Abstract
Ca-dependent affinity chromatography on phenothiazine-Sepharose 4B has been used to isolate a pure protein from the ciliate Tetrahymena pyriformis. This protein has been identified as calmodulin by demonstrating three of the Ca-dependent activities attributed to calmodulins. Tetrahymena calmodulin also has physicochemical properties similar to those of the previously characterized mammalian, coelenterate, and plant proteins, except for a lower molecular weight (15,000) and slightly different CNBr fragments compared to bovine brain calmodulin. Calmodulin is a constituent of demembranated Tetrahymena cilia from which it can be extracted with the crude dynein fraction. Sucrose density gradient fractionation indicated its presence in fractions containing the 14S dynein ATPase. It is concluded that the essential properties of calmodulin have been highly conserved during much of eukaryotic evolution, and it is suggested that calmodulin plays a role in the control of ciliary motility in Tetrahymena.
Full text
PDF
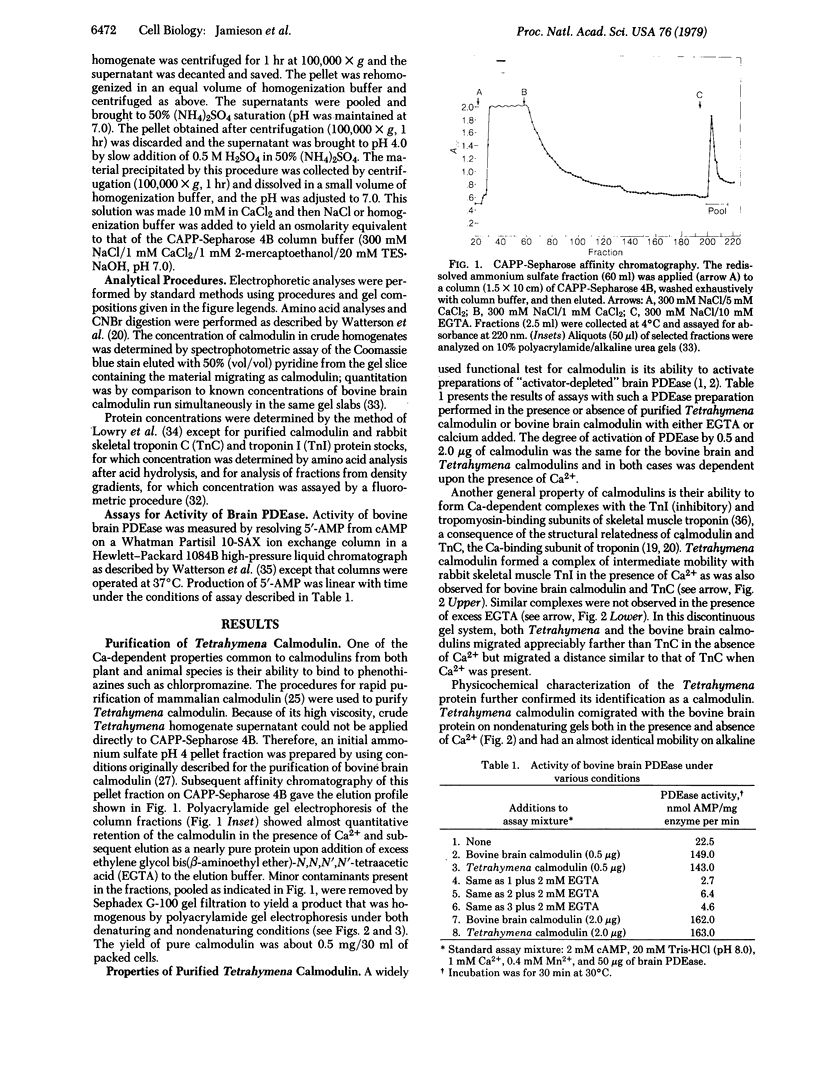

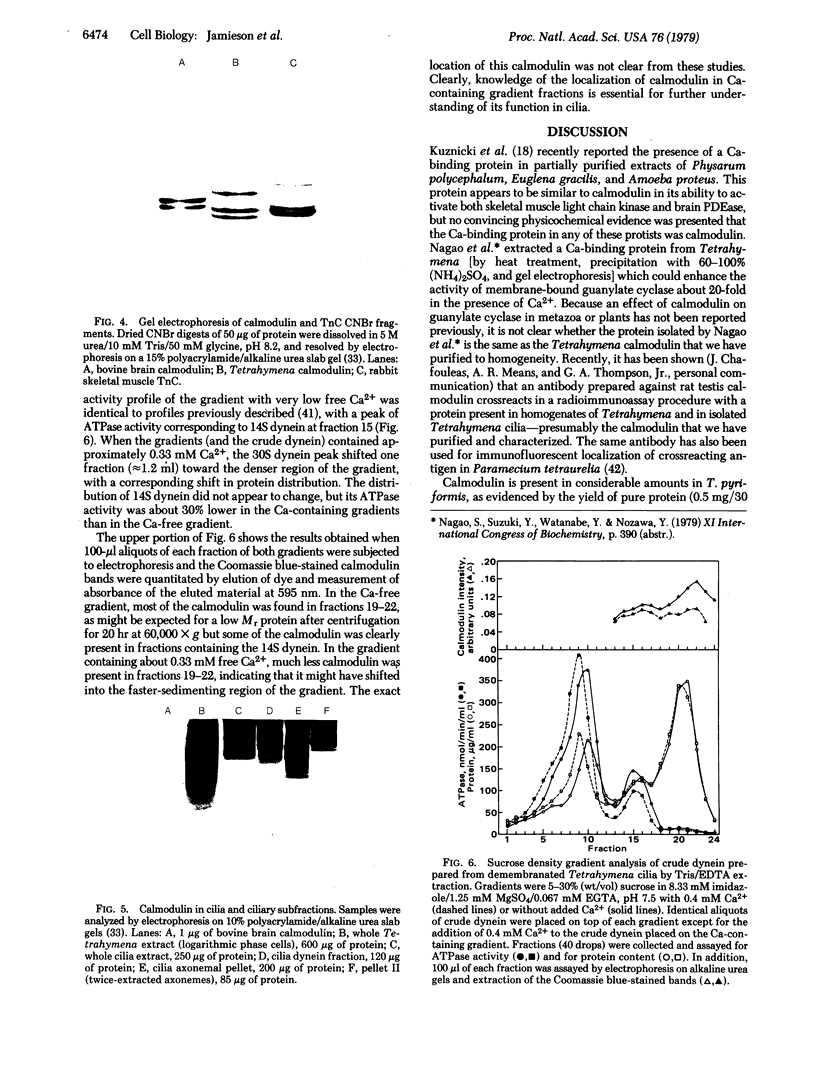

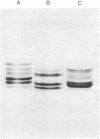
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Vanaman T. C., Perry S. V. Effect of the troponin C-like protein from bovine brain (brain modulator protein) on the Mg2+-stimulated ATPase of skeletal muscle actinomyosin. FEBS Lett. 1976 Dec 15;72(1):163–168. doi: 10.1016/0014-5793(76)80836-8. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Cormier M. J. Calcium-dependent regulation of NAD kinase. Biochem Biophys Res Commun. 1978 Oct 16;84(3):595–602. doi: 10.1016/0006-291x(78)90747-7. [DOI] [PubMed] [Google Scholar]
- Blum J. J. ATPase activity of Tetrahymena cilia before and after extraction of dynein. Arch Biochem Biophys. 1973 May;156(1):310–320. doi: 10.1016/0003-9861(73)90369-x. [DOI] [PubMed] [Google Scholar]
- Blum J. J., Hayes A. A comparison of the effects of gentle heating, acetone, and the sulfhydryl reagent bis (4-fluoro-3-nitrophenyl) sulfone on the ATPase activity and pellet height response of tetrahymena cilia. J Supramol Struct. 1977;6(2):155–167. doi: 10.1002/jss.400060202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. J., Hayes A. Effect of calcium on the pellet height response of Tetrahymena cilia. J Supramol Struct. 1977;7(2):205–211. doi: 10.1002/jss.400070205. [DOI] [PubMed] [Google Scholar]
- Blum J. J., Hayes A. Some changes in the properties of dynein ATPase in situ and after extraction following heat treatment of cilia. J Supramol Struct. 1976;5(1):15–25. doi: 10.1002/jss.400050103. [DOI] [PubMed] [Google Scholar]
