Abstract
Background and Objectives
Tobacco and cannabis use are both highly prevalent worldwide. Their co-use is also common in adults and adolescents. Despite this frequent co-occurrence, cessation from both substances is rarely addressed in randomized clinical trials. Given evidence that tobacco use may increase during cannabis cessation attempts, and additionally that tobacco users have poorer cannabis cessation outcomes, we explored tobacco outcomes, specifically cigarette smoking, from an adolescent cannabis cessation trial that tested the efficacy of N-acetylesteine (NAC).
Methods
Cannabis-dependent adolescents (ages 15–21; n=116) interested in cannabis treatment were randomized to NAC (1200 mg bid) or matched placebo for 8 weeks. Participants did not need to be cigarette smokers or be interested in smoking cessation to qualify for inclusion.
Results
Approximately 59% of enrolled participants were daily and non-daily cigarette smokers, and only differed from non-smoking participants on the compulsion sub-scale of the Marijuana Craving Questionnaire. Among cigarette smokers who were retained in the study, there was no change in cigarettes per day for either NAC or placebo groups during the 8-week treatment phase. Being a cigarette smoker did not appear to influence the effects of NAC on cannabis abstinence, though there was a trend in the placebo group of poorer cannabis outcomes for cigarette smokers vs. non-smokers.
Conclusions
No evidence was found of compensatory cigarette smoking during this cannabis cessation trial in adolescents. Further work assessing interventions to reduce both cannabis and tobacco use in this population is greatly needed.
Keywords: N-acetylcysteine, cannabis, marijuana, cigarette, tobacco, smoking, adolescents, cessation, co-occurring
Introduction
Cigarette smoking remains the leading cause of preventable death in the United States (1), with the majority of adult smokers (88%) starting prior to the age of 18 (2). Cannabis use is also highly prevalent and is the most commonly used illicit substance among adolescents, with use continuing to increase and the perceived risk of harm decreasing (3). The adverse effects associated with cannabis use are not as well-established as with tobacco, though data reveal adverse influences of cannabis on several aspects of health, work, and interpersonal relationships (e.g., mental health, respiration, relationships, employment) (4). Additionally, reports demonstrate problems associated with prolonged use of cannabis and progression to dependence (5–9).
Both tobacco and cannabis use are typically initiated during adolescence or young adulthood (17.2 and 17.5 years of age, respectively) (10), and tend to persist well into adulthood. The co-occurrence of cannabis and tobacco use is highly prevalent and has been demonstrated for adult and adolescent populations (11–19). Several adverse outcomes are associated with cannabis and tobacco co-use, including, the exacerbation of mental health symptoms (20) and prevalence of psychiatric problems (21), the initiation of regular tobacco use and dependence when tobacco is mixed in cannabis preparations (22), and a reduction in the ability to successfully quit both tobacco and cannabis (23,24). Tobacco users have been shown in laboratory and outpatient studies to have greater odds of relapse to cannabis compared to non- tobacco users (25,26). Consistent with those results, a recent review by Peters and colleagues (27) showed that use of tobacco and cannabis, compared to cannabis use alone, was associated with poorer cannabis cessation outcomes, as well as more psychosocial problems, and greater likelihood of a cannabis use disorder. Some preliminary evidence also suggests that tobacco may be substituted and may increase during cannabis reductions or abstinence (28,29). Another study did not find evidence of tobacco substitution, but found that tobacco use only decreased in participants with >50% reduction in cannabis use (30). Taken together, these results indicate that tobacco and cannabis co-users represent a vulnerable group that may begin cessation attempts already at a disadvantage and at risk for increases in tobacco use.
The purpose of this secondary analysis was to explore tobacco outcomes, specifically cigarette smoking during a cannabis cessation trial. The parent trial assessed the efficacy of N-acetylcysteine (NAC), an over-the-counter antioxidant supplement with glutamatergic properties, as a potential pharmacotherapy for cannabis cessation in adolescents (31). Cigarette smoking was explored for two main reasons. First, there is ample evidence from the literature that cannabis -tobacco co-users have poorer cannabis treatment outcomes and preliminary evidence that tobacco substitution during cannabis reductions or cessation may occur (discussed above). Second, there is preclinical and preliminary clinical evidence that NAC may be an effective pharmacotherapy for smoking cessation (32–35). Specifically, this study evaluated; 1) cigarette smoking characteristics of a treatment-seeking sample of adolescents enrolled in a cannabis cessation trial, 2) cigarette use changes during a cannabis cessation attempt with the use of NAC or placebo, and 3) if being a cigarette smoker resulted in poorer cannabis treatment outcomes.
Methods
Participants
Participants (n=116) were between the ages of 15–21, met criteria for cannabis dependence, used cannabis regularly (≥3 days/week), and were interested in cannabis cessation treatment. Participants were excluded if they were enrolled in substance abuse treatment, had comorbid substance dependence (other than nicotine), or had any unstable psychiatric or medical issue. Participants did not have to be seeking tobacco treatment in order to be enrolled in the study. Further details of study participants and cannabis abstinence outcomes can be found elsewhere (31).
Procedures
Eligible participants were randomized to active treatment (NAC, 1200 mg bid) or matched placebo. The study intervention lasted for 8 weeks, with one follow-up visit occurring at 12 weeks. In addition to study medication, contingency management procedures were used to reinforce adherence to study procedures, attendance at study visits, and abstinence from cannabis throughout the 8-week intervention. Brief cannabis cessation counseling was provided weekly, but no psychosocial treatment targeted cigarette smoking.
