Abstract
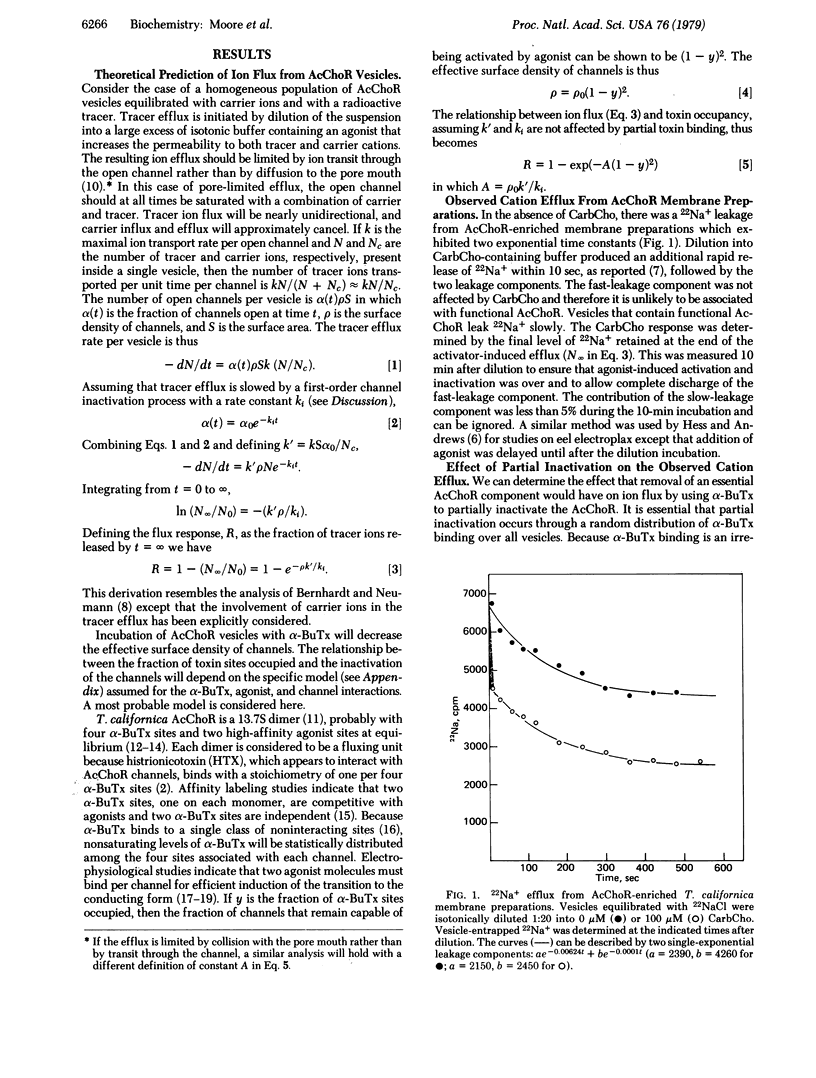
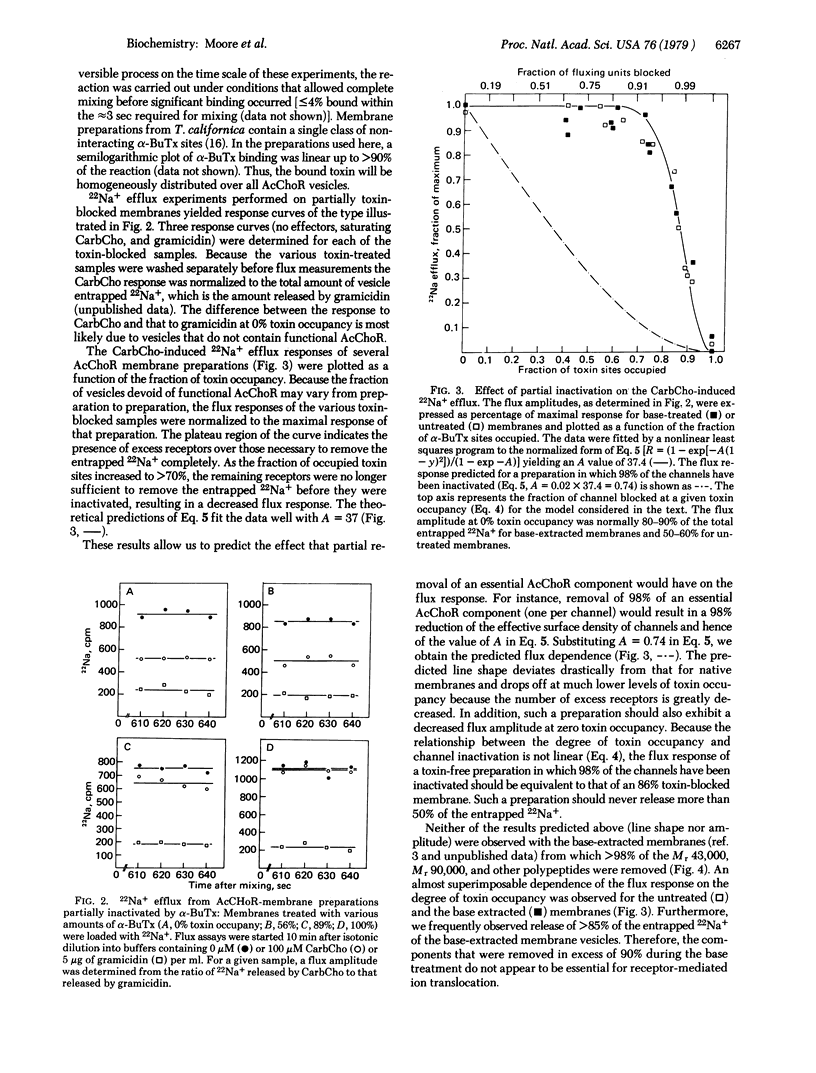
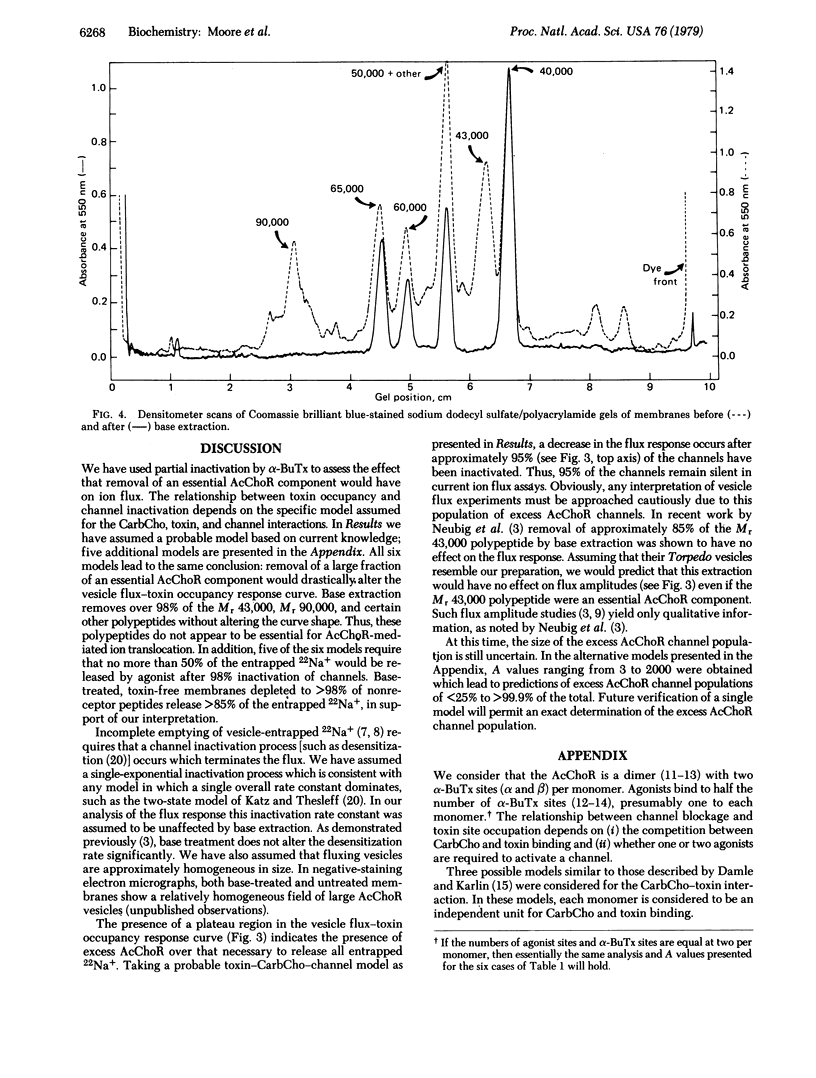
Membrane vesicles containing partially inactivated acetylcholine receptor (AcChoR) channels may produce a full 22Na+ flux response because an excess of channels may exist above the level needed to completely empty the vesicles of ions. Therefore, attempts to use ion flux amplitudes as indicators of AcChoR function may fail due to the presence of these excess AcChoR channels. Random inactivation of variable fractions of AcChoR channels in vesicles by the irreversible binding of the neurotoxin alpha-bungarotoxin provides a tool for assessing the size of the excess receptor population. Using this approach, we predict that the dependence of the flux response on partial inactivation by alpha-bungarotoxin will drastically change if an essential AcChoR component is substantially removed from the membranes. Membranes from which Mr 43,000, Mr 90,000, and other polypeptides had been substantially removed by base extraction exhibited a flux response after random inactivation that was indistinguishable from that of untreated membranes. Therefore, those components which are substantially removed by base extraction do not appear to be essential for AcChoR-mediated ion flux.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. An analysis of the dose-response curve at voltage-clamped frog-endplates. Pflugers Arch. 1975 Oct 28;360(2):145–153. doi: 10.1007/BF00580537. [DOI] [PubMed] [Google Scholar]
- Bernhardt J., Neumann E. Kinetic analysis of receptor-controlled tracer efflux from sealed membrane fragments. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3756–3760. doi: 10.1073/pnas.75.8.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. W., Bock E. Molecular forms of acetylcholine receptor. Effects of calcium ions and a sulfhydryl reagent on the occurrence of oligomers. Biochemistry. 1977 Oct 4;16(20):4513–4520. doi: 10.1021/bi00639a028. [DOI] [PubMed] [Google Scholar]
