Abstract
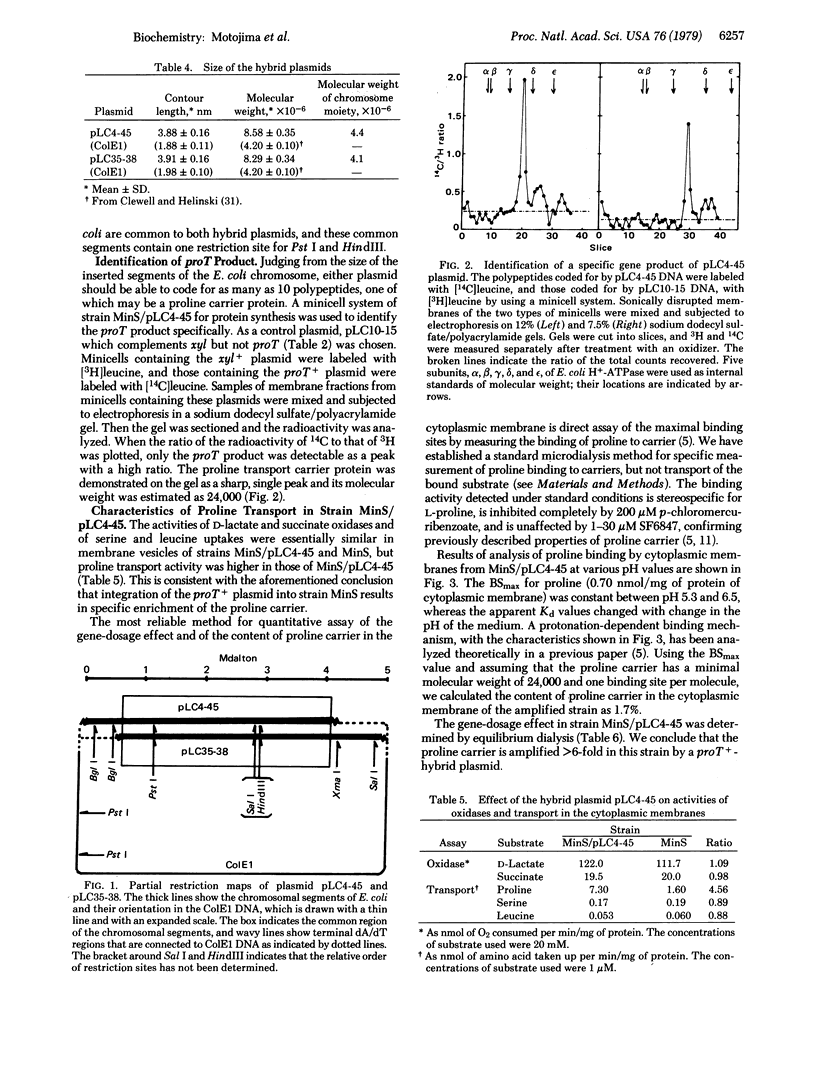
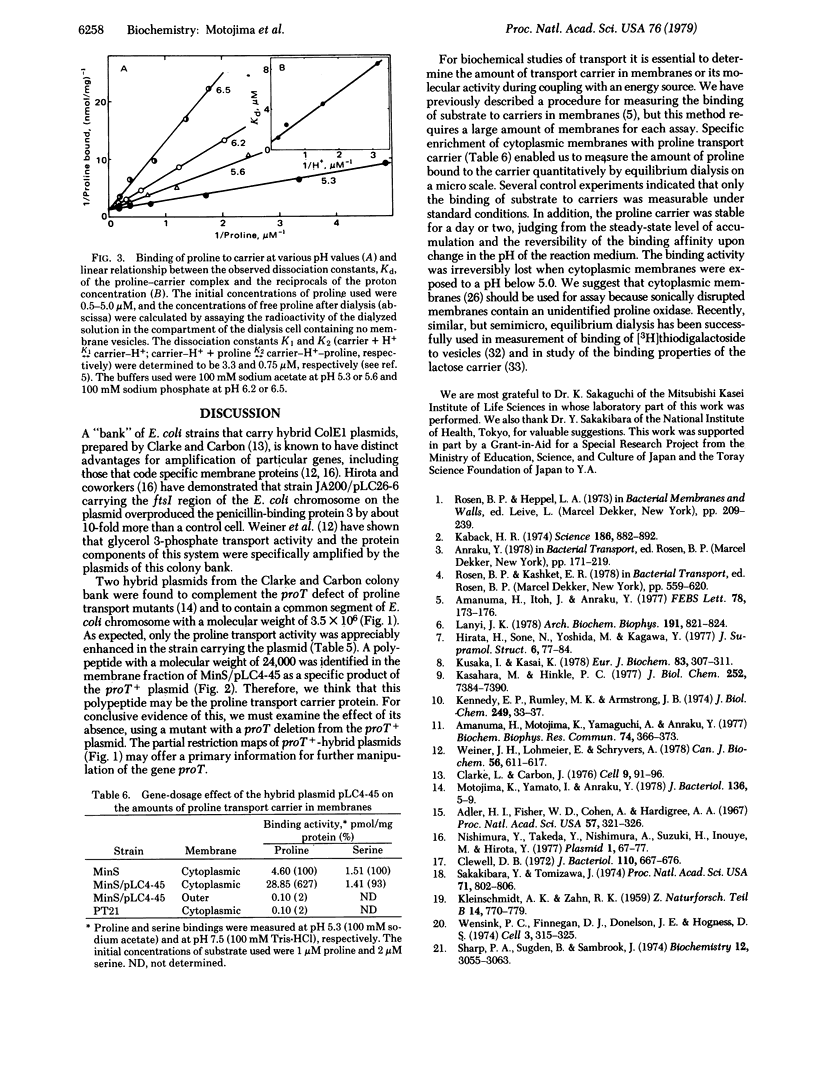
A previous report [Motojima, K., Yamato, I. & Anraku, Y. (1978) J. Bacteriol. 136, 5-9) described the characteristics of mutants (proT-) of Escherichia coli K-12 that are defective in proline transport carrier activity. Two hybrid plasmids from the Clarke and Carbon colony bank were found to complement the mutation by conjugation and transformation. Analysis with restriction endonucleases showed that both plasmid DNAs carried the same fragment of the E. coli chromosome. A polypeptide with a molecular weight of 24,000, specifically coded for by the proT+ plasmid, was identified in the cytoplasmic membrane by using a double-isotope labeling method in a minicell system. The strain harboring the proT+ plasmid has 6 times as much proline transport carrier as the wild strain. This amplification of the carrier makes it possible to measure proline binding to the carrier by microequilibrium dialysis. Detailed analysis of binding indicated that the maximal amount of proline bound to the carrier is 0.70 nmol/mg of protein of the cytoplasmic membrane of the amplified strain. From this value and the assumption that the carrier has one binding site per minimal molecular weight of 24,000, the content of the proline transport carrier in the cytoplasmic membrane was estimated to be 1.7%.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanuma H., Itoh J., Anraku Y. Proton-dependent binding of proline to carrier in Escherichia coli membrane. FEBS Lett. 1977 Jun 15;78(2):173–176. doi: 10.1016/0014-5793(77)80299-8. [DOI] [PubMed] [Google Scholar]
- Amanuma H., Itoh J., Anraku Y. Transport of sugars and amino acids in bacteria. XVII. On the existence and nature of substrate amino acids bound to purified branched chain amino acid-binding proteins of Escherichia coli. J Biochem. 1976 Jun;79(6):1167–1182. doi: 10.1093/oxfordjournals.jbchem.a131172. [DOI] [PubMed] [Google Scholar]