Measures
Cigarette smoking was measured through qualitative urinary cotinine (COT; NicAlert® test strips, Nymox Pharmaceutical Corporation, Montreal, Quebec) and self-reported cigarettes per day (cigs/day). Urine samples were submitted at each study visit, and urinary COT results were read immediately and coded as negative or positive (NicAlert test strip score of 3 = 100–200 ng/ml). During the 8-week treatment phase, participants recorded their cigs/day via daily diaries. Timeline Follow-back methods (TLFB) were used to measure cigs/day 30 days prior study enrollment and through the follow-up period (36). Calculations of average cigs/day included non-smoking days. Questionnaires assessing smoking characteristics included: the Modified Fagerström Tolerance Questionnaire (mFTQ) (37), a questionnaire to assess participants’ level of motivation to quit smoking cigarettes (locally developed), and the Questionnaire on Smoking Urges-Brief (QSU-B) (38). Use of other tobacco products was not systematically assessed.
Study participants self-reported if they were a cigarette smoker or non-smoker at the screening visit. Smoking-related questionnaires were not administered to self-reported non-smokers, but all participants provided breath CO samples, urinary COT samples, and were asked about any cigarettes smoked during the TLFB administered at the screening visit and at study visits. Mixing tobacco in cannabis preparations was not systematically assessed, though participants were asked at the screening visit how they typically used cannabis (i.e., bong, bowl, joint, blunt, and/or other). Based on COT, CO, and reported cigarettes smoked prior to screening, six participants were re-classified from non-smoking to smoking status. Three of these six participants also endorsed smoking cannabis as blunts, which would have contributed to positive COT values, but non-daily cigarette smoking was also endorsed among these participants. Three additional participants were re-classified from smokers to non-smokers.
Statistical Analysis
Standard descriptive statistics were used to summarize demographic, clinical and smoking characteristics for cigarette smokers and non-smokers. A Wilcoxon rank sum test statistic assessed differences among continuous variables at screening while differences in categorical variables were assessed using a Pearson chi-square test statistic. The efficacy of NAC versus placebo on secondary abstinence from cigarette smoking was analyzed over the 8-week treatment period. A repeated measures risk regression model using the methods of generalized estimating equations was applied to assess the overall treatment effect on self-reported (COT confirmed) cigarette smoking during active treatment. Risk ratios and asymptotic 95% confidence intervals were computed. Simple models contained treatment group assignment, visit, positive cannabinoid levels assessed weekly, and the interaction between treatment group and time. The effects of treatment on smoking outcomes were tested using treatment by visit interaction terms within each model. Adjusted models additionally controlled for race and mean cigs/day prior to study entry. Similar linear mixed effects models were developed to assess the influence of NAC on cigs/day and percent of days smoked between visits. Changes in mFTQ and QSU-B scores were assessed at screening and end of treatment (Week 8) and were analyzed using linear mixed effects models. To assess the possible moderating effects of cigarette smoking status on the efficacy of NAC on cannabis abstinence, models were developed with appropriate interactions (cigarette smoker by treatment assignment) and stratified by treatment assignment. To better control for inflation of the Type I error rate due to multiple testing, a Bonferroni correction was applied to each within family alpha cut-off. Within the family of hypotheses relating to smoking (percent of days smoked and overall abstinence), the alpha level needed for significance was α=0.017. Within the family of hypotheses relating smoking characteristics (mFTQ and QSU-B) the alpha level needed for significance was α=0.025. All statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Missing Data
In the analysis of the moderating effects of cigarette smoking status on cannabis abstinence, all participants were included and an intent to treat approach was used. Missing cannabis use data was assumed to be positive for cannabinoids at each treatment visit. Of the cigarette smokers enrolled in the study, 19/68 (28%) were lost to follow up prior to the start of the treatment phase of the study and/or did not have cigarette data. Of the remaining 49 participants with cigs/day data, the median (interquartile range) number of weekly treatment visits with available cigarette data was 6 (5–7) of 8 total visits. Additionally, 273/392 (70%) of the weekly TLFB cigarette smoking data were present; NAC=134/200 (67%) and PBO=139/192 (72%). Methods of Maximum Likelihood (ML) are known to provide estimates that are consistent, asymptotically normal and efficient in the presence of missing repeated measures data (39), and were used as the primary parameter estimation method for the examination of cigarette use and smoking measures.
Results
Participants (n=116) were on average (SD) 18.9 (1.5) years of age, 72% were male, and 84% were Caucasian. Additional treatment group characteristics and differences are reported elsewhere (31). Demographic, psychiatric, and cannabis use characteristics between cigarette smokers and non-smokers are presented in Table 1. Out of 116 participants, 68 (59%) were current cigarette smokers. Cigarette smokers and non-smokers were similar with respect to age, race, gender, impulsivity, cannabis use characteristics, and in psychiatric comorbidities. The only significant difference between these two groups was on the Compulsion sub-scale of the Marijuana Craving Questionnaire (40), in which cigarette smokers had higher compulsion scores compared to non-smokers.
Table 1.
