Abstract
The glycosaminoglycan (GAG) family of polysaccharides includes the unsulfated hyaluronan and the sulfated heparin, heparan sulfate, keratan sulfate, and chondroitin/dermatan sulfate. GAGs are biosynthesized by a series of enzymes, the activities of which are controlled by complex factors. Animal cells alter their responses to different growth conditions by changing the structures of GAGs expressed on their cell surfaces and in extracellular matrices. Because this variation is a means whereby the functions of the limited number of protein gene products in animal genomes is elaborated, the phenotypic and functional assessment of GAG structures expressed spatially and temporally is an important goal in glycomics. On-line mass spectrometric separations are essential for successful determination of expression patterns for the GAG compound classes due to their inherent complexity and heterogeneity. Options include size exclusion, anion exchange, reversed phase, reversed phase ion pairing, hydrophilic interaction, and graphitized carbon chromatographic modes and capillary electrophoresis. This review summarizes the application of these approaches to on-line MS analysis of the GAG classes.
Keywords: glycosaminoglycan, heparin, heparan sulfate, keratan sulfate, chondroitin sulfate, dermatan sulfate, hyaluronan, mass spectrometry, liquid chromatography, capillary electrophoresis
I. BIOLOGICAL SIGNIFICANCE OF GAGs
The glycosaminoglycan (GAG) family of linear polysaccharides, found in mast cell granules, on cell surfaces, in extracellular matrices and in basement membranes, was first identified over 100 years ago from cartilage. GAGs play structural roles in connective tissue, tethering cell surfaces to protein and proteoglycan molecules through interactions with lectin domains (Yang et al., 1994). The high concentration of sulfated GAGs also provides swelling pressure that is necessary for visco-elasticity in connective tissue. In addition, cell surface GAGs interact with a large number of growth factor families, growth factor receptors, cytokines and chemokines (Bernfield et al., 1999; Perrimon & Bernfield, 2000). GAGs are believed to act as co-receptors for many growth factors and growth factor receptors, interacting with both partners, either specifically or non-specifically (Lander, 1998; Herndon, Stipp, & Lander, 1999). Although it is believed that specific patterns of sulfation on the GAG backbone mediate growth factor–receptor interactions, efforts to gain a thorough understanding of these events have been limited by difficulties in obtaining sequences. GAGs are polydisperse with respect to chain length and sulfation pattern, rendering them analytically challenging.
Traditionally, a GAG oligosaccharide must be purified to homogeneity before analysis using a combination of chemical and enzymatic degradation (Conrad, 1998) and chromatographic, electrophoretic (Turnbull, Hopwood, & Gallagher, 1999), or mass spectrometric (Ernst et al., 1998; Rhomberg et al., 1998a,b; Venkataraman et al., 1999) detection to obtain the sequence. Complete structural analysis entails purification of sufficient material to allow for multiple degradative steps. On-line separations coupled with mass spectrometry have the significant advantage that they are sensitive and do not require a purified sample.
II. OVERVIEW OF GAG STRUCTURE
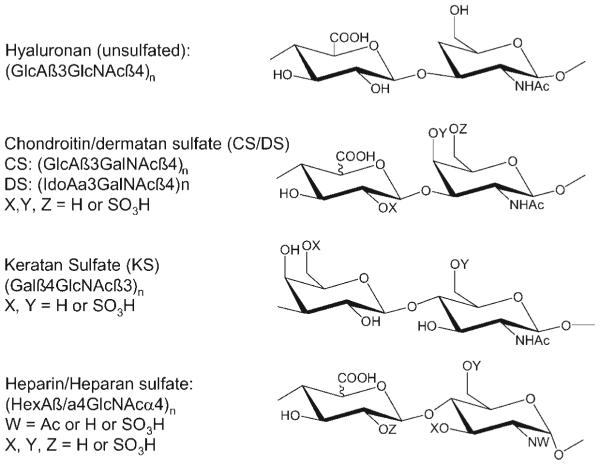
All GAGs consist of repeating disaccharide structures. Mature GAG chains reflect modification events that occur shortly after initial chain polymerization (Esko & Selleck, 2002; Bulow & Hobert, 2006). Heparin, heparan sulfate (HS), keratan sulfate (KS), chondroitin sulfate (CS), and dermatan sulfate (DS) are elaborated by sulfation of hydroxyl groups. The heparin, HS, CS, and DS classes are elaborated by epimerization of some uronic acid residues at the C5 position. The heparin and HS classes are acted upon by N-deacetylase, N-sulfotransferase activity. The disaccharide repeat structures of the four classes of GAGs found in mammalian systems are shown in Figure 1.
FIGURE 1.
Repeating disaccharide structures for the glycosaminoglycans.
A. Hyaluronan
The simplest GAG from the chemical point of view is hyaluronan, a molecule synthesized at and extruded from the plasma membrane (Toole, 2000), consisting of [GlcAβ3GlcNAc β4]n with n > 2,000 in extracellular matrices. The disaccharides are not chemically modified and the molecule serves as a tether between a variety of extracellular matrix molecules and cell surfaces by virtue of hyaluronan-specific lectin domains on cell surface receptor CD44 and ECM proteins, glycoproteins and proteoglycans. Oligosaccharides of hyaluronan have important biological activities and therefore molecular weight analysis of hyaluronan is an important consideration (Mahoney et al., 2001). Methods used most often for molecular weight analysis of hyaluronans include size exclusion chromatography (SEC) and gel electrophoresis as reviewed in (Kakehi, Kinoshita, & Yasueda, 2003). Both MALDI (Sakai et al., 2007) and ESI-MS techniques are appropriate for determining the size of oligosac-charides derived from hyaluronan (Roboz et al., 2000; Mahoney et al., 2001). Hyaluronan oligosaccharides may be distinguished from isobaric heparosan oligosaccharides using tandem MS (Zhang et al., 2008).
B. Chondroitin/Dermatan Sulfate
Chondroitin sulfate (CS) consists of repeating units of [GlcAb3-GalNAcb4] and may be sulfated on the 4- and/or 6-postions of GalNAc. DS is a CS variant in which a substantial fraction of GlcA residues are epimerized to IdoA, and the IdoA may be sulfated at the 2-position. CS/DS oligosaccharides are expressed in patterned domains with respect to the distribution of uronic acid epimers and with respect to sulfation patterns (Cheng et al., 1994). In addition, the capping regions of CS chains from cartilage aggrecan have been found to vary specifically with development and during the onset of osteoarthritis (Plaas et al., 1998; West et al., 1999). MS has the potential to vastly increase the information concerning spatial and temporal expression of CS/DS domains in biological tissue (Seidler et al., 2007).
C. Keratan Sulfate
Keratan sulfate (KS) is a sulfated polylactosamine chain, the disaccharide repeat of which, [Galb4GlcNAcb3], is identical to that found in antenna extensions of N- and O-linked glycans (Funderburgh, 2000). KS consists of approximately 50 disaccharide residues (Stuhlsatz et al., 1981) and is linked to the protein either as an extension to an N-linked glycan (KS I, cornea), or through a Ser/Thr bound linker structure (KS II, skeletal tissue). Biosynthesis of KS chains occurs with simultaneous polymerization and sulfation, forming a pattern of unsulfated, monosulfated (Gal-GlcNAc6S) and disulfated (Gal6S-GlcNAc6S) disaccharide units. The non-reducing ends of corneal KS chains are modified with a variety of capping structures including sialic acids and a3Gal (Tai, Huckerby, & Nieduszynski, 1996; Tai et al., 1997; Huckerby, Tai, & Nieduszynski, 1998). The existence of biologically regulated sequence epitopes of KS has been demonstrated in antigenicity studies (Mehmet et al., 1986; Tang et al., 1986) and the structures of KS chains are also known to vary with age (Lauder et al., 1998). A number of important papers concerning MS of KS have appeared (Oguma et al., 2001a; Karlsson et al., 2005; Zhang et al., 2005a,b), and it is clear that on-line separations will play an important role for future structural studies.
