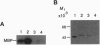Abstract
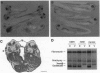
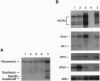
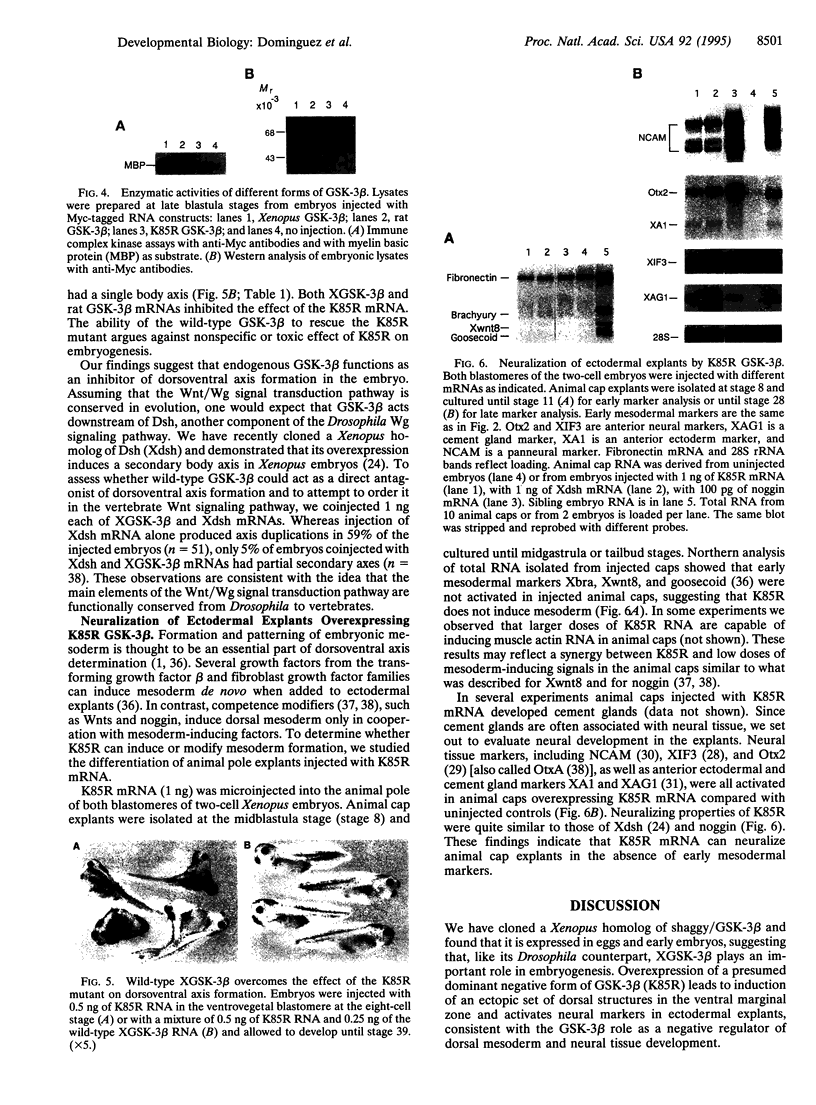
The dorsoventral axis is established early in Xenopus development and may involve signaling by Wnts, a family of Wnt1-protooncogene-related proteins. The protein kinase shaggy functions in the wingless/Wnt signaling pathway, which operates during Drosophila development. To assess the role of a closely related kinase, glycogen synthase kinase 3 beta (GSK-3 beta), in vertebrate embryogenesis, we cloned a cDNA encoding a Xenopus homolog of GSK-3 beta (XGSK-3 beta). XGSK-3 beta-specific transcripts were detected by Northern analysis in Xenopus eggs and early embryos. Microinjection of the mRNA encoding a catalytically inactive form of rat GSK-3 beta into a ventrovegetal blastomere of eight-cell embryos caused ectopic formation of a secondary body axis containing a complete set of dorsal and anterior structures. Furthermore, in isolated ectodermal explants, the mutant GSK-3 beta mRNA activated the expression of neural tissue markers. Wild-type XGSK-3 beta mRNA suppressed the dorsalizing effects of both the mutated GSK-3 beta and Xenopus dishevelled, a proposed upstream signaling component of the same pathway. These results strongly suggest that XGSK-3 beta functions to inhibit dorsoventral axis formation in the embryo and provide evidence for conservation of the Wnt signaling pathway in Drosophila and vertebrates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berra E., Diaz-Meco M. T., Dominguez I., Municio M. M., Sanz L., Lozano J., Chapkin R. S., Moscat J. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell. 1993 Aug 13;74(3):555–563. doi: 10.1016/0092-8674(93)80056-k. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Blumberg B., Steinbeisser H., De Robertis E. M. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991 Dec 20;67(6):1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian J. L., McMahon J. A., McMahon A. P., Moon R. T. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991 Apr;111(4):1045–1055. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- Dickinson M. E., McMahon A. P. The role of Wnt genes in vertebrate development. Curr Opin Genet Dev. 1992 Aug;2(4):562–566. doi: 10.1016/s0959-437x(05)80172-8. [DOI] [PubMed] [Google Scholar]
- Elinson R. P., Rowning B. A transient array of parallel microtubules in frog eggs: potential tracks for a cytoplasmic rotation that specifies the dorso-ventral axis. Dev Biol. 1988 Jul;128(1):185–197. doi: 10.1016/0012-1606(88)90281-3. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Danilchik M., Doniach T., Roberts S., Rowning B., Stewart R. Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development. 1989;107 (Suppl):37–51. doi: 10.1242/dev.107.Supplement.37. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Harwood A. J., Plyte S. E., Woodgett J., Strutt H., Kay R. R. Glycogen synthase kinase 3 regulates cell fate in Dictyostelium. Cell. 1995 Jan 13;80(1):139–148. doi: 10.1016/0092-8674(95)90458-1. [DOI] [PubMed] [Google Scholar]
- He X., Saint-Jeannet J. P., Woodgett J. R., Varmus H. E., Dawid I. B. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995 Apr 13;374(6523):617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- Heasman J., Crawford A., Goldstone K., Garner-Hamrick P., Gumbiner B., McCrea P., Kintner C., Noro C. Y., Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994 Dec 2;79(5):791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Holowacz T., Elinson R. P. Cortical cytoplasm, which induces dorsal axis formation in Xenopus, is inactivated by UV irradiation of the oocyte. Development. 1993 Sep;119(1):277–285. doi: 10.1242/dev.119.1.277. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990 May;8(5):528–535. [PubMed] [Google Scholar]
- Kessler D. S., Melton D. A. Vertebrate embryonic induction: mesodermal and neural patterning. Science. 1994 Oct 28;266(5185):596–604. doi: 10.1126/science.7939714. [DOI] [PubMed] [Google Scholar]