Demographic, psychiatric, and cannabis use characteristics for the overall study sample and compared between cigarette smokers and non-smokers.
| Overall (n=116) |
Cigarette smokers (n=68) |
Non-smokers (n=48) |
P Value | ||||
|---|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | Mean | Std | ||
| Demographics and Cannabis Use | |||||||
| Age | 18.9 | 1.5 | 18.8 | 1.4 | 19.0 | 1.6 | 0.62 |
| Caucasian % | 83.6 (97) | 80.9 (55) | 87.5 (42) | 0.47 | |||
| Male % | 72.4 (84) | 76.5 (52) | 66.7 (32) | 0.17 | |||
| Years Smoking Cannabis | 4.2 | 1.8 | 4.4 | 2.0 | 3.9 | 1.6 | 0.18 |
| Years Smoking Cigarettes | - | - | 2.9 | 2.5 | - | - | - |
| # of Quit attempts (Cannabis) | 2.4 | 3.4 | 2.5 | 3.5 | 2.2 | 3.4 | 0.57 |
| Grams of Cannabis Used per Day | 1.6 | 2.2 | 1.7 | 1.9 | 1.5 | 2.6 | 0.55 |
| Days of Cannabis Use (Past 30) | 23.3 | 6.7 | 24.0 | 6.9 | 22.5 | 6.2 | 0.25 |
| Positive THC at screening % | 90.5 (105) | 92.6 (63) | 87.5 (42) | 0.27 | |||
| Psychiatric Comorbidity | |||||||
| ADHD % -yes | 6.0 (7) | 5.9 (4) | 6.3 (3) | 0.62 | |||
| CD/ODD % -yes | 6.0 (7) | 7.4 (5) | 4.2 (2) | 0.34 | |||
| MDD % -yes | 7.8 (9) | 8.8 (6) | 6.3 (3) | 0.45 | |||
| Impulsivity | |||||||
| Barratt Impulsiveness Score | 67.5 | 10.1 | 68.3 | 10.0 | 66.3 | 10.4 | 0.29 |
| Marijuana Craving Questionnaire | |||||||
| Total | 47.4 | 14.4 | 49.0 | 14.4 | 45.2 | 14.2 | 0.18 |
| Compulsion | 7.7 | 4.1 | 8.6 | 4.3 | 6.5 | 3.6 | 0.007 |
| Emotion | 12.2 | 5.2 | 12.7 | 5.3 | 11.5 | 4.9 | 0.20 |
| Expectancy | 14.0 | 4.7 | 13.9 | 4.6 | 14.0 | 4.9 | 0.93 |
| Purposefulness | 13.6 | 4.4 | 13.7 | 4.3 | 13.5 | 4.5 | 0.80 |
Notes: THC=Tetrahydrocannabinol; ADHD=Attention deficit hyperactivity disorder; CD/ODD=Conduct/Oppositional Defiant Disorder; MDD=Major Depressive Disorder.
Among cigarette smoking participants, cigarette and cannabis-related characteristics across medication groups (NAC or placebo) are shown in Table 2. Treatment groups were similar in years of cigarette and cannabis use and prior quit attempts, cigs/day, CO, and in their motivation and intention to quit smoking. Nicotine dependence (mFTQ) was slightly higher among those randomized to placebo as compared to NAC, though this difference did not reach significance (p=0.08). Overall, cigarette smokers enrolled in a cannabis cessation trial had been smoking cannabis for an average (SD) of 4.4 (2.0) years and had attempted to quit an average of 2.5 (3.4) times in the past. They had been smoking cigarettes for an average of 2.9 (2.5) years, smoked approximately 6.3 (6.9) cigs/day, and had attempted to quit smoking an average of 2.4 (3.5) times in the past. Approximately half of the sample (46%) were daily smokers, and participants reported smoking on an average (SD) of 21 (11) days out of the past 30 days.
Table 2.
Cigarette and cannabis characteristics for the overall sample of cigarette smokers and compared across NAC and placebo treatment groups.
| Overall (n=68) Mean Std | NAC (n=34) | Placebo (n=34) | P Value | ||||
|---|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | Mean | Std | ||
| Years Smoking Cigarettes | 2.9 | 2.5 | 2.6 | 2.1 | 3.2 | 2.9 | 0.30 |
| Years Smoking Cannabis | 4.4 | 2.0 | 4.3 | 1.7 | 4.5 | 2.3 | 0.67 |
| # of Quit attempts (Cigarettes) | 2.4 | 3.5 | 1.8 | 1.9 | 3.0 | 4.4 | 0.18 |
| # of Quit attempts (Cannabis) | 2.5 | 3.5 | 2.1 | 2.6 | 3.0 | 4.1 | 0.27 |
| Cigarettes Per Day | 6.3 | 6.9 | 5.8 | 7.1 | 6.8 | 6.8 | 0.60 |
| Days of Smoking (Past 30) | 21.0 | 11.0 | 21.3 | 10.7 | 20.2 | 11.2 | 0.69 |
| Daily Smokers -% | 45.6 (31) | 48.4 (15) | 51.6 (16) | 0.50 | |||
| Carbon Monoxide (ppm) | 6.8 | 5.2 | 7.4 | 6.1 | 6.2 | 4.2 | 0.38 |
| Questionnaire of Smoking Urges (QSU) -Total Score | 3.2 | 1.4 | 3.0 | 1.4 | 3.5 | 1.4 | 0.21 |
| QSU -F1 | 4.1 | 1.8 | 3.7 | 1.6 | 4.5 | 1.9 | 0.10 |
| QSU -F2 | 2.2 | 1.2 | 2.1 | 1.3 | 2.3 | 1.1 | 0.50 |
| mFTQ Total Score | 3.4 | 1.5 | 3.1 | 1.3 | 3.8 | 1.6 | 0.08 |
| Intention to quit in 30 days (0 = no intention, 10 = very much intend) | 5.4 | 3.6 | 5.9 | 3.4 | 4.9 | 3.8 | 0.34 |
| Thinking of quitting within 30 days - | 28.6 (16) | 25.0 (7) | 32.1 (9) | 0.64 | |||
| No intention to quit -% | 7.1 (4) | 3.6 (1) | 10.7 (3) | 0.64 | |||
Notes: mFTQ = Modified Fagerström Tolerance Questionnaire.
