Abstract
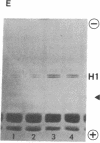
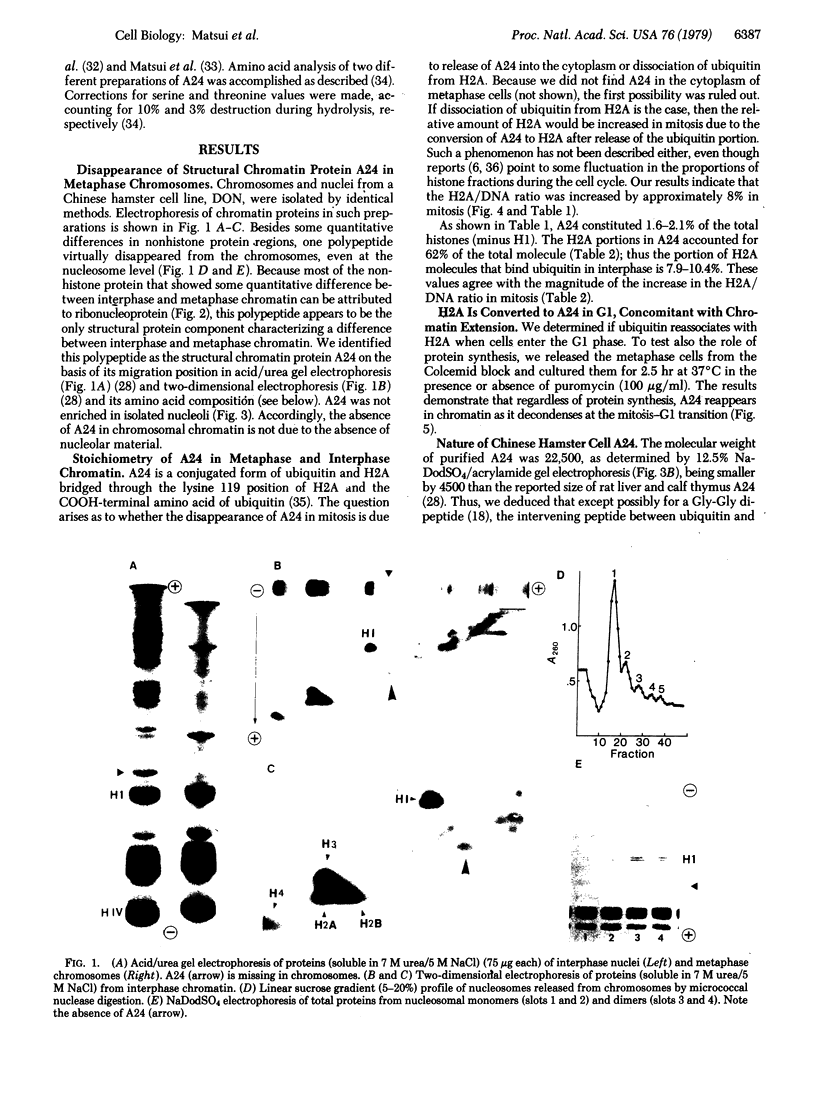
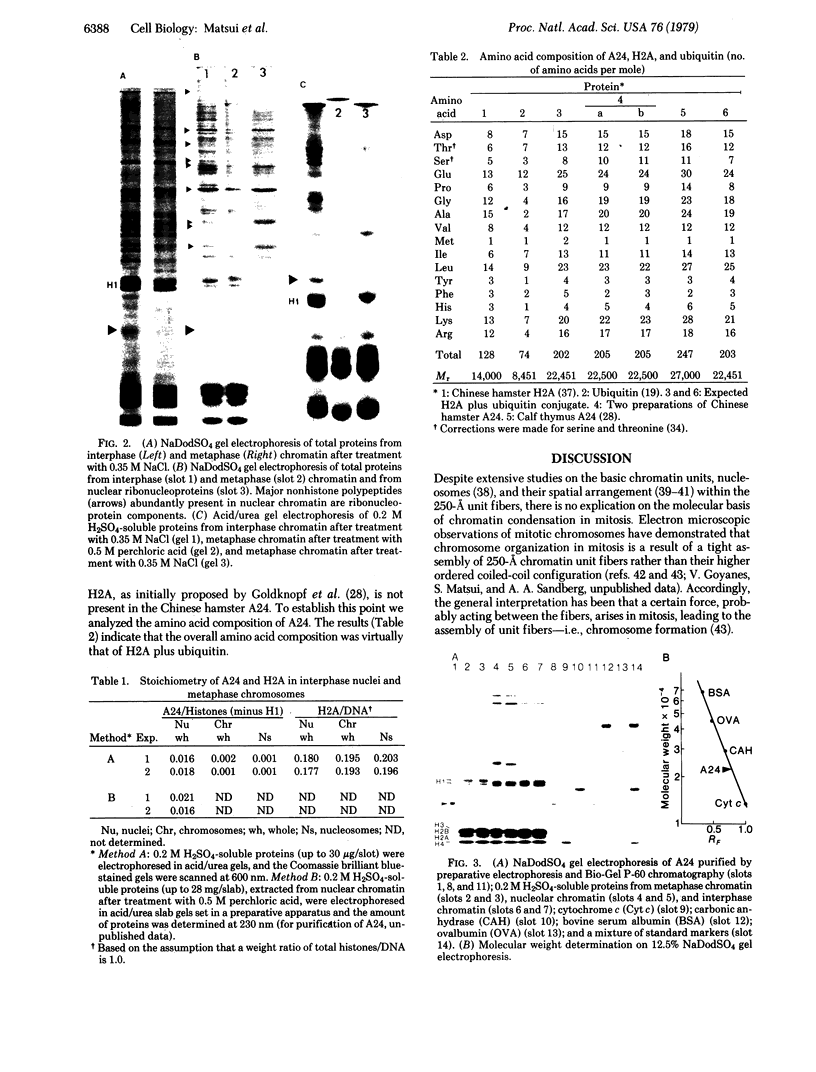
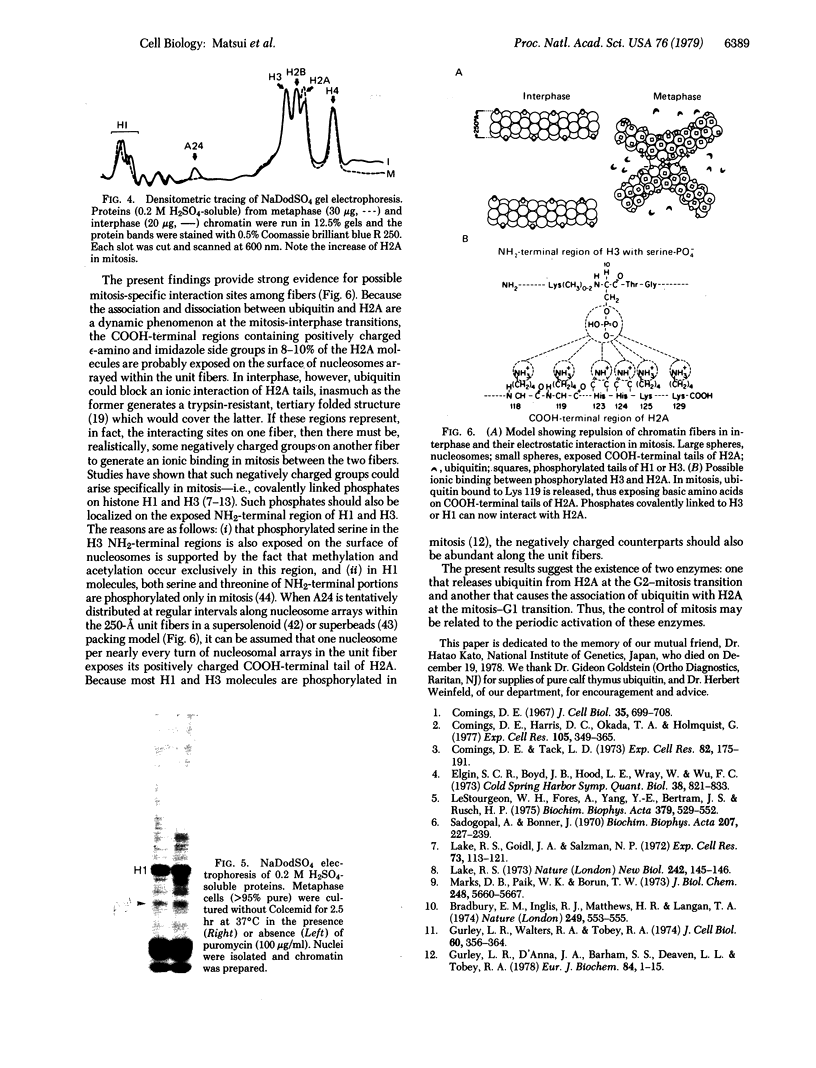
A chromatin protein, A24, a conjugate of histone H2A and evolutionally conserved ubiquitin, was virtually the only structural polypeptide that was present in interphase but missing in mitosis of a Chinese hamster cell line (DON). Because a 10% increase in the H2A/DNA ratio observed in interphase-mitosis transition explained the stoichiometric conversion of A24 to H2A, it appears that ubiquitin bound to H2A of nucleosomal surfaces in interphase is released at mitosis whereas the total H2A remains as a structural component of nucleosomes. Regardless of protein synthesis, ubiquitin was again bound to H2A when cells entered the G1 phase. Based on the electrostatic nature of the COOH-terminal region of H2A, where ubiquitin binds, and the mitosis-specific rise of covalently linked phosphates in histones H1 and H3, we propose that an ionic interaction between the positively charged H2A COOH-terminal regions on fibers and negatively charged phosphates linked to serine or threonine of H1 and H3 molecules on adjacent fibers could generate an assembly of chromatin fibers in mitosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhorjee J. S., Pederson T. Chromatin: its isolation from cultured mammalian cells with particular reference to contamination by nuclear ribnucleoprotein particles. Biochemistry. 1973 Jul 3;12(14):2766–2773. doi: 10.1021/bi00738a033. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R. Control of cell division by very lysine rich histone (F1) phosphorylation. Nature. 1974 Feb 1;247(5439):257–261. doi: 10.1038/247257a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R., Langan T. A. Molecular basis of control of mitotic cell division in eukaryotes. Nature. 1974 Jun 7;249(457):553–556. doi: 10.1038/249553a0. [DOI] [PubMed] [Google Scholar]
- Comings D. E., Harris D. C., Okada T. A., Holmquist G. Nuclear proteins. IV. Deficiency of non-histone proteins in condensed chromatin of Drosophila virilis and mouse. Exp Cell Res. 1977 Mar 15;105(2):349–365. doi: 10.1016/0014-4827(77)90133-1. [DOI] [PubMed] [Google Scholar]