- Blum J. J., Hines M. Biophysics of flagellar motility. Q Rev Biophys. 1979 May;12(2):103–180. doi: 10.1017/s0033583500002742. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B. J., Byrne B. C. Behavior and the excitable membrane in Paramecium. CRC Crit Rev Microbiol. 1978 Sep;6(1):53–108. doi: 10.3109/10408417809090620. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Cormier M. J. Purification of plant calmodulin by fluphenazine-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1039–1047. doi: 10.1016/0006-291x(79)91931-4. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y., Bradham L. S., Lynch T. J., Lin Y. M., Tallant E. A. Protein activator of cyclic 3':5'-nucleotide phosphodiesterase of bovine or rat brain also activates its adenylate cyclase. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1055–1062. doi: 10.1016/0006-291x(75)90747-0. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Cohen P., Burchell A., Foulkes J. G., Cohen P. T., Vanaman T. C., Nairn C. Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 1978 Aug 15;92(2):287–293. doi: 10.1016/0014-5793(78)80772-8. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Sherry J. M., Aromatorio D. K., Hartshorne D. J. Modulator protein as a component of the myosin light chain kinase from chicken gizzard. Biochemistry. 1978 Jan 24;17(2):253–258. doi: 10.1021/bi00595a010. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D., Yohe W. B., Maurer S. C. Stimulation of Ca2+-dependent neurotransmitter release and presynaptic nerve terminal protein phosphorylation by calmodulin and a calmodulin-like protein isolated from synaptic vesicles. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1838–1842. doi: 10.1073/pnas.76.4.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedman J. R., Jackson R. L., Schreiber W. E., Means A. R. Sequence homology of the Ca2+-dependent regulator of cyclic nucleotide phosphodiesterase from rat testis with other Ca2+-binding proteins. J Biol Chem. 1978 Jan 25;253(2):343–346. [PubMed] [Google Scholar]
- GUTTMAN H. N., FRIEDMAN W. PROTOZOA AS PHARMACOLOGICAL TOOLS: THE PHENOTHIAZINE TRANQUILIZERS. Trans N Y Acad Sci. 1963 Nov;26:75–89. doi: 10.1111/j.2164-0947.1963.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Gopinath R. M., Vincenzi F. F. Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1203–1209. doi: 10.1016/s0006-291x(77)80107-1. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V., Weeks R. A. Troponin C-like proteins (calmodulins) from mammalian smooth muscle and other tissues. Biochem J. 1979 Feb 1;177(2):521–529. doi: 10.1042/bj1770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson G. A., Jr, Vanaman T. C. Calcium-dependent affinity chromatography of calmodulin on an immobilized phenothiazine. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1048–1056. doi: 10.1016/0006-291x(79)91932-6. [DOI] [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Partial purification of the Ca2+-Mg2+ ATPase activator from human erythrocytes: its similarity to the activator of 3':5' - cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1210–1216. doi: 10.1016/s0006-291x(77)80108-3. [DOI] [PubMed] [Google Scholar]