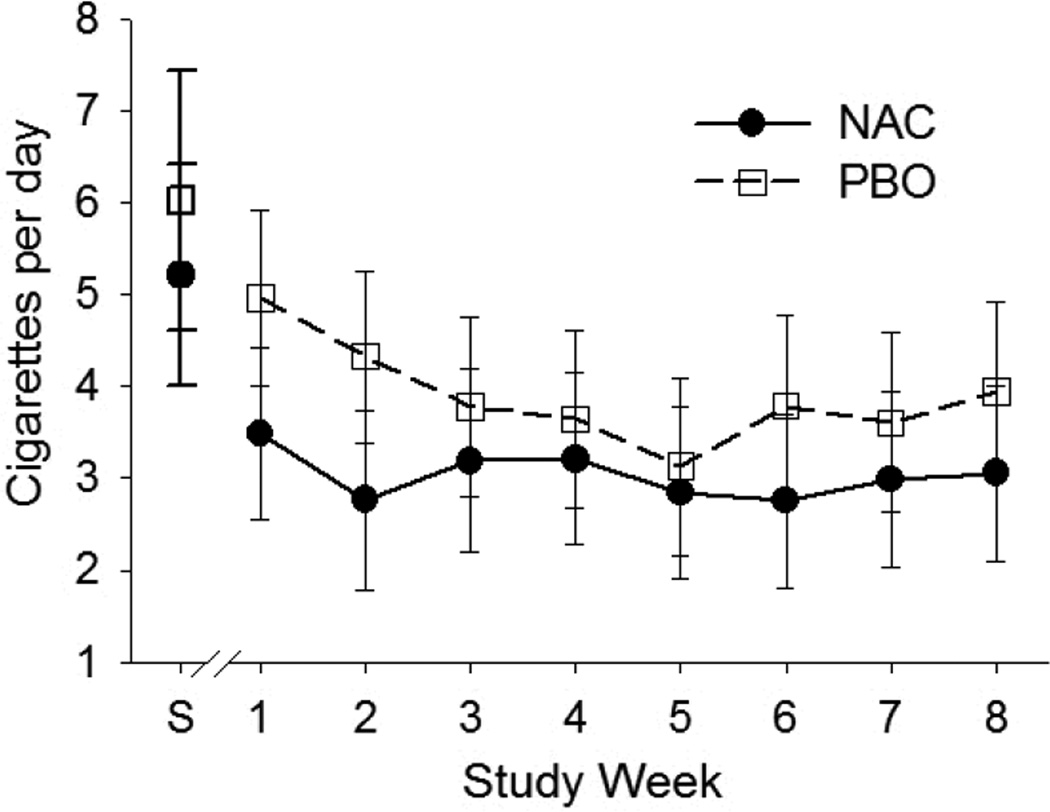
Cigs/day during the 30 days prior to screening and during the active 8-week treatment phase for NAC and placebo groups were compared by treatment assignment and are shown in Figure 1. Participants (with available smoking data) reported smoking approximately 3.6 (4.2) cigs/day across 8 weeks of the active treatment phase. Though there appear to be slight decreases in cigs/day during the 8-week treatment phase, the within subjects time effect was not significant (p=0.19). During active treatment, rates of cigarette smoking at each study visit did not differ between NAC or placebo treatment groups. There were similar rates of cigs/day (F1, 46=0.28; p=0.60), percent of days smoked (F1,46=0.02; p=0.88), and abstinence from smoking (χ21=0.78; p=0.38) across the 8-week active treatment study visits. There were no differences in the pattern of cigs/day (F7, 209=0.51; p=0.82), percent of days smoked (F7, 209=0.79; p=0.59) or overall abstinence (χ27=5.53; p=0.60) over time between the two treatment groups.
Figure 1.
Self-reported cigarettes per day collected the 30 days prior to screening (S) and across the 8-week treatment phase for NAC and placebo (PBO) groups. Averaged cigarettes per day include smoking and non-smoking days. Only participants that completed at least one treatment phase study visit were included here (n=49). Error bars represent standard error of the mean.