- Comings D. E. Histones of genetically active and inactive chromatin. J Cell Biol. 1967 Dec;35(3):699–708. doi: 10.1083/jcb.35.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings D. E., Tack L. O. Non-histone proteins. The effect of nuclear washes and comparison of metaphase and interphase chromatin. Exp Cell Res. 1973 Nov;82(1):175–191. doi: 10.1016/0014-4827(73)90260-7. [DOI] [PubMed] [Google Scholar]
- Elgin S. C., Boyd J. B., Hood L. E., Wray W., Wu F. C. A prologue to the study of the nonhistone chromosomal proteins. Cold Spring Harb Symp Quant Biol. 1974;38:821–833. doi: 10.1101/sqb.1974.038.01.085. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Noll M., Kornberg R. D. Electron microscopy of defined lengths of chromatin. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3320–3322. doi: 10.1073/pnas.72.9.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24. Proc Natl Acad Sci U S A. 1977 Mar;74(3):864–868. doi: 10.1073/pnas.74.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldknopf I. L., French M. F., Musso R., Busch H. Presence of protein A24 in rat liver nucleosomes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5492–5495. doi: 10.1073/pnas.74.12.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldknopf I. L., Taylor C. W., Baum R. M., Yeoman L. C., Olson M. O., Prestayko A. W., Busch H. Isolation and characterization of protein A24, a "histone-like" non-histone chromosomal protein. J Biol Chem. 1975 Sep 25;250(18):7182–7187. [PubMed] [Google Scholar]
- Golomb H. M., Bahr G. F. Human chromatin from interphase to metaphase: a scanning electron microscopic study. Exp Cell Res. 1974 Mar 15;84(1):79–87. doi: 10.1016/0014-4827(74)90382-6. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Keevert J. B. Absence of histone F1 in a mitotically dividing, genetically inactive nucleus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2672–2676. doi: 10.1073/pnas.72.7.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley L. R., D'Anna J. A., Barham S. S., Deaven L. L., Tobey R. A. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem. 1978 Mar;84(1):1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Hardin J. M. The metabolism of histone fractions. I. Synthesis of histone fractions during the life cycle of mammalian cells. Arch Biochem Biophys. 1968 Nov;128(2):285–292. doi: 10.1016/0003-9861(68)90034-9. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Cell cycle-specific changes in histone phosphorylation associated with cell proliferation and chromosome condensation. J Cell Biol. 1974 Feb;60(2):356–364. doi: 10.1083/jcb.60.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann P., Tobey R. A., Gurley L. R. Phosphorylation of distinct regions of f1 histone. Relationship to the cell cycle. J Biol Chem. 1976 Jun 25;251(12):3685–3692. [PubMed] [Google Scholar]