- Damle V. N., Karlin A. Affinity labeling of one of two alpha-neurotoxin binding sites in acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2039–2045. doi: 10.1021/bi00604a002. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Dunn S. M., Blanchard S. G., Raftery M. A. Specific binding of perhydrohistrionicotoxin to Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2576–2579. doi: 10.1073/pnas.76.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Raftery M. A. Binding of perhydrohistrionicotoxin to intact and detergent-solubilized membranes enriched in nicotinic acetylcholine receptor. Biochemistry. 1979 May 15;18(10):1868–1874. doi: 10.1021/bi00577a004. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., McLaughlin M., Karlin A. Disulfide bond cross-linked dimer in acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1977 Dec 7;79(3):692–699. doi: 10.1016/0006-291x(77)91167-6. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P. Functional acetylcholine receptor--electroplax membrane microsacs (vesicles): purification and characterization. Proc Natl Acad Sci U S A. 1977 Feb;74(2):482–486. doi: 10.1073/pnas.74.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Diffusion-limited ion flow through pores. Biochim Biophys Acta. 1976 Dec 2;455(2):493–509. doi: 10.1016/0005-2736(76)90320-5. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Moore H. P., Hartig P. R., Raftery M. A. Fast cation flux from Torpedo californica membrane preparations: implications for a functional role for acetylcholine receptor dimers. Biochem Biophys Res Commun. 1978 Nov 29;85(2):632–640. doi: 10.1016/0006-291x(78)91209-3. [DOI] [PubMed] [Google Scholar]