D. Heparin and Heparan Sulfate
HS, like CS, is bound to proteoglycan core proteins through Ser/Thr residues via a xylosyl linker (Varki et al., 1999). HS and heparin chains are synthesized as [GlcAb4GlcNAca4] and subsequently modified by an N-deacetylase/N-sulfotranferase enzyme that removes GlcN acetyl groups and replaces them with sulfate groups. This enzyme produces domains of high N-sulfate content, interspersed with those containing high N-acetate content. The N-sulfated domains may be acted upon by glucuronic acid C5 epimerase, resulting in the conversion of GlcA residues to IdoA and be subsequently sulfated at the 3O- and/or 6O-positions of GlcN and/or the 2O-postion of HexA. The mature chains thus display a pattern of N-acetylated domains with low degrees of O-sulfation and N-sulfated domains with high degrees of O-sulfation. Heparin is expressed exclusively in mast cells bound to the serglycin proteoglycan, the carbohydrate chains of which are highly sulfated, corresponding predominantly to [IdoA2S-GlcNS6S]n (Gallagher & Walker, 1985; Kjellen & Lindahl, 1991). HS expressed on cell surfaces and in basement membranes is more diverse than heparin in that it contains a greater percentage of N-acetylated domains. The structures of these HS chains vary among different core proteins, cell types and cellular environments.
III. STRUCTURAL ANALYSIS OF GAGs
Successful mass spectrometric analysis of GAGs depends on an extraction method that is compatible with the separations system and MS detection. Classical biochemical extraction procedures, summarized in (Iozzo, 2001; Vynios, Karamanos, & Tsiganos, 2002; Didraga, Barroso, & Bischoff, 2006; Volpi, 2006), typically require further development to enable use of MS-based detection. In particular, levels of salts and contaminants such as other carbohydrate classes, nucleic acids or lipids that may not be a problem when using optical detection may cause unacceptable background ion counts when using MS. LC/MS-compatible extraction methods for analysis of tissue GAGs have recently been demonstrated (Zhang et al., 2005a; Hitchcock et al., 2006).
A. Depolymerization of GAGs
Purified GAGs are often too large in size and heterogeneous to permit direct mass spectral analysis and are usually chemically or enzymatically depolymerized. The extent of such digestion may be engineered to achieve complete depolymerization, in which disaccharides are produced. The analysis of disaccharide composition is typically done to characterize the overall chemical character of a given GAG preparation. Specific enzymes are available for depolymerization of each GAG class, and details regarding their sources and activities are given in (Ernst et al., 1995). Generally speaking, lyase enzymes act to cleave hexosaminic bonds and the newly liberated non-reducing ends contain a 4,5-unsaturated (Δ-unsaturated) uronosyl residue. Oligosaccharides produced by lyase action are distinguished by the loss of water, relative to a fully saturated structure, that accompanies glycosidic cleavage. Chondroitin lyases and heparin lyases are specific, available commercially, and often used in depolymerization of GAGs. GAG hydrolase enzymes cleave hexosaminic bonds by addition of water to produce fully saturated uronic acid at the non-reducing chain termini. Keratanases and testicular hyaluronidase are commonly used GAG hydrolases. Nitrous acid is used to cleave N-sulfated hexosaminic bonds at pH 1.5 or free hexosamine at pH 4 (Conrad, 1998). This chemical cleavage is used for analysis of heparins but may be used for analysis of CS/DS after de-acetylation of GalNAc residues. Nitrous acid degradation products contain an anhydromannose residue at the reducing end.
IV. MASS SPECTROMETRY OF GAGs
The topic of mass spectrometric analysis of the GAGs has been reviewed recently (Zaia, 2004, 2005; Chi, Amster, & Linhardt, 2005; Minamisawa & Hirabayashi, 2006). Mass spectrometric ionization of carbohydrates, including GAGs, has been reviewed recently (Zaia, 2006). The following is a summary of some general points germane to LC/MS methods. In summary, GAGs are acidic molecules that produce abundant negative ions. Their acidity also makes them significantly more fragile than peptides or less acidic glycans. Acidic residues are most stable when ionized in deprotonated form or paired with a cation. Thus, GAG oligosaccharides may be analyzed directly using negative mode MS techniques or in the positive mode when paired with a cation. For on-line separations, ESI is the primary ionization method used.
A. FAB MS
The use of FAB in the analysis of GAG oligosaccharides has been reviewed (Zaia, 2004, 2006). FAB was used to develop fundamental mass spectrometric principles for analysis of the GAG class. Unfortunately, it imparts sufficient internal energy to GAG ions to cause fragmentation of the sulfate groups. In addition, the FAB technique is significantly less sensitive than either MALDI or ESI. For these and other reasons, it is no longer widely used for analysis of GAG oligosaccharides.
B. MALDI-TOF MS
The amount of energy imparted during the vacuum MALDI process suffices to fragment polysulfated oligosaccharides (Juhasz & Biemann, 1994, 1995). This problem may be circumvented by pairing the sulfated oligosaccharides with basic proteins or peptides, resulting in the observation of complexes between peptide and oligosaccharide in the positive mode. This technique has been widely used for the determination of sulfated GAG oligosaccharide mass values (Venkataraman et al., 1999). The potential for the use of room temperature ionic liquids as MALDI matrices for analysis of uncomplexed polysulfated oligosaccharides has been explored recently (Laremore et al., 2006). MALDI MS may be used for analysis of chromatographic fractions containing GAG oligosaccharides in an off-line mode.
C. ESI-MS
The development of ESI enabled mass spectrometric analysis of GAGs without in-source fragmentation problems (Takagaki et al., 1992, 1994; Chai et al., 1998; Kim et al., 1998). Provided that source desolvation conditions are carefully optimized using commercially available standards, polysulfated oligosaccharides may be analyzed in the negative mode with minimal fragmentation to the sulfate groups occurring during the desolvation process (Chai et al., 1998; Kim et al., 1998; Desaire, Sirich, & Leary, 2001; Zaia & Costello, 2001; Zaia, McClellan, & Costello, 2001; Saad & Leary, 2003; Naggar, Costello, & Zaia, 2004; Saad & Leary, 2004; Saad et al., 2005). As a flowing technique, ESI allows direct detection of chromatographic effluents. Chromatographic mobile phases must contain only volatile components to be compatible with ESI. Therefore, the chromatography system must either be optimized using only volatile solvent components or an on-line solvent desalting device. For GAGs, SEC, reversed phase ion pairing, normal phase, or graphitized carbon chromatography may be operated using mobile phases compatible with ESI. Capillary electrophoresis may also be used with on-line MS detection. These applications are reviewed in detail below.
Recently, the electron detachment dissociation (EDD) has been used to dissociate GAG oligosaccharides using Fourier transform MS (Wolff et al., 2007a,b; Chi et al., 2008). EDD is a technique whereby a beam of electrons detaches an electron from a negatively charged precursor ion in the FTMS cell, resulting in the formation of an odd-electron species that dissociates to form product ions (Budnik, Haselmann, & Zubarev, 2001; Zubarev, 2003). It has been demonstrated that native glycoconjugate glycans dissociate to form structurally informative cross-ring cleavages in higher abundances than observed using conventional collision-induced dissociation (McFarland et al., 2005; Adamson & Hakansson, 2007). High abundances of cross-ring cleavages were also observed for GAG oligosaccharides (Wolff et al., 2007a), and it is possible to distinguish uronic acid epimers based on diagnostic product ions (Wolff et al., 2007b). EDD has been applied to the analysis of the CS/DS chain of the bikunin proteoglycan (Chi et al., 2008).
V. LC/MS OF GAGs
Some of the chromatography modes used for on-line LC/MS of N- and O-glycans are also used for GAGs. Because in many cases work on N- and O-glycans predates that on GAGs, a brief discussion of general glycan applications is provided at the beginning of each section below. The intent is to summarize the principles necessary to understand the applications to GAG separations. It is beyond the scope of this discussion to present an exhaustive review for N- O-, and lipid-linked glycan LC/MS.
A. SEC –LC/MS of GAGs
Classical large format size exclusion chromatography (SEC) has long been used for separation of the oligosaccharide products of GAG depolymerization reactions, and recent work has described the use of high performance SEC for this purpose (Ziegler & Zaia, 2006). High performance SEC columns are available in formats that operate below 100 mL/min flow rate range, the effluent of which may be infused with or without a post-column splitter into the mass spectrometer source.
