Abstract
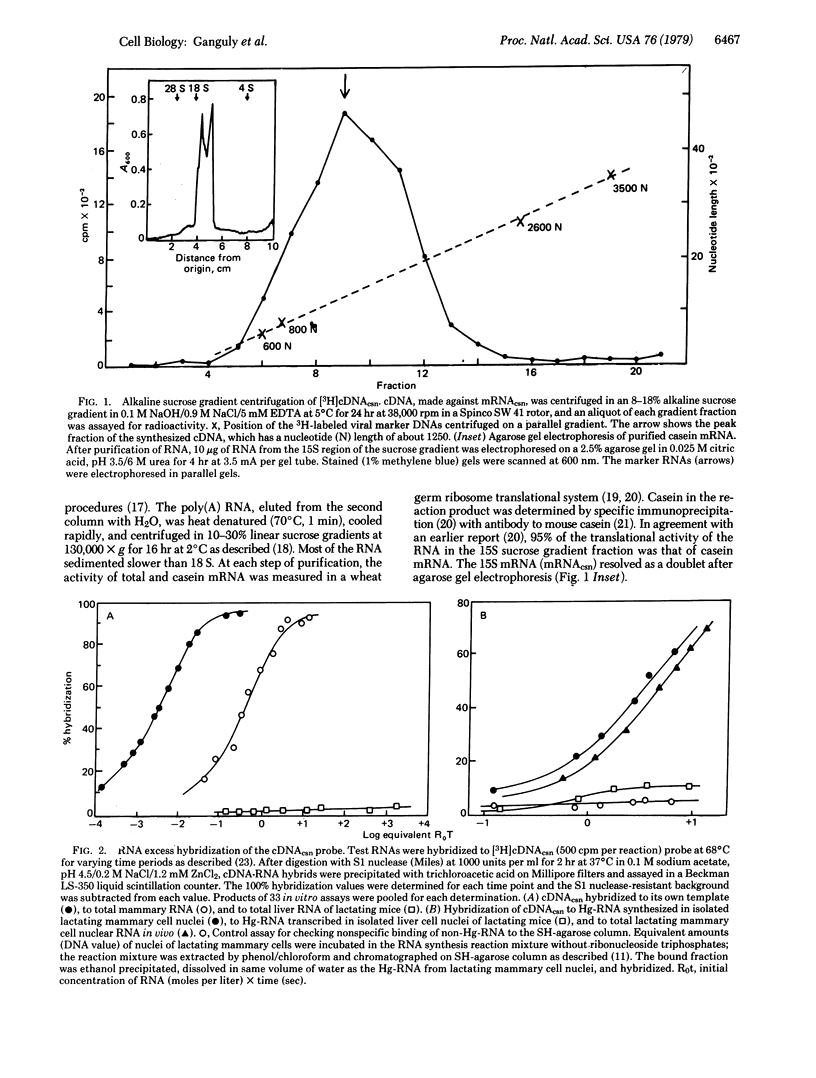
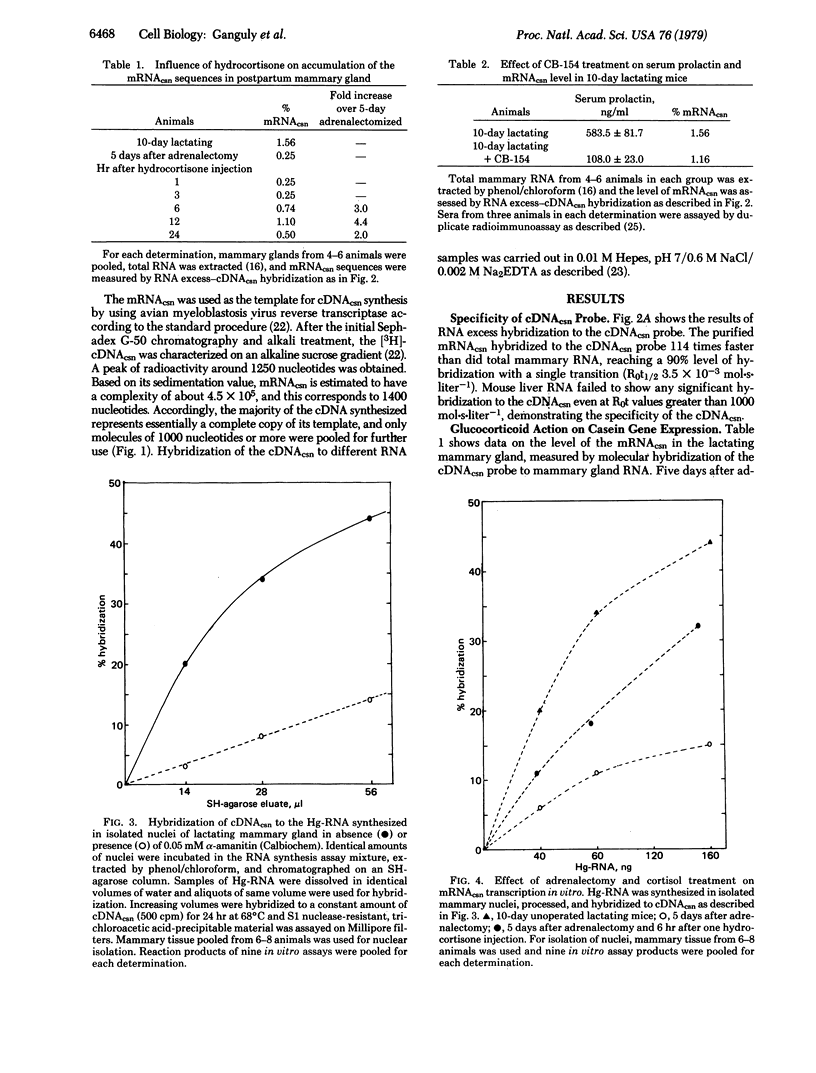
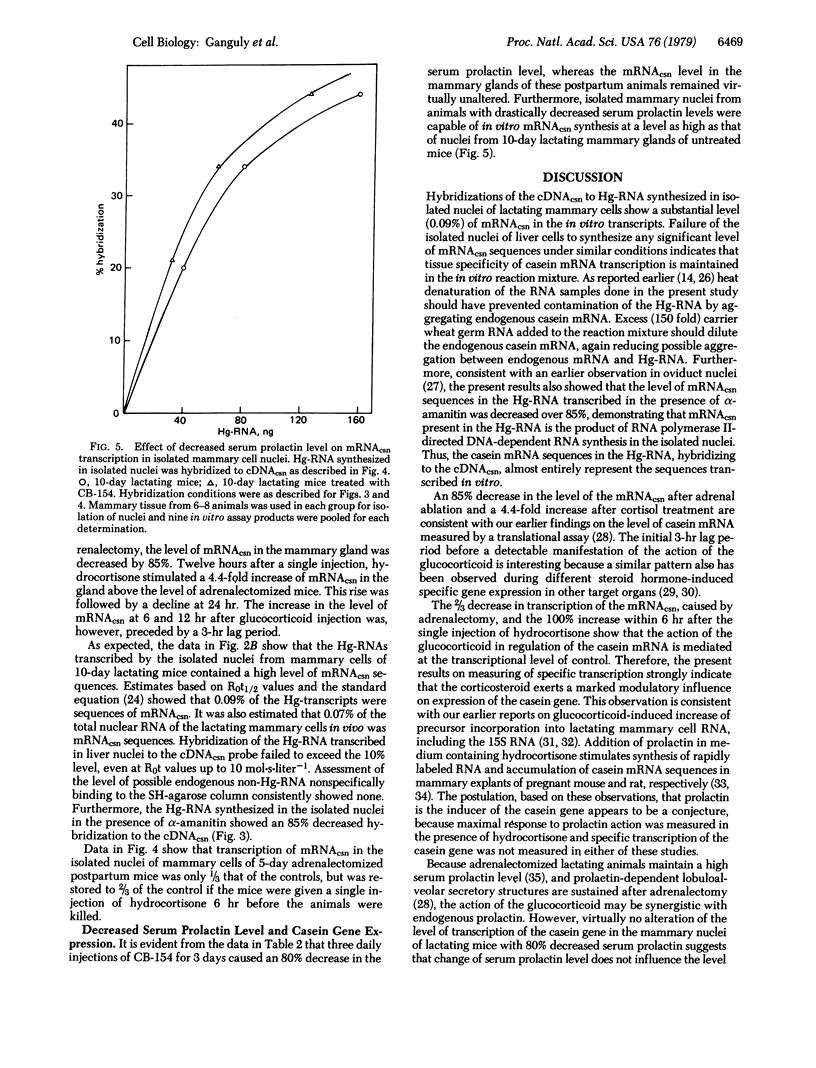
The influence of cortisol and prolactin on casein gene expression in the mammary gland of lactating BALB/c mice was measured by using a specific cDNA probe to 15S casein mRNA (cDNAcsn). Casein mRNA (mRNAcsn) level in the mammary gland was decreased by 85% 5 days after adrenal ablation, but then was increased 4.4-fold 12 hr after a single injection of hydrocortisone-21-acetate. An 80% decrease in serum prolactin level, induced by the prolactin inhibitor 2-bromo-alpha-ergocryptin (CB-154), did not alter the level of mRNAcsn in the gland. Specific transcription of the casein gene in nuclei isolated from lactating mammary glands was measured by cDNAcsn hybridization to the in vitro synthesized Hg-CTP-containing RNA (Hg-RNA), which was purified by SH-agarose chromatography. The level of the mRNAcsn in Hg-RNA synthesized in the isolated nuclei was 0.09% and this was decreased 85% by alpha-amanitin, indicating that the mRNAcsn sequences in the Hg-RNA were the products of RNA polymerase II-directed DNA-dependent RNA synthesis. Transcription of the mRNAcsn in isolated nuclei was decreased by 70% 5 days after adrenalectomy and a single injection of the glucocorticoid then increased the transcription level 2-fold at 6 hr. Essentially no alteration of the level of transcription was detectable in mammary nuclei isolated from lactating mice with 80% decreased serum prolactin level, induced by CB-154 treatment. The results thus demonstrate a glucocorticoid involvement on the modulation of casein gene expression at the transcriptional level of control.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M. R. Responses of mammary cells to hormones. Int Rev Cytol. 1976;47:1–97. doi: 10.1016/s0074-7696(08)60086-8. [DOI] [PubMed] [Google Scholar]
- Banerjee M. R., Rogers F. M., Banerjee D. N. Hormonal regulation of RNA and protein synthesis in the mouse mammary gland before and during lactation. J Endocrinol. 1971 Jun;50(2):281–291. doi: 10.1677/joe.0.0500281. [DOI] [PubMed] [Google Scholar]