- Moody T., Schmidt J., Raftery M. A. Binding of acetylcholine and related compounds to purified acetylcholine receptor from Torpedo Californica electroplax. Biochem Biophys Res Commun. 1973 Aug 6;53(3):761–772. doi: 10.1016/0006-291x(73)90158-7. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Hartig P. R., Wu W. C., Raftery M. A. Rapid cation flux from Torpedo californica membrane vesicles: comparison of acetylcholine receptor enriched and selectively extracted preparations. Biochem Biophys Res Commun. 1979 May 28;88(2):735–743. doi: 10.1016/0006-291x(79)92109-0. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci U S A. 1979 Feb;76(2):690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast U., Schimerlik M., Lee T., Witzemann T. L., Blanchard S., Raftery M. A. Ligand-induced conformation changes in Torpedo californica membrane-bound acetylcholine receptor. Biochemistry. 1978 Jun 13;17(12):2405–2414. doi: 10.1021/bi00605a024. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Schmidt J., Clark D. G. Specificity of -bungarotoxin binding to Torpedo californica electroplax. Arch Biochem Biophys. 1972 Oct;152(2):882–886. doi: 10.1016/0003-9861(72)90285-8. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Vandlen R. L., Reed K. L., Lee T. Characterization of Torpedo californica acetylcholine receptor: its subunit composition and ligand-binding properties. Cold Spring Harb Symp Quant Biol. 1976;40:193–202. doi: 10.1101/sqb.1976.040.01.021. [DOI] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Rates and equilibria at the acetylcholine receptor of Electrophorus electroplaques: a study of neurally evoked postsynaptic currents and of voltage-jump relaxations. J Gen Physiol. 1977 Aug;70(2):187–219. [PMC free article] [PubMed] [Google Scholar]