The first work showing on-line SEC–LC/MS of GAGs was accomplished using an Amersham/Pharmacia/GE Health Sciences Superdex peptide column with a mobile phase consisting of 30% methanol, 10 mM HCl (Zaia & Costello, 2001). In subsequent work, the use of ammonium salts as a solvent modifier has been found to be preferable. On-line SEC– LC/MS using a Superdex peptide column with 10% acetonitrile, 50 mM ammonium formate at 40 mL/min has been used to characterize CS/DS glycoform distributions from purified proteoglycans from different sources (Hitchcock, Costello, & Zaia, 2006). The same LC/MS system has been used for the characterization of normal and osteoarthritic cartilage samples (Hitchcock et al., 2006). The significance of this work is that SEC–LC/MS is compatible with the tissue extraction procedure and that on-line tandem mass spectrometry determines the CS/DS glycoform distribution in the tissue. One limitation of on-line SEC–LC/MS is that the chromatographic resolution depends on the column volume when other factors are held constant. As a result, scaling down of the chromatography dimensions comes at the expense of resolution.
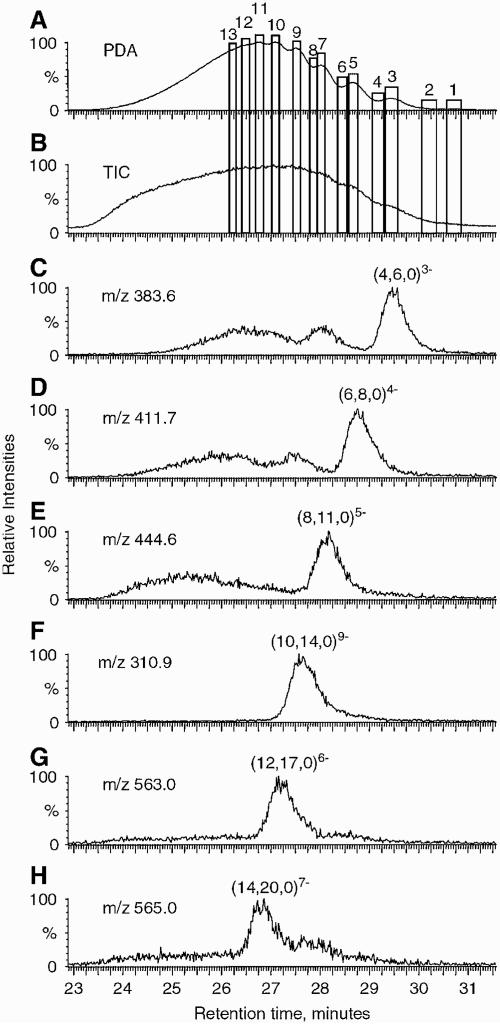
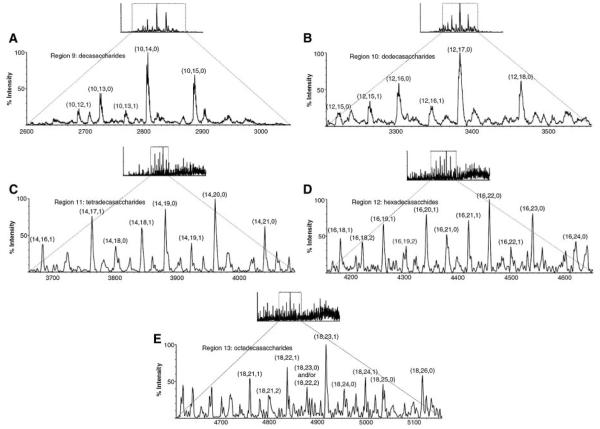
The use of an ion suppression device for reducing the concentration of ammonium ions for the purposes of separation of heparin oligosaccharides has been shown (Henriksen, Ringborg, & Roepstorrf, 2004). The ion suppressor serves to remove the ammonium cations from the SEC column mobile phase, thus improving the signal strength from the heparin oligosaccharides. Using this system, extracted ion chromatograms have been produced for heparin oligosaccharides up to degree of polymerization (dp) 14. Such mixtures are extremely complex, and the separation system enables deconvolution of the heparin oligomers. Figure 2 shows SEC–LC/MS results obtained on Tinzaparin, the active substance in the anticoagulant drug Innohep1 a low molecular weight heparin drug produced from partial heparin lyase depolymerization of intact heparin (Henriksen, Ringborg, & Roepstorrf, 2004). The UV (A) and total ion chromatogram (B) traces show a partially resolved distribution ranging from dp2 to >dp14. Traces (C–H) show extracted ion chromatograms for the ion corresponding to the most abundant composition of each oligomer size. The most abundant disaccharide repeat in heparin is (IdoA2Sa4GlcNS6S)n and trace (C) shows dp4 containing this repeat. Traces (D–H) show that the number of sulfate groups on heparin oligomers = 3n - 1, where n is the number of dis-accharide repeats. For example, (E) shows dp8, corresponding to 4 disaccharide repeats and 11 sulfate groups. Such compositions are typical of heparins. Mass spectra were summed for the 13 regions indicated in (A and B), and the results are shown in Figure 3. Regions 9–13 correspond to decasaccharides through octadecasaccharides, respectively. SEC–LC/MS with an ion suppressor has also been applied to the analysis of the antithrombin binding characteristics of various heparin preparations (Seyrek, Dubin, & Henriksen, 2007). Although the SEC resolution is low, the combination with MS detection allows extremely useful characterization of low molecular weight heparin samples.
FIGURE 2.
A: Photodiode array (PDA) chromatogram of tinzaparin recorded at 231–233 nm. B: ESI total ion chromatogram of tinzaparin. C–H: Extracted ion traces corresponding to abundant GAGs with different degree of polymerization (dp). Mass spectra were summed within the 13 regions shown in (a–b). Compositions are given as (X, Y, Z) where X = number of monosaccharide units, Y = number of sulfate groups, and Z = number of acetyl groups (Henriksen, Ringborg, & Roepstorrf, 2004). © 2004 John Wiley and Sons, Limited. Reproduced with permission.
FIGURE 3.
Summed mass spectra from different regions in the TIC chromatogram shown in Figure 2 (Henriksen, Ringborg, & Roepstorrf, 2004). The components are categorized by (X, Y, Z), as defined in Figure 2 legend. © 2004 John Wiley and Sons, Limited. Reproduced with permission.
B. Strong Anion Exchange Chromatography LC/MS of Glycans
GAG chains are often subjected to anion exchange-based separations for both preparative and analytical purposes. HPLC-based strong anion exchange methods for separation of GAG chains have been described (Pye et al., 1998; Vives, Goodger, & Pye, 2001). Although on-line LC/MS analysis typically requires a gradient of sodium chloride that precludes direct detection using mass spectrometry, an ion suppression device may be used to remove salts before analytes enter the ion source (Simpson et al., 1990; Conboy & Henion, 1992). Recently a sub-millimeter diameter strong anion exchange high pH anion exchange column was used on-line with a Nafion cation exchange capillary desalting device to enable detection using an ESI ion trap mass spectrometer (Bruggink et al., 2005a,b). Results showed baseline chromatographic resolution with MS detection for a series of fructans from dp3 to dp13. It was also possible to analyze GM1-gangliosides from human urine samples. There is potential for use of such a desalting device for strong anion exchange LC/MS of GAGs and other acidic glycans.
C. Reversed Phase LC/MS of GAGs
Although native carbohydrates are not retained on reversed phase stationary phases, derivatization with a hydrophobic group improves their chromatographic properties. Reductive amination is a robust method for attaching a single amine-containing alkyl group to the reducing end of native oligosaccharides, and several different tags have been used for this purpose (Anumula, 2000, 2006). The tag increases reversed phase retention and adds a chromophore and/or fluorophore to the analyte carbohydrates to improve optical detection. Reductive amination also improves mass spectrometric ionization responses (Harvey, 2000).
Reductive amination with pyridyl amine (PA) serves to increase the hydrophobicity of N- and O-linked glycans to the point that they are retained using a reversed phase chromatography column (Yamamoto et al., 1989; Kuraya & Hase, 1996). In principle, the most highly retained compound in such an analysis is the reductive amination reagent itself. As the size of the glycan portion increases, so does the hydrophilicity of the tagged molecule. A two-dimensional chromatography system featuring SEC and reversed phase separation has been used to map PA-labeled oligosaccharides from glycoproteins (Kuraya & Hase, 1996; Yamamoto et al., 1989). This method has been applied to analysis of partial acid hydrolysis products of glycoprotein glycans (Makino et al., 1996). An additivity rule was applied for the correlation of two-dimensional chromatographic elution position with glycan chemical structure (Nakagawa et al., 1995). An off-line RP HPLC-MALDI TOF MS method has been used to compare the expression of PA-labeled N-linked glycans in murine dermis and epidermis (Uematsu et al., 2005). The 2-aminobenzoic acid label has also been used for RP LC/MS of N-linked glycans (Chen & Flynn, 2007). For further details on use of RP LC/MS for reductively aminated glycans see (Wuhrer, Deelder, & Hokke, 2005).