- Banerjee M. R., Terry P. M., Sakai S., Lin F. K., Ganguly R. Hormonal regulation of casein messenger RNA (mRNA). In Vitro. 1978 Jan;14(1):128–139. doi: 10.1007/BF02618179. [DOI] [PubMed] [Google Scholar]
- Ben-David M., Danon A., Benveniste R., Weller C. P., Sulman F. G. Results of radioimmunoassays of rat pituitary and serum prolactin after adrenalectomy and perphenazine treatment in rats. J Endocrinol. 1971 Aug;50(4):599–606. doi: 10.1677/joe.0.0500599. [DOI] [PubMed] [Google Scholar]
- Chambon P. Summary: the molecular biology of the eukaryotic genome is coming of age. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1209–1234. doi: 10.1101/sqb.1978.042.01.122. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Feigelson P. Glucocorticoid induction of alpha2u-globulin protein synthesis and its mRNA in rat hepatocytes in vitro. J Biol Chem. 1978 Nov 10;253(21):7880–7885. [PubMed] [Google Scholar]
- Cox R. F., Haines M. E., Emtage J. S. Quantitation of ovalbumin mRNA in hen and chick oviduct by hybridization to complementary DNA. Accumulation of specific mRNA in response to estradiol. Eur J Biochem. 1974 Nov 1;49(1):225–236. doi: 10.1111/j.1432-1033.1974.tb03827.x. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- Ganguly R., Banerjee M. R. RNA synthesis in isolated nuclei of lactating mammary cells in presence of unmodified and mercury-labeled CTP. Nucleic Acids Res. 1978 Nov;5(11):4463–4477. doi: 10.1093/nar/5.11.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. E., Rosen J. M., Means A. R., O'Malley B. W. Use of a specific probe for ovalbumin messenger RNA to quantitate estrogen-induced gene transcripts. Biochemistry. 1975 May 20;14(10):2072–2081. doi: 10.1021/bi00681a006. [DOI] [PubMed] [Google Scholar]
- Juergens W. G., Stockdale F. E., Topper Y. J., Elias J. J. Hormone-dependent differentiation of mammary gland in vitro. Proc Natl Acad Sci U S A. 1965 Aug;54(2):629–634. doi: 10.1073/pnas.54.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYONS W. R., LI C. H., JOHNSON R. E. The hormonal control of mammary growth and lactation. Recent Prog Horm Res. 1958;14:219–254. [PubMed] [Google Scholar]