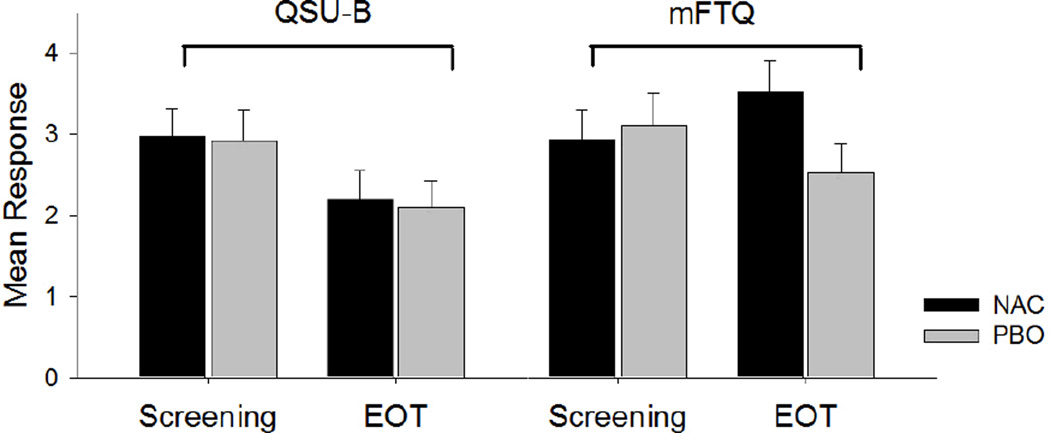
Mean ratings on the QSU-B and mFTQ from the screening visit to the end of treatment (EOT; Week 8) are shown for NAC and placebo groups in Figure 2. The overall decrease in QSU-B score was significant (F1,27=7.0, p=0.02), but the mean decrease in QSU-B score was not different between the NAC and placebo groups (F1,27=0.01, p=0.93). The mean change in mFTQ score was moderately different between the NAC and placebo groups from screening to the end of treatment (F1,26=5.1, p=0.03). These results indicate that participants in the placebo group had a more pronounced decrease in mFTQ score over the course of treatment than those in the NAC group, though this did not translate to a greater reduction in cigs/day over the course of treatment.
Figure 2.
Mean ratings on the Questionnaire on Smoking Urges-Brief (QSU-B) and the Modified Fagerström Tolerance Questionnaire (mFTQ) from the screening visit to the end of treatment (EOT; Week 8) for NAC (n=15) and Placebo (PBO; n=17) groups. Error bars represent standard error of the mean.
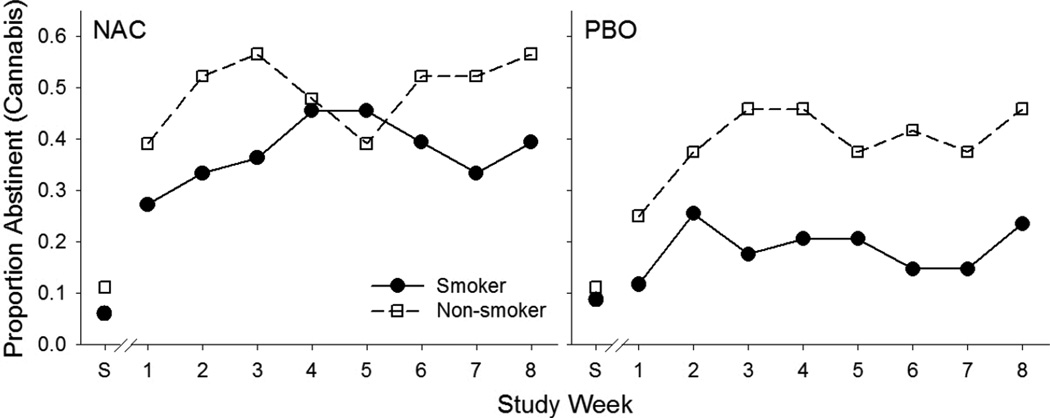
Cannabis abstinence outcomes compared between cigarette smokers and non-smokers during the 8-week treatment phase are shown in Figure 3 stratified by treatment group assignment. For the entire study cohort, cigarette smokers had slightly lower rates of cannabis abstinence during the study period (RR=0.81; 95% CI: 0.62–1.05; p=0.101). Among the participants randomized to NAC, being a cigarette smoker did not affect abstinence from cannabis during the 8-week treatment intervention (RR=0.87; 95% CI: 0.58–1.30; p=0.427). Among participants randomized to placebo, cigarette smokers had slightly lower rates of cannabis abstinence compared to non-smokers, though this finding failed to reach statistical significance (RR=0.76; 95% CI: 0.56–1.03; p=0.073).
Figure 3.
Proportion of participants abstinent from cannabis at screening (S) and across study week for cigarette smokers and non-smokers separated by NAC and PBO groups. All participants were included in this analysis (NAC cigarette smokers, n=34; NAC cigarette non-smokers, n=34; PBO cigarette smokers, n=24; PBO cigarette non-smokers, n=24).
Discussion
Following 8 weeks of treatment for cannabis dependence with NAC or placebo in adolescents, the current findings demonstrated no increase in cigarette smoking, indicating that participants were not substituting tobacco during a cannabis cessation attempt. This differs from increases in tobacco use during cannabis abstinence or reductions found in other studies (28,29). Those preliminary studies, however, relied on self-report, were retrospective, and the cannabis quit attempts were most likely unassisted. In contrast, the current study provided pharmacological and behavioral treatment for cannabis dependence, and though cigarette smoking was not specifically targeted, some strategies may have generalized to cigarette smoking to reduce increases in use. This study also found no evidence that being a cigarette smoker influenced the efficacy of NAC to aid in cannabis cessation. Within the placebo group, cigarette smokers appeared to have slightly lower rates of abstinence compared to non-smokers. This result did not reach statistical significance and the study was not adequately powered to detect this finding.