- Kintner C. R., Melton D. A. Expression of Xenopus N-CAM RNA in ectoderm is an early response to neural induction. Development. 1987 Mar;99(3):311–325. doi: 10.1242/dev.99.3.311. [DOI] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Lloyd P., Rapp U. R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991 Jan 31;349(6308):426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Developmental regulation of a gastrula-specific gene injected into fertilized Xenopus eggs. EMBO J. 1985 Dec 16;4(13A):3463–3471. doi: 10.1002/j.1460-2075.1985.tb04105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. M., Knecht A. K., Smith W. C., Stachel S. E., Economides A. N., Stahl N., Yancopolous G. D., Harland R. M. Neural induction by the secreted polypeptide noggin. Science. 1993 Oct 29;262(5134):713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Lemaire P., Garrett N., Gurdon J. B. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell. 1995 Apr 7;81(1):85–94. doi: 10.1016/0092-8674(95)90373-9. [DOI] [PubMed] [Google Scholar]
- McCrea P. D., Brieher W. M., Gumbiner B. M. Induction of a secondary body axis in Xenopus by antibodies to beta-catenin. J Cell Biol. 1993 Oct;123(2):477–484. doi: 10.1083/jcb.123.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Wnt genes. Cell. 1992 Jun 26;69(7):1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Pannese M., Polo C., Andreazzoli M., Vignali R., Kablar B., Barsacchi G., Boncinelli E. The Xenopus homologue of Otx2 is a maternal homeobox gene that demarcates and specifies anterior body regions. Development. 1995 Mar;121(3):707–720. doi: 10.1242/dev.121.3.707. [DOI] [PubMed] [Google Scholar]
- Perrimon N. The genetic basis of patterned baldness in Drosophila. Cell. 1994 Mar 11;76(5):781–784. doi: 10.1016/0092-8674(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Pierce S. B., Kimelman D. Regulation of Spemann organizer formation by the intracellular kinase Xgsk-3. Development. 1995 Mar;121(3):755–765. doi: 10.1242/dev.121.3.755. [DOI] [PubMed] [Google Scholar]
- Plyte S. E., Hughes K., Nikolakaki E., Pulverer B. J., Woodgett J. R. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim Biophys Acta. 1992 Dec 16;1114(2-3):147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- Ruel L., Bourouis M., Heitzler P., Pantesco V., Simpson P. Drosophila shaggy kinase and rat glycogen synthase kinase-3 have conserved activities and act downstream of Notch. Nature. 1993 Apr 8;362(6420):557–560. doi: 10.1038/362557a0. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L. K., De Robertis E. M. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994 Dec 2;79(5):779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe C. R., Pluck A., Gurdon J. B. XIF3, a Xenopus peripherin gene, requires an inductive signal for enhanced expression in anterior neural tissue. Development. 1989 Dec;107(4):701–714. doi: 10.1242/dev.107.4.701. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Hattori K., Weintraub H. Progressive determination during formation of the anteroposterior axis in Xenopus laevis. Cell. 1989 Jul 14;58(1):171–180. doi: 10.1016/0092-8674(89)90413-3. [DOI] [PubMed] [Google Scholar]
- Smith J. C. Mesoderm-inducing factors in early vertebrate development. EMBO J. 1993 Dec;12(12):4463–4470. doi: 10.1002/j.1460-2075.1993.tb06135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Green J. B., Weigel D., Herrmann B. G. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991 Oct 4;67(1):79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992 Sep 4;70(5):829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991 Nov 15;67(4):753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Sokol S. Y., Klingensmith J., Perrimon N., Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995 Jun;121(6):1637–1647. doi: 10.1242/dev.121.6.1637. [DOI] [PubMed] [Google Scholar]
- Sokol S. Y., Melton D. A. Interaction of Wnt and activin in dorsal mesoderm induction in Xenopus. Dev Biol. 1992 Dec;154(2):348–355. doi: 10.1016/0012-1606(92)90073-p. [DOI] [PubMed] [Google Scholar]
- Sokol S. Y. Mesoderm formation in Xenopus ectodermal explants overexpressing Xwnt8: evidence for a cooperating signal reaching the animal pole by gastrulation. Development. 1993 Aug;118(4):1335–1342. doi: 10.1242/dev.118.4.1335. [DOI] [PubMed] [Google Scholar]
- Sokol S., Christian J. L., Moon R. T., Melton D. A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991 Nov 15;67(4):741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Wang Q. M., Fiol C. J., DePaoli-Roach A. A., Roach P. J. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem. 1994 May 20;269(20):14566–14574. [PubMed] [Google Scholar]