The following example demonstrates an LC/MS method for quantification of GAG metabolites from urine or plasma after derivatization using 1-phenyl-3-methyl pyrazolone (PMP) (Ramsay, Meikle, & Hopwood, 2003). Lyophilized samples of urine or plasma were derivatized with PMP, excess reagent was removed by chloroform extraction, and the aqueous layer bound to a C18 cartridge. The cartridge was dried and washed with chloroform, dried again, and eluted with 50% acetonitrile. PMP-derivatized sulfated mono- and disaccharides were then analyzed by infusion using negative ion ESI tandem MS. This method has been used to quantify mono- and di-sulfated HexNAc and monosulfated HexNAc-HexA in plasma of mucopolysaccharidosis patients (Ramsay, Meikle, & Hopwood, 2003). The method has been used to monitor dose response in enzyme replacement therapy for mucopolysaccharidosis type VI using an animal model (Crawley et al., 2004). On-line reversed phase LC/MS has been used to profile PMP-derivatized oligosaccharides from mucopolysaccharidosis type IIIA patient urine (Mason et al., 2006). In this example, urine GAGs were partially purified using anion exchange chromatography followed by size fractionation. The GAG fractions were derivatized with PMP, extracted with chloroform and analyzed using on-line reversed phase LC/MS. GAG oligosaccharides including some hexasac-charides were detected. A series of di- to hexadecasaccharides were detected. The PMP label has also been used to characterize GAG disaccharide markers in organ tissue in a mouse mucopolysaccharidosis animal model (King et al., 2006). The use of ion pairing agents to facilitate reverse phase binding interactions is also reported for separation of sulfated GAG oligosaccharides, see below.
D. Reversed Phase Ion Pairing LC/MS of GAGs
Carbohydrates do not interact with hydrophobic stationary phases because they interact favorably with water in the mobile phase. GAGs, with their acidic character, are amenable to use of ion pairing agents to increase the degree to which binding occurs to the reversed phase. As reviewed (Garcia, 2005), retention of charged analytes using ion pairing may be viewed as partitioning of the uncharged ion-pairs onto the hydrophobic stationary phase. An alternative view is that the ion pairing agent coats the hydrophobic phase and retention of analytes occurs through an ion exchange mechanism. Although ion pairing agents improve chromatographic properties, they often interfere with mass spectrometric detection due to their propensity to produce strong signals, thereby suppressing those of the analytes.
The inclusion of quaternary ammonium salts in the mobile phase enables direct separation of GAG disaccharides using a reversed phase chromatography column (Lee & Tieckelmann, 1980). Such reversed phase ion pairing chromatography systems remain popular for disaccharide analysis, particularly when combined with fluorescence-based detection. The formation of fluorescent derivatives through post-column addition of cyanoacetamide (Toyoda et al., 1991) have enabled GAG disaccharides from sub-microgram quantities of biological samples. This method has enabled progress in understanding of GAG expression in model organisms including Caenorhabditis elegans and Drosophila (Toyoda, Kinoshita-Toyoda, & Selleck, 2000; Toyoda et al., 2000). It has also been applied to heparan sulfate disaccharide analysis from human liver samples (Vongchan et al., 2005), among other biological systems. RPIP HPLC using tributylamine in the mobile phase has been shown to produce similar chromatographic resolution of a complex mixture of heparin oligosaccharides as observed using anion exchange.
Retention in RPIP HPLC is dependent on electrostatic interactions between the acidic GAG oligosaccharides and the amine amphiphile (El Rassi, 1996). In order for RPIP to be useful, a mobile phase system must be found that produces adequate ion pairing and remains volatile enough to be compatible for on-line MS detection. A systematic study of the properties of di-, tri, and tetra alkyl ammonium ions for on-line LC/MS of GAG oligosaccharides identified 5 mM dibutylamine as a promising ion pairing agent (Kuberan et al., 2002). In an acidic mobile phase, it has sufficient cationic character to pair with GAG oligosaccharides and is volatile. A capillary HPLC LC/MS separation was shown for unsulfated heparosan from dp6 to dp40. It was also used for analysis of synthetic heparin pentamers.
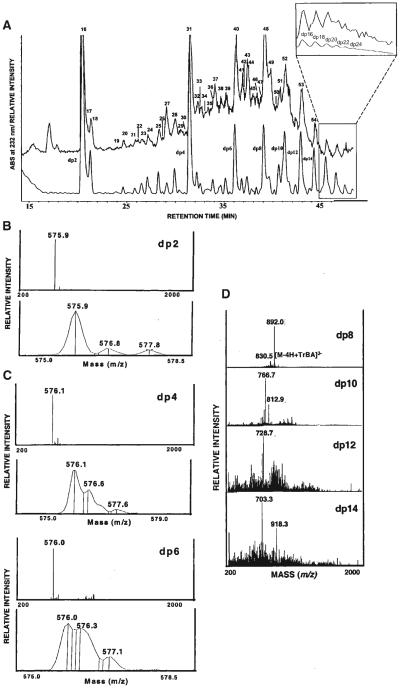
Other researchers prefer 15 mM tributylamine/50 mM ammonium acetate as the ion pairing agent for on-line LC/MS of heparin-derived oligosaccharides (Thanawiroon et al., 2004). As shown in Figure 4, these authors analyzed oligosaccharides from a 30% heparin lyase depolymerization of heparin. The UV and total ion mass chromatograms are compared in (A). The UV trace allows absolute determination of the molar quantity of Δ-unsaturated disaccharides using 232 nm absorbance. The mass dimension of the data enables a series of oligosaccharide compositions to be identified that contain either the reducing or the non-reducing end of the parent heparin chain. This is made possible by the fact that the reducing and the non-reducing ends of the parent chain have unique masses with respect to those deriving from the internal portion. Thus, a series of compositions may be identified that correspond to a sequence. The ability to identify such structures from GAG preparations is clearly an enabling technology for the proteoglycan field. Summed mass spectra for dp2-dp14 are shown in (B–E). Ions corresponding to dp2–dp6 consisting of (HexA2Sb/aGlcNS6S)n, where n = 1–3 are observed at the same m/z value, 576, distinguished by charge state. Ions corresponding to dp8 –14 (E) were detected with a tributylamine adduct. This method has been applied to disaccharide analysis for pharmacokinetics of oral heparin dose in human subjects (Mousa et al., 2007). Similar chromatographic conditions were used to analyze CS oligosaccharides derived from the bikunin proteoglycan (Chi et al., 2008).
FIGURE 4.
A: RPIP-HPLC separation of heparin oligosaccharides obtained from controlled (30%) heparinase depolymerization of bovine lung heparin. A total ion chromatogram using negative ESI-MS detection (upper trace) with peaks numbered and a UV chromatogram at 232 nm (lower trace) with degree of polymerization (dp) of peaks are shown. The inset shows the expanded view of both the total ion chromatogram and the UV chromatograms of higher oligosaccharides assigned to dp16 –dp28 by peak counting. Negative mode ESI mass spectra of the fully sulfated heparin oligosaccharides ranging in size from disaccharide (dp2, B), hexasaccharide (dp6, C) to tetradecasaccharide (dp14, D). The full scan spectra (upper panel) and narrow range spectra showing isotope distribution (lower panel) are presented for the oligosaccharides of dp2–dp6 (Thanawiroon et al., 2004). © 2004 The American Society for Biochemistry and Molecular Biology, Inc., Reproduced with permission.
Direct mass spectrometric analysis of chromatographic effluents containing millimolar concentrations of amines is feasible for mass spectrometers dedicated solely to analysis of GAGs. To analyze other compounds, extensive cleaning of the MS source and optics is necessary to reduce the strength of signals produced from the amines to acceptable background levels. Because such extensive cleaning can take considerable time, laboratories wishing to analyze compound classes for which ion pairing is not necessary are posed with a challenge. It is best not to infuse ion pairing agents into an instrument for which compound classes not requiring such additives must be analyzed.