Decreases in the urge to smoke cigarettes as indicated by reductions in ratings on the QSU-B were found, but did not differ among treatment groups, and did not correspond to reductions in cigarette smoking. The reductions in cigarette craving found in the current report are similar to findings in a previous report from this study cohort that showed reductions in cannabis craving across the treatment period, though those craving data also did not differ across treatment groups (41). These findings suggest that craving ratings for both cannabis and cigarettes reduced over time, but was not related to treatment condition and not attributable to NAC. Rather, this could be due to the added attention given to substance use by research staff, motivational enhancement through counseling or contingency management, or simply an effect of time.
There is justification in the literature to support the simultaneous treatment of cannabis and tobacco dependence. Evidence from the substance abuse treatment literature has shown that smoking cessation interventions provided during a treatment episode improves the likelihood of prolonged abstinence from drugs and alcohol (42). Among adolescents, cigarette smokers have been shown to have a greater likelihood of relapse to alcohol and cannabis during a treatment episode (25). There is also a demonstrated pattern of cannabis use preceding tobacco initiation and regular use in the literature (22). The current study found that among enrolled participants, years of regular cannabis use averaged around four years, while years of regular cannabis smoking averaged about three years. Cannabis use that precedes and potentially contributes to tobacco use represents perhaps the most detrimental adverse effect of early cannabis use, and is especially relevant for an adolescent population that will most likely continue smoking into adulthood. Tobacco exposure in cannabis-dependent individuals may come from sources other than cigarettes as well. Blunts are growing increasingly common, especially among young adults (ages 18–25) (43). Electronic cigarettes are also becoming popular as a means of cannabis administration; though no prevalence data currently exist on this practice. Tobacco use in forms other than cigarettes should not be overlooked in cannabis research and treatment.
Efficacy trials for cannabis cessation rarely address tobacco use as part of the formal intervention and interventions that address cannabis have not yet been modified and tested to evaluate tobacco intervention as well (44). It is not clear how targeting both substances may influence treatment outcomes. It has been shown that more severe withdrawal symptoms have been demonstrated from tobacco and cannabis cessation more so than from the withdrawal produced by each substance alone (45). Exacerbation of withdrawal may play a large role in relapse and should be carefully considered when developing dual-substance treatment interventions. A recent pilot study evaluating treatment for both cannabis and tobacco found that cognitive behavioral therapy targeting both cannabis and tobacco, along with nicotine replacement patches, resulted in decreases in tobacco use, but not in cannabis use (46). That study found no increases in cannabis use associated with decreases in tobacco use, suggesting that dual treatments are safe to pursue, although dual pharmacotherapy may also be required in addition to behavioral interventions.
There were several limitations to the current analysis. First, attrition rates were high, which limited the available data for cigs/day across the treatment period. Only 72% of data from cigarette smokers could be used to assess changes in cigs/day, and the median (interquartile range) number of study visits following randomization was 6 (5–7) of a possible 8 for those retained. Given the amount of missing data due to high attrition, multiple imputation (MI) analyses were conducted in concert with maximum likelihood methods. The MI models confirmed our results in the presence of poor retention rates. Second, this study was not specifically designed or adequately powered to assess cigarette smoking as a predictor of cannabis treatment success. The study was also not designed to assess for changes in cigarette smoking, as cannabis abstinence was the primary outcome. As such, any trends towards significance should be interpreted with caution. Finally, cigarette smoking was not being specifically targeted in this study, which may be a necessary condition to see reductions in use. Medical clinicians met with participants at each study visit, but tobacco use was rarely addressed, unless the participant expressed motivation to reduce or quit tobacco.
The majority of cannabis-dependent adolescents who were enrolled in this study were current cigarette smokers (59%). Their rates of cigarette smoking did not change over the course of the study intervention, which provides evidence that participants were not substituting tobacco during cannabis cessation. This also reveals that without targeted and specific interventions, cigarette smoking most likely will not reduce during cannabis cessation. Further work should address interventions that target both tobacco and cannabis to determine how treatment success is impacted. The parent trial, from which this secondary analysis was derived, showed that NAC increased the odds of abstinence from cannabis (31). There is also preclinical literature on the glutamatergic system as a potential pharmacotherapeutic target in the treatment of nicotine dependence (32,33) and preliminary clinical studies that have shown that NAC may hold promise as a smoking cessation pharmacotherapy (34,35). Based on those results, NAC may hold the potential to be used as a pharmacotherapy for both cannabis and tobacco dependence. However, NAC may require pairing with comprehensive and specialized psychosocial treatment focused on reducing the use of several substances concurrently. Participants enrolled in the current study showed interest in cannabis cessation with the use of pharmacotherapy, and may have been amenable to smoking cessation as well with or without pharmacotherapy. Adolescent tobacco-cannabis co-users are a potentially vulnerable group and research efforts should be aggressively focused on their poly-substance use, improving their motivation to quit with evidence-based strategies, cessation efforts, and dual interventions to improve treatment outcomes.
Acknowledgements
The authors wish to acknowledge the funding sources for this study. Funding was provided by NIDA grant R01DA026777 via the American Recovery and Reinvestment Act of 2009 (PI, Kevin M. Gray). Additional funding was also provided by the South Carolina Clinical and Translational Institute at the Medical University of South Carolina (UL1TR000062), and from NIDA grants U01DA031779 and U10DA013727. The funding source had no role other than financial support. The authors also wish to thank the clinical research team at the Medical University of South Carolina, including Christine Horne, Jessica Lydiard, Sarah Farber, Jessica Olsen, and Priscilla Muldrow.