- Jones H. P., Bradford M. M., McRorie R. A., Cormier M. J. High levels of a calcium-dependent modulator protein in spermatozoa and its similarity to brain modulator protein. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1264–1272. doi: 10.1016/0006-291x(78)90324-8. [DOI] [PubMed] [Google Scholar]
- Jones H. P., Matthews J. C., Cormier M. J. Isolation and characterization of Ca2+-dependent modulator protein from the marine invertebrate Renilla reniformis. Biochemistry. 1979 Jan 9;18(1):55–60. doi: 10.1021/bi00568a009. [DOI] [PubMed] [Google Scholar]
- Kuźnicki J., Kuźnicki L., Drabikowski W. Ca2+-binding modulator protein in protozoa and myxomycete. Cell Biol Int Rep. 1979 Jan;3(1):17–23. doi: 10.1016/0309-1651(79)90064-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLAN J. M., SHENOY K. G., CAMPBELL J. A. Some apparent drug-vitamin interrelationships in Lactobacillus leichmannii and Tetrahymena pyriformis. J Pharm Sci. 1961 Jan;50:59–63. doi: 10.1002/jps.2600500114. [DOI] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of troponin and the effects of interactions between the components of the complex. Biochem J. 1974 Sep;141(3):733–743. doi: 10.1042/bj1410733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C. G. Uptake of 32p-orthophosphate and incorporation into phospholipids in Tetrahymena pyriformis W exposed to phenothiazine derivatives. Can J Biochem. 1968 Apr;46(4):331–339. doi: 10.1139/o68-049. [DOI] [PubMed] [Google Scholar]
- Rylatt D. B., Embi N., Cohen P. Glycogen synthase kinase-2 from rabbit skeletal muscle is activated by the calcium-dependent regulator protein. FEBS Lett. 1979 Feb 1;98(1):76–80. doi: 10.1016/0014-5793(79)80155-6. [DOI] [PubMed] [Google Scholar]
- Schulman H., Greengard P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by "calcium-dependent regulator". Proc Natl Acad Sci U S A. 1978 Nov;75(11):5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisman D., Stevens F. C., Wang J. H. The distribution of the Ca++-dependent protein activator of cyclic nucleotide phosphodiesterase in invertebrates. Biochem Biophys Res Commun. 1975 Aug 4;65(3):975–982. doi: 10.1016/s0006-291x(75)80481-5. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Watterson D. M., Van Eldik L. J., Smith R. E., Vanaman T. C. Calcium-dependent regulatory protein of cyclic nucleotide metabolism in normal and transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2711–2715. doi: 10.1073/pnas.73.8.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]