The chemical nature of different GAG preparations may necessitate optimization of separate RPIP mobile phase compositions (Henriksen, Roepstorff, & Ringborg, 2006). One RPIP method was developed using 25 mM tripropyl amine, 30 mM acetic acid with a water/methanol gradient to separate partially depolymerized heparin preparations containing in excess of 200 components. A second method was developed using 40 mM butyl amine, 40 mM acetic acid with a water/methanol gradient to separate size-fractionated heparin oligomers. An on-line ion suppressor was used to remove the relatively high concentration of ion pairing agents prior to the MS source. A partially depolymerized heparin mixture produced extracted ion chromatograms with peak widths of approximately 1 min. Peaks corresponding to dp12 with 16, 17, and 18 sulfates produced distinct extracted ion peaks with retention times increasing with number of sulfate groups. Deconvoluted mass spectra showed the presence of dp10–dp30 oligosaccharides. It therefore appears that the separation system is a useful means for profiling extremely complex, partially depolymerized heparin mixtures. The second method, using butlyamine, is useful for separating size fractionated heparin. Different HPLC gradient programs were optimized for dp4 and dp6 oligosaccharides.
E. Hydrophilic Interaction Chromatography LC/MS of GAGs
Although normal phase chromatography is an older chromatographic technique than reversed phase, it is used far less often for biomolecular separations. As described in a recent review (Hemström & Irgum, 2006), hydrophilic interaction chromatography (HILIC) is normal phase chromatography in which a polar stationary phase is used with a less polar mobile phase and in which water is used as the strongly eluting solvent (Alpert, 1990). HILIC has been widely used for separation of carbohydrates (Churms, 1996). It offers the ability to bind and separate both charged and uncharged carbohydrates using a gradient from high to low organic content. For LC/MS of GAGs, amine and amide stationary phases are the most widely used for HILIC separations. Solvent modifiers are required, and MS-compatible ammonium salts are often used for this purpose. The uses of these two stationary phases for on-line LC/MS of GAGs are described below.
1. HILIC LC/MS of GAGs Using Amine Stationary Phases
Amino propyl silica was the first stationary phased used for HILIC separation of carbohydrates. Because formation of a Schiff base between the primary amino groups and the glycan reducing end aldehyde is a concern, amine HILIC columns are often used for separation of reduced or reductively aminated carbohydrates. Many manufacturers offer amino propyl silica columns and non-silica packings are available. Such phases are effective for separation of Δ-unsaturated GAG disaccharides (Hjerpe, Antonopoulos, & Engfeldt, 1979; Lee & Tieckelmann, 1979). Although the amine-bonded stationary phase may be considered to act as a weak ion exchanger, isocratic elution conditions were used for separation of the disaccharides. A method employing a gradient of increasing concentration of NaH2PO4 was developed for separation of Δ-unsaturated disaccharides (Yoshida et al., 1989) and used for separation of reductively aminated GAG oligosaccharides (Kinoshita & Sugahara, 1999). Amine stationary phases have been used with gradients of acetonitrile/water without modifier to separate mixtures of glycan alditols liberated from gastric mucins (Hanisch et al., 1993). Such conditions would be compatible with on-line MS detection.
The oligosaccharide profiles of glycoprotein glycans may be resolved using an amine-type HPLC technology using a dextran ladder as a reference (Guile et al., 1996). When used with fluorescent reductive amination with 2-aminobenzamide, this system produces a sensitive and reproducible correlation of released glycan mixtures with glucose unit values from the dextran ladder. The chromatography system has been used to construct a database of O-linked glycan structures (Royle et al., 2002). An amine-based HPLC method has also been used to map N-glycans reductively aminated with 2-anthranilic acid (Anumula & Dhume, 1998). Oligosaccharides were separated based on the number of sialic acids. Fucose variants for each sialylated glycoform produced distinct chromatographic peaks. For further details on the use of amine-type normal phase chromatography in mapping of glycans, see (Wuhrer, Deelder, & Hokke, 2005). Mono-sulfated N-glycans have been analyzed using aminobonded HPLC–ESI-MS using a water/acetonitrile/ammonium hydrogen carbonate pH 8.0 solvent system (Thomsson, Karlsson, & Hansson, 1999). The high pH of the solvent system facilitates analysis of the glycans using negative mode ESI.
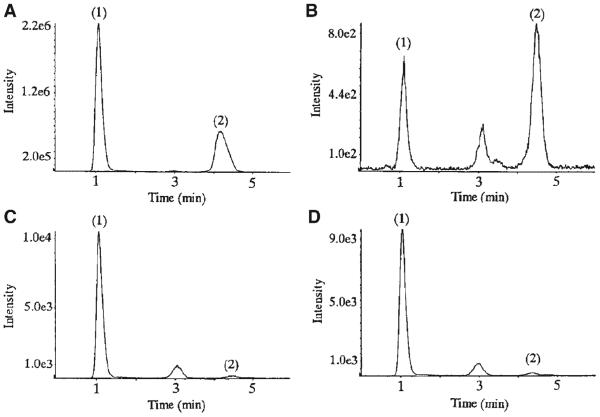
The following examples demonstrate the usefulness of amino columns for separation of sulfated glycans in combination with a multiple reaction monitoring (MRM) method for quantification of specific digestion products among different tissue samples. An MS-compatible amine HPLC method was developed for on-line negative ESI-LC/MS of KS oligosaccharides derived from keratanase II digestion (Oguma et al., 2001a). The mobile phase consisted of 0.01 M ammonium formate pH 9.4/acetonitrile operated under isocratic conditions. Under these conditions, two disaccharides differing by a sulfate group: Galb4GlcNAc(6S) and Gal6Sb4GlcNAc(6S) were easily separated using amine HILIC chromatography, as shown in Figure 6 (Oguma et al., 2001a). The source conditions were set so as to favor loss of SO3 on the part of the disulfated disaccharide, forming an ion at m/z 462 that is isobaric with the [M – H]− ion for the monosulfated disaccharide. The m/z precursor ion was selected and dissociated under relatively high energetic conditions whereby an abundant product ion at m/z 98 was observed. The LC–mass chromatograms in (a–d) show the abundances for the two disaccharides among four tissue samples. This example illustrates both the usefulness of HILIC for LC/MS of sulfated GAGs and MRM for quantifying expressed oligosaccharides or fragments thereof in different tissues. The same chromatography method was applied with LC/MRM MS to the analysis of the Δ-unsaturated disaccharides produced from lyase digestion of HS extracted from brain, liver and tumor samples (Oguma et al., 2001b). Both MS and tandem MS were acquired on-line to differentiate some of the isomeric Δ-unsaturated HS disaccharide structures.
FIGURE 6.
Representative MRM chromatogram of (1) Galb4GlcNAc(6S) and (2)Gal(6S)b4GlcNAc(6S) obtained by keratanase II digestion of (A) bovine cornea, (B) bovine nasal cartilage, (C) mouse brain, and (D) rat brain by turbo-ionspray LC-MS/MS using precursor ion m/z 462.0 and product ion m/z 96.8 (Oguma et al., 2001a). © 2001 Elsevier Limited. Reproduced with permission.
2. HILIC LC/MS of GAGs Using Amide Stationary Phases
Because the amide group is less basic and reactive than the primary amine, amide stationary phases have advantages for separation of acidic glycans. Retention on amide type HILIC columns is less sensitive to eluant pH and Schiff base formation with native carbohydrates does not occur (Hemström & Irgum, 2006). At this writing, there are few commercial choices for amide-type HILIC columns, with the TSK-gel Amide-80 (Tosoh Bioscience, Montgomeryville, PA) and Glycosep-N (Prozyme LLC, San Leandro, CA) used most frequently.
Amide HILIC HPLC was applied to separation of Δ-unsaturated GAG oligosaccharides for the purposes of disaccharide analysis using an acetate buffer with isocratic elution (Akiyama et al., 1992). Partial lyase digestion of CS/DS GAGs produces a series of Δ-unsaturated oligosaccharide products differing by disaccharide units. Such products may be separated very effectively using amide HILIC columns using a gradient of decreasing organic content with an acetate modifier (Saitoh et al., 1995). In this work, the GAG oligosaccharides were reductively aminated using PA (Kon et al., 1991) to enable fluorescence detection. The resulting chromatograms consist of a series of peaks corresponding to CS/DS dp2–dp22 with baseline separation. Offline ESI mass spectra were acquired directly on the fractions to determine the m/z values and compositions of the GAG oligosaccharides. This off-line LC/MS method was used in a series of studies on enzymatic reconstruction of CS/DS and hyaluronan oligosaccharides using the transglycosylase activity of testicular hyaluronidase (Takagaki et al., 1999, 2000a,b, 2002; Takagaki & Ishido, 2000). A similar method has been used to fractionate CS isomers of urinary trypsin inhibitor (Kakizaki et al., 2007).