Footnotes
Declaration of Interest. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper. All authors contributed to the design and execution of the study, analyses of data, and manuscript preparation. All authors have read and approved the manuscript.
References
- 1.Centers for Disease Control and Prevention (CDC) Smoking-attributable mortality, years of potential life lost, and productivity losses--united states, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–1228. [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. Preventing tobacco use among youth and young adults: A report of the surgeon general. 2012 [PubMed] [Google Scholar]
- 3.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on drug use: 2012 overview, key findings on adolescent drug use. 2013 [Google Scholar]
- 4.Budney AJ, Moore BA, Vandrey R. Health consequences of marijuana use. In: Brick J, editor. Handbook of the medical consequences of drug abuse. Binghamton, NY: Haworth Press; 2008. pp. 171–218. [Google Scholar]
- 5.Chen CY, Anthony JC. Possible age-associated bias in reporting of clinical features of drug dependence: Epidemiological evidence on adolescent-onset marijuana use. Addiction. 2003;98(1):71–82. doi: 10.1046/j.1360-0443.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 6.Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009;92(4):559–565. doi: 10.1016/j.pbb.2009.04.001. doi:10.1016/j.pbb.2009.04.001; 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: An overview of animal and human research. Curr Drug Abuse Rev. 2008;1(2):114–123. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- 8.Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):E2657–E2664. doi: 10.1073/pnas.1206820109. doi:10.1073/pnas.1206820109; 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rey JM, Martin A, Krabman P. Is the party over? cannabis and juvenile psychiatric disorder: The past 10 years. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1194–1205. doi: 10.1097/01.chi.0000135623.12843.60. [DOI] [PubMed] [Google Scholar]
- 10.Substance Abuse and Mental Health Services Administration. Results from the 2011 national survey on drug use and health: Summary of national findings. 2012:12–4713. NSDUH Series H-44. [PubMed] [Google Scholar]
- 11.Agrawal A, Budney AJ, Lynskey MT. The co-occurring use and misuse of cannabis and tobacco: A review. Addiction. 2012;107(7):1221–1233. doi: 10.1111/j.1360-0443.2012.03837.x. doi:10.1111/j.1360-0443.2012.03837.x; 10.1111/j.1360-0443.2012.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal A, Lynskey MT. Tobacco and cannabis co-occurrence: Does route of administration matter? Drug Alcohol Depend. 2009;99(1–3):240–247. doi: 10.1016/j.drugalcdep.2008.08.007. doi:10.1016/j.drugalcdep.2008.08.007; 10.1016/j.drugalcdep.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leatherdale ST, Ahmed R, Kaiserman M. Marijuana use by tobacco smokers and nonsmokers: Who is smoking what? CMAJ. 2006;174(10):1399". doi: 10.1503/cmaj.051614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin CS, Clifford PR, Clapper RL. Patterns and predictors of simultaneous and concurrent use of alcohol, tobacco, marijuana, and hallucinogens in first-year college students. J Subst Abuse. 1992;4(3):319–326. doi: 10.1016/0899-3289(92)90039-z. [DOI] [PubMed] [Google Scholar]
- 15.Richter KP, Kaur H, Resnicow K, Nazir N, Mosier MC, Ahluwalia JS. Cigarette smoking among marijuana users in the united states. Subst Abus. 2004;25(2):35–43. doi: 10.1300/j465v25n02_06. [DOI] [PubMed] [Google Scholar]
- 16.Rigotti NA, Lee JE, Wechsler H. US college students' use of tobacco products: Results of a national survey. JAMA. 2000;284(6):699–705. doi: 10.1001/jama.284.6.699. [DOI] [PubMed] [Google Scholar]
- 17.Leatherdale ST, Hammond DG, Kaiserman M, Ahmed R. Marijuana and tobacco use among young adults in canada: Are they smoking what we think they are smoking? Cancer Causes Control. 2007;18(4):391–397. doi: 10.1007/s10552-006-0103-x. [DOI] [PubMed] [Google Scholar]
- 18.Tullis LM, Dupont R, Frost-Pineda K, Gold MS. Marijuana and tobacco: A major connection? J Addict Dis. 2003;22(3):51–62. doi: 10.1300/J069v22n03_05. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal A, Lynskey MT, Pergadia ML, et al. Early cannabis use and DSM-IV nicotine dependence: A twin study. Addiction. 2008;103(11):1896–1904. doi: 10.1111/j.1360-0443.2008.02354.x. doi:10.1111/j.1360-0443.2008.02354.x; 10.1111/j.1360-0443.2008.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramo DE, Liu H, Prochaska JJ. Tobacco and marijuana use among adolescents and young adults: A systematic review of their co-use. Clin Psychol Rev. 2012;32(2):105–121. doi: 10.1016/j.cpr.2011.12.002. doi:10.1016/j.cpr.2011.12.002; 10.1016/j.cpr.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters EN, Schwartz RP, Wang S, O'Grady KE, Blanco C. Psychiatric, psychosocial, and physical health correlates of co-occurring cannabis use disorders and nicotine dependence. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2013.10.003. doi:10.1016/j.drugalcdep.2013.10.003; 10.1016/j.drugalcdep.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction. 2005;100(10):1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- 23.Amos A, Wiltshire S, Bostock Y, Haw S, McNeill A. 'You can't go without a fag...you need it for your hash'--a qualitative exploration of smoking, cannabis and young people. Addiction. 2004;99(1):77–81. doi: 10.1111/j.1360-0443.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 24.Highet G. The role of cannabis in supporting young people's cigarette smoking: A qualitative exploration. Health Educ Res. 2004;19(6):635–643. doi: 10.1093/her/cyg089. [DOI] [PubMed] [Google Scholar]