- Matusik R. J., Rosen J. M. Prolactin induction of casein mRNA in organ culture. A model system for studying peptide hormone regulation of gene expression. J Biol Chem. 1978 Apr 10;253(7):2343–2347. [PubMed] [Google Scholar]
- McKnight G. S. The induction of ovalbumin and conalbumin mRNA by estrogen and progesterone in chick oviduct explant cultures. Cell. 1978 Jun;14(2):403–413. doi: 10.1016/0092-8674(78)90125-3. [DOI] [PubMed] [Google Scholar]
- NANDI S., BERN H. A. The hormones responsible for lactogenesis in BALB/cCrgl mice. Gen Comp Endocrinol. 1961 Sep;1:195–210. doi: 10.1016/0016-6480(61)90029-6. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Tsai M. J., Tsai S. Y., Towle H. C. Regulation of gene expression in chick oviduct. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):605–615. doi: 10.1101/sqb.1978.042.01.063. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. M., Barker S. W. Quantitation of casein messenger ribonucleic acid sequences using a specific complementary DNA hybridization probe. Biochemistry. 1976 Nov 30;15(24):5272–5280. doi: 10.1021/bi00669a012. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Harris S. E., Rosenfeld G. C., Liarakos C. D., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. 3. Hybridization studies with (3H) messenger RNA and (3H) complementary DNA under conditions of DNA excess. Cell Differ. 1974 Jul;3(2):103–116. doi: 10.1016/0045-6039(74)90032-3. [DOI] [PubMed] [Google Scholar]
- Rosen J. M. Isolation and characterization of purified rat casein messenger ribonucleic acids. Biochemistry. 1976 Nov 30;15(24):5263–5271. doi: 10.1021/bi00669a011. [DOI] [PubMed] [Google Scholar]
- Schütz G., Nguyen-Huu M. C., Giesecke K., Hynes N. E., Groner B., Wurtz T., Sippel A. E. Hormonal control of egg white protein messenger RNA synthesis in the chicken oviduct. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):617–624. doi: 10.1101/sqb.1978.042.01.064. [DOI] [PubMed] [Google Scholar]
- Shyamala G., Dickson C. Relationship between receptor and mammary tumour virus production after stimulation by glucocorticoid. Nature. 1976 Jul 8;262(5564):107–112. doi: 10.1038/262107a0. [DOI] [PubMed] [Google Scholar]
- Shyamala G. Specific cytoplasmic glucocorticoid hormone receptors in lactating mammary glands. Biochemistry. 1973 Jul 31;12(16):3085–3090. doi: 10.1021/bi00740a022. [DOI] [PubMed] [Google Scholar]