Mobile phase conditions originally developed for off-line amide-based chromatographic glycan mapping (Guile et al., 1996) have been applied for on-line LC/MS (Mattu et al., 2000). A gradient of decreasing acetonitrile concentration relative to water is used with an ammonium formate modifier to separate glycans. Results were correlated relative to glucose unit values, and exoglycosidase treatments were used to sequence neutrophil gelatinase B O-glycans. The system is also applicable to structural analysis of N-glycans (Royle et al., 2003).
Capillary amide-HILIC chromatography has recently been used for on-line LC/MS of underivatized N-glycans (Wuhrer et al., 2004). These authors packed 5 mm amide HILIC particles into a 75 mm × 100 mm fused silica column and used a mobile phase containing ammonium formate with a gradient of decreasing acetonitrile content relative to water. A dextran ladder was well separated from dp3 –dp10. The elution times increased with N-glycan size and acidity. The total ion mass spectrum showed a combination of protonated and alkaliadducted ions. The chromatography allows on-line tandem MS of eluting glycans. Thus, normal phase LC/MS is a complement to use of reversed phase or graphitized carbon chromatography (GCC) LC/MS for analysis of permethylated glycans. This chromatography system was used to analyze non-specific protease digestion products of glycoproteins (Wuhrer et al., 2005). The chemical properties of such glycopeptide products are dominated by the glycan portion of the molecules. As a result, separation of glycopeptide glycoforms was observed.
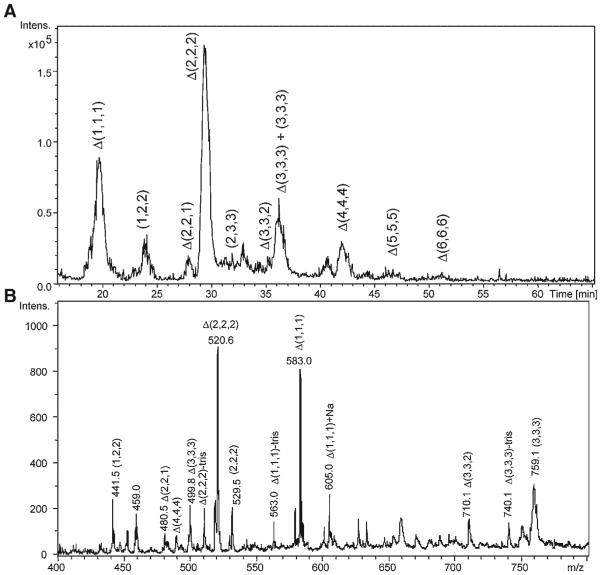
Capillary amide-HILIC LC/MS has been used to characterize CS/DS oligosaccharides from connective tissue (Hitchcock, Costello, & Zaia, 2008). The tissue was digested with pronase and the GAGs isolated using an MS-compatible workup procedure (Hitchcock et al., 2006). As shown in Figure 5A, it was possible to detect dp2–dp12 oligosaccharides with baseline resolution using an ion trap mass spectrometer in the negative mode. In (B), a mass spectrum summed over 18–55 min is shown, and the monosaccharide compositions of the GAG ions are indicated. Similar chromatographic conditions were used to determine antithrombin-binding heparin oligosaccharides using a hybrid ion trap-Orbitrap mass spectrometer (Naimy et al., 2008). These examples demonstrate the usefulness of amide HILIC for LC/MS of GAG classes.
FIGURE 5.
A: Amide-HILIC base peak mass-chromatogram (100–800 m/z) of 30% chondroitin lyase depolymerized CS/DS from juvenile bovine cartilage. GAG oligosaccharide chains ranging from disaccharide to dodecasaccharide elute from 15 to 55 min. Oligosaccharide compositions are given as (HexA, GalNAc, SO3) (X, Y, Z), with 4,5-unsaturation of HexA shown as Δ B: The extracted mass spectrum of all eluted oligosaccharides in the sample mixture. Label of tris indicates reductive amination product with tris buffer (Hitchcock, Costello, & Zaia, 2008). © 2008 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Reproduced with permission.
F. Graphitized Carbon LC/MS of GAGs
Packed charcoal liquid chromatography columns have been used in preparative separation of oligosaccharides for decades, but do not have acceptable physical properties for HPLC (Koizumi, 1996). Graphitized carbon chromatography (GCC) columns were developed for HPLC separations of isomeric and closely related compounds and applied to separation of carbohydrate compounds, including mono- and disaccharides and cyclo-dextrins (Koizumi, Okada, & Fukuda, 1991). Retention by GCC has been described to occur by an adsorption mechanism and planar molecules exhibit increased retention over non-planar ones. For oligosaccharides of the same repeating unit structure, retention increases with degree of polymerization. GCC exhibits greater hydrophobicity than octadecylsilyl reversed phase stationary phases. The structured graphite surface of the GCC material confers exceptional physical and chemical stability. As a result, the entire pH range can be used with a variety of solvents and temperatures. It is not necessary to use salts in the mobile phase, and thus GCC is well suited for interface with mass spectrometry (Davies et al., 1992). Structural isomers may be separated using GCC. To avoid splitting of anomers, oligosac-charides are often reduced or reductively aminated prior to GCC.
Neutral and sialylated N-glycans may be separated using the same GCC mobile phase system (Davies et al., 1992). Sialo forms elute later in the gradient, indicating that the presence of acidic groups influences GCC retention. GCC LC/MS has been used to probe glycoprotein glycan heterogeneity (Kawasaki et al., 1999, 2000). Recently, native glycan separations using GCC in microbore (Itoh et al., 2002) and capillary (Kawasaki et al., 2003) scales with on-line MS detection have been reported. GCC has been packed into a microchip device of dimensions 50 mm × 75 mm width and 50 mm depth that incorporates a short trapping cartridge and an electrospray needle (Ninñonuevo et al., 2005). Such devices are very robust due to the fact that the fluidics connections are made robotically. These studies demonstrate the potential of GCC as an MS-compatible separation system that may be scaled down and adapted to meet the needs of glycomics. GCC has been used for LC/MS of permethylated oligosaccharide alditols under conditions whereby oligosaccharide isomers are separated routinely (Costello, Contado-Miller, & Cipollo, 2007).
Sulfated N-linked glycans have been analyzed using GCC (Kawasaki et al., 2001). Monosulfated N-glycans have been analyzed using GCC-negative ESI-MS using a water/acetonitrile mobile phase system with 5 mM ammonium formate pH 9.3 as a modifier (Thomsson, Karlsson, & Hansson, 1999; Karlsson et al., 2004). The high pH of the mobile phase facilitates detection using negative mode ESI. The peak shapes are narrower than observed for separation of the same compounds using an amino HILIC column. GCC LC/MS using the same conditions has also been applied to analysis of O-glycans from glycoproteins and salivary mucins (Schulz, Packer, & Karlsson, 2002). A significant fraction of the O-glycans detected were monosulfated and one low abundance ion was detected the m/z of which was consistent with two sulfate groups.