- Amanuma H., Motojima K., Yamaguchi A., Anraku Y. Solubilization of a functionally active proline carrier from membranes of Escherichia coli with an organic solvent. Biochem Biophys Res Commun. 1977 Jan 24;74(2):366–373. doi: 10.1016/0006-291x(77)90313-8. [DOI] [PubMed] [Google Scholar]
- Belaich A., Simonpietri P., Belaich J. P. Microcalorimetric study of the binding of thiodigalactoside to the lactose permease M protein of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6735–6738. [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Sternweis P. C., Heppel L. A. Purification and properties of reconstitutively active and inactive adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2725–2729. doi: 10.1073/pnas.71.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto F., Anraku Y. Transport of sugars and amino acids in bacteria. IX. Studies on the active transport reaction in sodium azide- and 2,4-dinitrophenol-sensitive mutants of Escherichia coli. J Biochem. 1974 Feb;75(2):243–251. doi: 10.1093/oxfordjournals.jbchem.a130391. [DOI] [PubMed] [Google Scholar]
- Hirata H., Sone N., Yoshida M., Kagawa Y. Isolation of the alanine carrier from the membranes of a thermophilic bacterium and its reconstitution into vesicles capable of transport. J Supramol Struct. 1977;6(1):77–84. doi: 10.1002/jss.400060106. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Armstrong J. B. Dierect measurement of the binding of labeled sugars to the lactose permease M protein. J Biol Chem. 1974 Jan 10;249(1):33–37. [PubMed] [Google Scholar]
- Kusaka I., Kanai K. Purification and characterization of alanine carrier isolated from H-proteins of Bacillus subtilis. Eur J Biochem. 1978 Feb 1;83(1):307–311. doi: 10.1111/j.1432-1033.1978.tb12095.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanyi J. K. Apparent cooperativity of amino acid transport in Halobacterium halobium: effect of electrical potential. Arch Biochem Biophys. 1978 Dec;191(2):821–824. doi: 10.1016/0003-9861(78)90425-3. [DOI] [PubMed] [Google Scholar]