- Kay R. R., Fraser D., Johnston I. R. A method for the rapid isolation of nuclear membranes from rat liver. Characterisation of the membrane preparation and its associated DNA polymerase. Eur J Biochem. 1972 Oct 17;30(1):145–154. doi: 10.1111/j.1432-1033.1972.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Krause M. O., Yoo B. Y., Macbeath L. Histones from exponential and stationary L-cells. Evidence for differential binding of lysine-rich and arginine-rich fractions in chromatin. Arch Biochem Biophys. 1974 Sep;164(1):172–178. doi: 10.1016/0003-9861(74)90019-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lake R. S. F1-histone phosphorylation in metaphase chromosomes of cultured Chinese hamster cells. Nat New Biol. 1973 Apr 4;242(118):145–146. doi: 10.1038/newbio242145a0. [DOI] [PubMed] [Google Scholar]
- Lake R. S., Goidl J. A., Salzman N. P. F1-histone modification at metaphase in Chinese hamster cells. Exp Cell Res. 1972 Jul;73(1):113–121. doi: 10.1016/0014-4827(72)90108-5. [DOI] [PubMed] [Google Scholar]
- Lestourgeon W. M., Forer A., Yang Y. Z., Bertram J. S., Pusch H. P. Contractile proteins. Major components of nuclear and chromosome non-histone proteins. Biochim Biophys Acta. 1975 Feb 27;379(2):529–552. [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D. B., Paik W. K., Borun T. W. The relationship of histone phosphorylation to deoxyribonucleci acid replication and mitosis during the HeLa S-3 cell cycle. J Biol Chem. 1973 Aug 25;248(16):5660–5667. [PubMed] [Google Scholar]
- Matsui S. I., Weinfeld H., Sandberg A. A. Quantitative conservation of chromatin-bound RNA polymerases I and II in mitosis. Implications for chromosome structure. J Cell Biol. 1979 Feb;80(2):451–464. doi: 10.1083/jcb.80.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. I., Yoshida H., Weinfeld H., Sandberg A. A. Induction of prophase in interphase nuclei by fusion with metaphase cells. J Cell Biol. 1972 Jul;54(1):120–132. doi: 10.1083/jcb.54.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S., Busch H. Isolation and characterization of rDNA-containing chromatin from nucleoli. Exp Cell Res. 1977 Oct 1;109(1):151–161. doi: 10.1016/0014-4827(77)90054-4. [DOI] [PubMed] [Google Scholar]