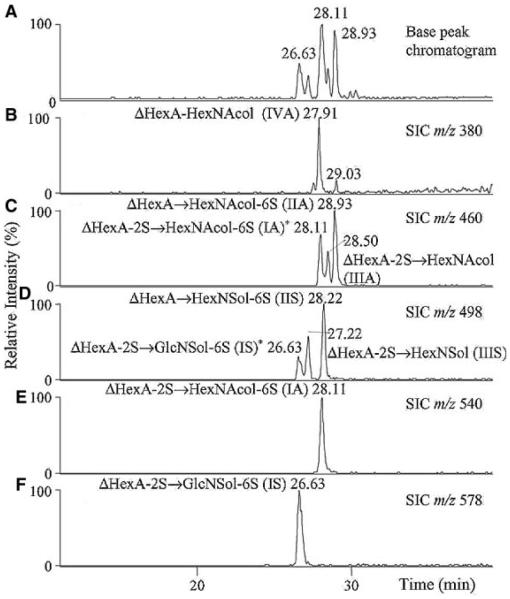
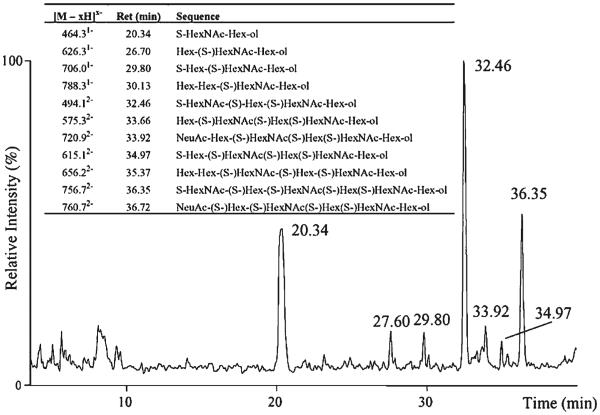
Native CS disaccharides were analyzed using GCC with UV detection using a water/acetonitrile gradient containing trifluoroacetic acid modifier (Davies et al., 1992). The results showed baseline resolution of α and β anomers for the disaccharides. GCC negative mode ESI-LC/MS has been applied to the analysis of enzymatic digestion products of hyaluronan, KS, heparin, and HS (Karlsson et al., 2005). The GCC column (100 mm × 0.32 mm) was eluted using a linear gradient from 0–24% acetonitrile with 20 mM ammonium bicarbonate modifier. Heparin/HS disaccharides were reduced prior to analysis to prevent chromatographic splitting of α and β anomers. As shown in Figure 7A and B, the disaccharide alditols elute between 26 and 29 min in the linear gradient with an order of elution of monosulfated < disulfated < trisulfated (Karlsson et al., 2005). The mono-sulfated disaccharide alditol IVA does not follow this trend, eluting at approximately the same time as one of the monosulfated disaccharides. As shown in (C) and (D), isomeric disaccharide alditols differing by sulfation position are separated. Some of the disaccharide alditols produce split peaks, and it is not clear if the reduction step was not complete or if this is due to other factors. As shown in Figure 8, GCC LC/MS was used to separate oligosaccharide alditols produced from keratanase digestion of bovine cornea KS (Karlsson et al., 2005). A series of peaks corresponding to monosulfated disaccharide (20.34 min), trisulfated dp4 (32.46 min), and pentasulfated dp6 (36.35 min) were observed. These results indicate that GCC may be used for separation of polysulfated GAG oligosaccharides.
FIGURE 7.
Separation and detection of heparan disaccharides (100 ng each) using negative ion graphitized carbon LC–MS. Panes (A–F) include base peak chromatogram and single ion chromatograms (SIC) of detected [M – H]− ions. Structures assigned with an asterisk (*) indicate that these structures were detected in these SIC due to in-source fragmentation with loss of sulfate (S) as [M – H – S]− ions (Karlsson et al., 2005). © 2005 Elsevier Limited. Reproduced with permission.
FIGURE 8.
LC–MS (base peak chromatogram) of keratanase digested keratan sulphate (1 μg) from bovine cornea with detected structures. Sulfate is abbreviated as S (Karlsson et al., 2005). © 2005 Elsevier Limited. Reproduced with permission.
G. Capillary Electrophoresis–MS of GAGs
Capillary electrophoresis (CE) has great potential as a separation system for mass spectrometric analysis of biomolecules including proteins (Gennaro, Salas-Solano, & Ma, 2006), glycans (Zamfir & Peter-Katalinicć, 2004), and small molecule metabolites (Monton & Soga, 2007). Advances in CE–MS of carbohydrates have been reviewed recently (Campa et al., 2006) and the present discussion will focus on applications to the GAG classes.
Di- and oligosaccharides derived from GAGs are readily amenable to gel electrophoretic (Calabro, Hascall, & Midura, 2000; Calabro et al., 2001; Karousou et al., 2004) and capillary electrophoretic (CE) separations (Mao, Thanawiroon, & Linhardt, 2002; Volpi & Maccari, 2006) due to their acidic, negatively charged nature. In normal polarity mode, samples are separated using uncoated fused silica capillaries and a basic buffer system in which analytes are carried toward the cathode by virtue of the electroosmotic flow. The least acidic compounds elute first using this mode of electrophoresis. In reversed polarity mode, the electroosmotic flow is minimized through the use of an acidic buffer system, and only analytes, such as GAGs, that retain anionic character are able to migrate toward the anode. Disaccharides produced by depolymerization of GAGs using lyase enzymes may be detected directly using the 232 absorbance of the Δ-unsaturated uronic acid residue. GAG disaccharides may be analyzed in normal polarity using a borate buffer at pH 8–9 (al-Hakim & Linhardt, 1991) or reversed polarity at pH 3–4 (Pervin, al-Hakim, & Linhardt, 1994). Substantially improved sensitivity of detection has been achieved through the use of reductive amination of the disaccharides to incorporate 2-aminoacridone (AMAC) as a laser-induced fluorophore (Lamari et al., 2002; Militsopoulou et al., 2002; Militsopoulou, Lamari, & Karamanos, 2003).
Direct coupling of CE to MS under conditions appropriate for GAG disaccharide analysis was first accomplished using ammonium acetate buffer to investigate the forward and reverse polarities (Duteil et al., 1999). Forward polarity using 20 mM ammonium acetate pH 9.2 and reversed polarity using 530 mM ammonium acetate pH 3.5 were shown to work with both positive and negative mode ESI-MS detection of heparin disaccharides. Positive mode ESI-MS entailed use of a sheath liquid containing formic acid and negative mode ESI one containing triethylamine. Similar negative ESI-MS conditions have been used to separate hyaluronan oligosaccharides (Kuhn et al., 2003). In this case, polyacrylamide coated fused silica capillaries were used with forward polarity separation in 40 mM ammonium acetate buffer, pH 9.0. The hyaluronan oligomers were all similar in their electrophoretic migration times, and the MS data serve to differentiate them based on mass. Reversed polarity CE using 30 mM formic acid pH 3.2 has been used for CE/MS with a sheath flow of 2-propanol, 5 mM formic acid pH 3.2 (Ruiz-Calero et al., 2001). Negative mode ESI-MS of a mixture of heparin/HS disaccharides showed separation based on number of sulfate groups.
An on-line sheathless CE/MS interface has been used for the analysis of acidic glycan mixtures using uncoated fused silica capillaries and a buffer system containing 50 mM ammonium acetate adjusted to pH 12.0 with ammonia (Zamfir & Peter- Katalinicć, 2001). On-line negative mode ESI mass spectra were acquired using a Q-TOF instrument. CS/DS oligosaccharides were analyzed first using CE with off-line ESI-MS (Zamfir et al., 2002) and then using an on-line sheathless interface (Zamfir et al., 2004). On-line tandem MS data were acquired for a dp18 CS/DS oligosaccharide with 11 sulfate groups, and the data used to assign the sulfation pattern. MS-compatible CE conditions have been optimized for separation of heparin oligosaccharides derived from a partial lyase digestion (Gunay & Linhardt, 2003). Use of ammonium salts at pH 8.5 shows baseline separation for heparin dp4 –14 in the normal polarity mode. The electrophoretic resolution was improved by the addition of 10 mM triethylamine to the buffer system.
Frontal analysis capillary electrophoresis (FACE) with MS detection has been used to detect complexes between antithrombin and a synthetic heparin pentasaccharide (Fermas et al., 2007). Using FACE, a mixture of the protein and oligosaccharide is subjected to continuous electrokinetic injection in ammonium carbonate pH 8.5. The unbound protein migrates fastest, followed by the protein-oligosaccharide complex. The unbound pentasaccharide migrates much more slowly than does the protein. The protein and protein-oligosaccharide complex were detected using positive ion ESI-MS. Unbound pentasaccharide was detected using the negative ion mode.
At this time, with relatively few examples published, the use of on-line CE MS for GAGs is still under development.