- Sinha Y. N., Selby F. W., Lewis U. J., VanderLaan W. P. Studies of prolactin secretion in mice by a homologous radioimmunoassay. Endocrinology. 1972 Oct;91(4):1045–1053. doi: 10.1210/endo-91-4-1045. [DOI] [PubMed] [Google Scholar]
- Terry P. M., Ball E. M., Ganguly R., Banerjee M. R. An indirect radioimmunoassay for mouse casein using 125I-labeled antigen. J Immunol Methods. 1975 Dec;9(2):123–134. doi: 10.1016/0022-1759(75)90102-7. [DOI] [PubMed] [Google Scholar]
- Terry P. M., Banerjee M. R., Lui R. M. Hormone-inducible casein messenger RNA in a serum-free organ culture of whole mammary gland. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2441–2445. doi: 10.1073/pnas.74.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry P. M., Lin F. K., Banerjee M. R. Responses of mouse mammary gland casein mRNA to corticosteroid action and suckling. Mol Cell Endocrinol. 1977 Dec;9(2):169–182. doi: 10.1016/0303-7207(77)90118-6. [DOI] [PubMed] [Google Scholar]
- Topper Y. J. Multiple hormone interactions in the development of mammary gland in vitro. Recent Prog Horm Res. 1970;26:287–308. doi: 10.1016/b978-0-12-571126-5.50011-x. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., Tsai S. Y., Chang C. W., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. In vitro transcription of the ovalbumin gene. Biochim Biophys Acta. 1978 Dec 21;521(2):689–707. doi: 10.1016/0005-2787(78)90309-x. [DOI] [PubMed] [Google Scholar]
- Turkington R. W. Hormonal regulation of rapidly labeled ribonucleic acid in mammary cells in vitro. J Biol Chem. 1970 Dec 25;245(24):6690–6697. [PubMed] [Google Scholar]
- Turkington R. W., Majumder G. C., Kadoama N., MacIndoe J. H., Frantz W. L. Hormonal regulation of gene expression in mammary cells. Recent Prog Horm Res. 1973;29:417–455. doi: 10.1016/b978-0-12-571129-6.50015-4. [DOI] [PubMed] [Google Scholar]
- Welsch C. W., Squiers M. D., Cassell E., Chen C. L., Meites J. Median eminence lesions and serum prolactin: influence of ovariectomy and ergocornine. Am J Physiol. 1971 Dec;221(6):1714–1717. doi: 10.1152/ajplegacy.1971.221.6.1714. [DOI] [PubMed] [Google Scholar]