- Matsui S., Weinfeld H., Sandberg A. A. Fate of chromatin of interphase nuclei subjected to "phosphasing" in virus-fused cells. J Natl Cancer Inst. 1972 Dec;49(6):1621–1630. doi: 10.1093/jnci/49.6.1621. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L. Nucleosomes: the structural quantum in chromosomes. Am Sci. 1978 Nov-Dec;66(6):704–711. [PubMed] [Google Scholar]
- Orrick L. R., Olson M. O., Busch H. Comparison of nucleolar proteins of normal rat liver and Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1973 May;70(5):1316–1320. doi: 10.1073/pnas.70.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Hamkalo B. A. Nucleosome packing in interphase chromatin. J Cell Biol. 1979 May;81(2):453–457. doi: 10.1083/jcb.81.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadgopal A., Bonner J. Proteins of interphase and metaphase chromosomes compared. Biochim Biophys Acta. 1970 Apr 28;207(1):227–239. doi: 10.1016/0005-2795(70)90154-6. [DOI] [PubMed] [Google Scholar]
- Schildkraut C. L., Maio J. J. Studies on the intranuclear distribution and properties of mouse satellite DNA. Biochim Biophys Acta. 1968 Jun 18;161(1):76–93. doi: 10.1016/0005-2787(68)90296-7. [DOI] [PubMed] [Google Scholar]
- Schlesinger D. H., Goldstein G., Niall H. D. The complete amino acid sequence of ubiquitin, an adenylate cyclase stimulating polypeptide probably universal in living cells. Biochemistry. 1975 May 20;14(10):2214–2218. doi: 10.1021/bi00681a026. [DOI] [PubMed] [Google Scholar]
- Seon B. K., Pressman D. Unique human glycoprotein, alpha1-microglycoprotein: isolation from the urine of a cancer patient and its characterization. Biochemistry. 1978 Jul 11;17(14):2815–2821. doi: 10.1021/bi00607a018. [DOI] [PubMed] [Google Scholar]
- Tanphaichitr N., Moore K. C., Granner D. K., Chalkley R. Relationship between chromosome condensation and metaphase lysine-rich histone phosphorylation. J Cell Biol. 1976 Apr;69(1):43–50. doi: 10.1083/jcb.69.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Benyajati C. Higher order coiling of DNA in chromatin. Cell. 1977 Sep;12(1):83–100. doi: 10.1016/0092-8674(77)90187-8. [DOI] [PubMed] [Google Scholar]
- von Holt C., Brandt W. F. Fractionation of histones on molecular sieve matrices. Methods Cell Biol. 1977;16:205–225. doi: 10.1016/s0091-679x(08)60101-6. [DOI] [PubMed] [Google Scholar]