: 10.1126/science.824729. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Purification and properties of nitrogenase from Rhodospirillum rubrum, and evidence for phosphate, ribose and an adenine-like unit covalently bound to the iron protein. Biochem J. 1978 Oct 1;175(1):251–259. doi: 10.1042/bj1750251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Eriksson U., Baltscheffsky H. Necessity of a membrane component for nitrogenase activity in Rhodospirillum rubrum. Biochim Biophys Acta. 1977 Oct 12;462(1):187–195. doi: 10.1016/0005-2728(77)90201-8. [DOI] [PubMed] [Google Scholar]
- Schick H. J. Substrate and light dependent fixation of molecular nitrogen in Rhodospirillum rubrum. Arch Mikrobiol. 1971;75(2):89–101. doi: 10.1007/BF00407997. [DOI] [PubMed] [Google Scholar]
- Walker G. A., Mortenson L. E. An effect of magnesium adenosine 5'-triphosphate on the structure of azoferredoxin from Clostridium pasteurianum. Biochem Biophys Res Commun. 1973 Aug 6;53(3):904–909. doi: 10.1016/0006-291x(73)90177-0. [DOI] [PubMed] [Google Scholar]
- Yuki H., Sempuku C., Park M., Takiura K. Fluorometric determination of adenine and its derivatives by reaction with glyoxal hydrate trimer. Anal Biochem. 1972 Mar;46(1):123–128. doi: 10.1016/0003-2697(72)90403-4. [DOI] [PubMed] [Google Scholar]