- Motojima K., Yamato I., Anraku Y. Proline transport carrier-defective mutants of Escherichia coli K-12: properties and mapping. J Bacteriol. 1978 Oct;136(1):5–9. doi: 10.1128/jb.136.1.5-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Takeda Y., Nishimura A., Suzuki H., Inouye M., Hirota Y. Synthetic ColE1 plasmids carrying genes for cell division in Escherichia coli. Plasmid. 1977 Nov;1(1):67–77. doi: 10.1016/0147-619x(77)90009-9. [DOI] [PubMed] [Google Scholar]
- Overath P., Teather R. M., Simoni R. D., Aichele G., Wilhelm U. Lactose carrier protein of Escherichia coli. Transport and binding of 2'-(N-dansyl)aminoethyl beta-D-thiogalactopyranoside and p-nitrophenyl alpha-d-galactopyranoside. Biochemistry. 1979 Jan 9;18(1):1–11. doi: 10.1021/bi00568a001. [DOI] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Tomizawa J. I. Replication of colicin E1 plasmid DNA in cell extracts. Proc Natl Acad Sci U S A. 1974 Mar;71(3):802–806. doi: 10.1073/pnas.71.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiner J. H., Lohmeier E., Schryvers A. Cloning and expression of the glycerol-3-phosphate transport genes of Escherichia coli. Can J Biochem. 1978 Jun;56(6):611–617. doi: 10.1139/o78-092. [DOI] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]
- Yamato I., Anraku Y., Hirosawa K. Cytoplasmic membrane vesicles of Escherichia coli. A simple method for preparing the cytoplasmic and outer membranes. J Biochem. 1975 Apr;77(4):705–718. doi: 10.1093/oxfordjournals.jbchem.a130774. [DOI] [PubMed] [Google Scholar]
- Yamato I., Anraku Y. Transport of sugars and amino acids in bacteria. XVIII. Properties of an isoleucine carrier in the cytoplasmic membrane vesicles of Escherichia coli. J Biochem. 1977 May;81(5):1517–1523. [PubMed] [Google Scholar]