- 25.de Dios MA, Vaughan EL, Stanton CA, Niaura R. Adolescent tobacco use and substance abuse treatment outcomes. J Subst Abuse Treat. 2009;37(1):17–24. doi: 10.1016/j.jsat.2008.09.006. doi:10.1016/j.jsat.2008.09.006; 10.1016/j.jsat.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haney M, Bedi G, Cooper ZD, et al. Predictors of marijuana relapse in the human laboratory: Robust impact of tobacco cigarette smoking status. Biol Psychiatry. 2013;73(3):242–248. doi: 10.1016/j.biopsych.2012.07.028. doi:10.1016/j.biopsych.2012.07.028; 10.1016/j.biopsych.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: A systematic review. Addiction. 2012;107(8):1404–1417. doi: 10.1111/j.1360-0443.2012.03843.x. doi:10.1111/j.1360-0443.2012.03843.x; 10.1111/j.1360-0443.2012.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copersino ML, Boyd SJ, Tashkin DP, et al. Quitting among non-treatment-seeking marijuana users: Reasons and changes in other substance use. Am J Addict. 2006;15(4):297–302. doi: 10.1080/10550490600754341. [DOI] [PubMed] [Google Scholar]
- 29.Schaub M, Gmel G, Annaheim B, Mueller M, Schwappach D. Leisure time activities that predict initiation, progression and reduction of cannabis use: A prospective, population-based panel survey. Drug Alcohol Rev. 2010;29(4):378–384. doi: 10.1111/j.1465-3362.2009.00156.x. doi:10.1111/j.1465-3362.2009.00156.x; 10.1111/j.1465-3362.2009.00156.x. [DOI] [PubMed] [Google Scholar]
- 30.Gray KM, Riggs PD, Min SJ, Mikulich-Gilbertson SK, Bandyopadhyay D, Winhusen T. Cigarette and cannabis use trajectories among adolescents in treatment for attention-deficit/hyperactivity disorder and substance use disorders. Drug Alcohol Depend. 2011;117(2–3):242–247. doi: 10.1016/j.drugalcdep.2011.02.005. doi:10.1016/j.drugalcdep.2011.02.005; 10.1016/j.drugalcdep.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805–812. doi: 10.1176/appi.ajp.2012.12010055. doi:10.1176/appi.ajp.2012.12010055; 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gipson CD, Reissner KJ, Kupchik YM, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A. 2013;110(22):9124–9129. doi: 10.1073/pnas.1220591110. doi:10.1073/pnas.1220591110; 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez-Nino AM, D'Souza MS, Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: Comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology (Berl) 2013;225(2):473–482. doi: 10.1007/s00213-012-2837-3. doi:10.1007/s00213-012-2837-3; 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knackstedt LA, LaRowe S, Mardikian P, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841–845. doi: 10.1016/j.biopsych.2008.10.040. doi:10.1016/j.biopsych.2008.10.040; 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmaal L, Berk L, Hulstijn KP, Cousijn J, Wiers RW, van den Brink W. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: A double-blind placebo-controlled pilot study. Eur Addict Res. 2011;17(4):211–216. doi: 10.1159/000327682. doi:10.1159/000327682; 10.1159/000327682. [DOI] [PubMed] [Google Scholar]
- 36.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: Assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83(4):393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 37.Prokhorov AV, De Moor C, Pallonen UE, Hudmon KS, Koehly L, Hu S. Validation of the modified fagerstrom tolerance questionnaire with salivary cotinine among adolescents. Addict Behav. 2000;25(3):429–433. doi: 10.1016/s0306-4603(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 38.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 39.Molenberghs G, Kenward MG. Missing data in clinical studies. 1st Edition ed. Chichester, UK: John Wiley & Sons, Ltd; 2007. [Google Scholar]
- 40.Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the marijuana craving questionnaire. Drug Alcohol Depend. 2009;102(1–3):35–40. doi: 10.1016/j.drugalcdep.2008.12.010. doi:10.1016/j.drugalcdep.2008.12.010; 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788–1791. doi: 10.1016/j.addbeh.2012.11.003. doi:10.1016/j.addbeh.2012.11.003; 10.1016/j.addbeh.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 43.Cullen J, Mowery P, Delnevo C, et al. Seven-year patterns in US cigar use epidemiology among young adults aged 18–25 years: A focus on race/ethnicity and brand. Am J Public Health. 2011;101(10):1955–1962. doi: 10.2105/AJPH.2011.300209. doi:10.2105/AJPH.2011.300209; 10.2105/AJPH.2011.300209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4(1):4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92(1–3):48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill KP, Toto LH, Lukas SE, et al. Cognitive behavioral therapy and the nicotine transdermal patch for dual nicotine and cannabis dependence: A pilot study. Am J Addict. 2013;22(3):233–238. doi: 10.1111/j.1521-0391.2012.12007.x. doi:10.1111/j.1521-0391.2012.12007.x; 10.1111/j.1521-0391.2012.12007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]