VI. SUMMARY
As reviewed here, there are several useful options for on-line separations with MS of the GAG compound classes. The universal nature of the SEC mechanism makes it a robust separations system for comparing the structures of GAG from sources where comparatively high sample quantities (>5 μg) are available. SEC is very robust and may be used effectively to separate a series of GAG preparations with high reproducibility. Anion exchange chromatography is a natural choice for separation of acidic glycans such as GAGs. It use requires an on-line ion supressor, and the robustness of such devices for removing the high concentrations of salt required have not been demonstrated. Reversed phase chromatography is attractive because the chromatographic conditions are similar to those widely used for peptides. The extent to which reductively aminated glycans are retained using reversed phase chromatography diminishes with the size and polarity of the molecules. As a result, the retention of reductively aminated GAG oligosaccharides > dp4 is uncertain. The inclusion of alkylamines in the mobile phase allows separation of GAGs and other acidic glycans using RPIP. On-line MS detection of GAGs using RPIP is widely used, either with or without an ion suppressor. Infusion of alkylamines is not recommended for instruments for which compounds not requiring this additive must be analyzed. HILIC separations work well for LC/MS of all glycan classes, including the GAGs. Amine-HILIC may be used for separation of reductively aminated oligosaccharides. Amide-HILIC has recently been applied to reduced format (75 mm internal diameter) chromatography of glycan classes and has been applied to LC/MS of CS/DS and heparin oligosaccharides. GCC produces the highest resolution of any chromatography system for glycans including GAGs. It use for on-line LC/MS has been demonstrated from GAG disaccharides and poly-sulfated oligosaccharides. There appears to be potential for expanded use of GCC for LC/MS of GAGs. Although GAG digestion products are amenable to CE separations due to their negative charge, only a few reports have shown on-line MS analysis of GAGs with CE, and it appears that technical challenges remain.
ACKNOWLEDGMENTS
The author acknowledges support from NIH grants P41RR10888 and R01HL74197.
Contract grant sponsor: NIH grants P41RR10888 and R01HL74179.
VII. ABBREVIATIONS
- CE
capillary electrophoresis
- CS
chondroitin sulfate
- dp
degree of polymerization
- DS
dermatan sulfate
- ESI
electrospray ionization
- FACE
frontal analysis capillary electrophoresis GAG glycosaminoglycan
- GCC
graphitized carbon chromatography HILIC hydrophilic interaction chromatography KS keratan sulfate
- LC
liquid chromatography
- MALDI
matrix-assisted laser desorption/ionization MS mass spectrometry
- PA
pyridyl amine
- RP
reversed phase
- RPIP
reversed phase ion pairing
- SEC
size exclusion chromatography
Biography
Joseph Zaia received his Ph.D. in organic chemistry from MIT in 1993, working in the laboratory of Klaus Biemann on peptide sequencing by mass spectrometry. After a one year post-doctoral fellowship at MIT, he did a second post-doc with Catherine Fenselau at the University of Maryland, focusing on mass spectrometry of metalloproteins. He joined the cartilage biochemistry group of Osiris Therapeutics, Inc., in June, 1996 and worked on the characterization of glycoproteins and proteoglycans from connective tissue. In June, 1999 he became Assistant Research Professor of Biochemistry at Boston University School of Medicine. Since this time he has served as Associate Director of the Mass Spectrometry Resource, an NIH/NCRR-funded research resource center. He was promoted to Associate Research Professor in 2005. His research focuses on correlating structure and function for heparan sulfates, and other glycosaminoglycans, using mass spectrometry as a primary tool.
REFERENCES
- Adamson JT, Hakansson K. Electron detachment dissociation of neutral and sialylated oligosaccharides. J Am Soc Mass Spectrom. 2007;18:2162–2172. doi: 10.1016/j.jasms.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Shidawara S, Mada A, Toyoda H, Toida T, Imanari T. Chemiluminescence high-performance liquid-chromatography for the determination of hyaluronic-acid, chondroitin sulfate and dermatan sulfate. J Chromatogr-Biomed Appl. 1992;579:203–207. doi: 10.1016/0378-4347(92)80383-2. [DOI] [PubMed] [Google Scholar]
- al-Hakim A, Linhardt RJ. Capillary electrophoresis for the analysis of chondroitin sulfate- and dermatan sulfate-derived disaccharides. Anal Biochem. 1991;195:68–73. doi: 10.1016/0003-2697(91)90296-6. [DOI] [PubMed] [Google Scholar]
- Alpert AJ. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr. 1990;499:177. doi: 10.1016/s0021-9673(00)96972-3. [DOI] [PubMed] [Google Scholar]
- Anumula KR. High-sensitivity and high-resolution methods for glycoprotein analysis. Anal Biochem. 2000;283:17–26. doi: 10.1006/abio.2000.4645. [DOI] [PubMed] [Google Scholar]
- Anumula KR. Advances in fluorescence derivatization methods for high-performance liquid chromatographic analysis of glycoprotein carbohydrates. Anal Biochem. 2006;350:1–23. doi: 10.1016/j.ab.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Anumula KR, Dhume ST. High resolution and high sensitivity methods for oligosaccharide mapping and characterization by normal phase high performance liquid chromatography following derivatization with highly fluorescent anthranilic acid. Glycobiology. 1998;8:685–694. doi: 10.1093/glycob/8.7.685. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bruggink C, Maurer R, Herrmann H, Cavalli S, Hoefler F. Analysis of carbohydrates by anion exchange chromatography and mass spectrometry. J Chromatogr A. 2005a;1085:104–109. doi: 10.1016/j.chroma.2005.03.108. [DOI] [PubMed] [Google Scholar]
- Bruggink C, Wuhrer M, Koeleman CA, Barreto V, Liu Y, Pohl C, Ingendoh A, Hokke CH, Deelder AM. Oligosaccharide analysis by capillary-scale high-pH anion-exchange chromatography with on-line ion-trap mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005b;829:136–143. doi: 10.1016/j.jchromb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Budnik BA, Haselmann KF, Zubarev RA. Electron detachment dissociation of peptide di-anions: An electron-hole recombination phenomenon. Chem Phys Lett. 2001;342:299–302. [Google Scholar]
- Bulow HE, Hobert O. The molecular diversity of glycosaminoglycans shapes animal development. Annu Rev Cell Dev Biol. 2006;22:375–407. doi: 10.1146/annurev.cellbio.22.010605.093433. [DOI] [PubMed] [Google Scholar]
- Calabro A, Hascall VC, Midura RJ. Adaptation of FACE methodology for microanalysis of total hyaluronan and chondroitin sulfate composition from cartilage. Glycobiology. 2000;10:283–293. doi: 10.1093/glycob/10.3.283. [DOI] [PubMed] [Google Scholar]
- Calabro A, Midura R, Wang A, West L, Plaas A, Hascall VC. Fluorophore-assisted carbohydrate electrophoresis (FACE) of glycosaminoglycans. Osteoarthritis Cartilage. 2001;9(Suppl A):S16–S22. doi: 10.1053/joca.2001.0439. [DOI] [PubMed] [Google Scholar]
- Campa C, Coslovi A, Flamigni A, Rossi M. Overview on advances in capillary electrophoresis-mass spectrometry of carbohydrates: A tabulated review. Electrophoresis. 2006;27:2027–2050. doi: 10.1002/elps.200500960. [DOI] [PubMed] [Google Scholar]
- Chai W, Luo J, Lim CK, Lawson AM. Characterization of heparin oligosaccharide mixtures as ammonium salts using electrospray mass spectrometry. Anal Chem. 1998;70:2060–2066. doi: 10.1021/ac9712761. [DOI] [PubMed] [Google Scholar]
- Chen X, Flynn GC. Analysis of N-glycans from recombinant immunoglobulin G by on-line reversed-phase high-performance liquid chromatography/mass spectrometry. Anal Biochem. 2007;370:147–161. doi: 10.1016/j.ab.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Cheng F, Heinegard D, Malmstrom A, Schmidtchen A, Yoshida K, Fransson LA. Patterns of uronosyl epimerization and 4-/6-O-sulphation in chondroitin/dermatan sulphate from decorin and biglycan of various bovine tissues. Glycobiology. 1994;4:685–696. doi: 10.1093/glycob/4.5.685. [DOI] [PubMed] [Google Scholar]
- Chi LL, Amster J, Linhardt RJ. Mass spectrometry for the analysis of highly charged sulfated carbohydrates. Curr Anal Chem. 2005;1:223–240. doi: 10.2174/157341105774573929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Wolff JJ, Laremore TN, Restaino OF, Xie J, Schiraldi C, Toida T, Amster IJ, Linhardt RJ. Structural analysis of bikunin glycosaminoglycan. J Am Chem Soc. 2008 doi: 10.1021/ja0778500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churms SC. Recent progress in carbohydrate separation by highperformance liquid chromatography based on hydrophilic interaction. J Chromatogr. 1996;720:75. [Google Scholar]
- Conboy JJ, Henion J. High-performance anion-exchange chromatography coupled with mass spectrometry for the determination of carbohydrates. Biol Mass Spectrom. 1992;21:397–407. doi: 10.1002/bms.1200210806. [DOI] [PubMed] [Google Scholar]
- Conrad HE. Heparin binding proteins. Academic Press; New York: 1998. [Google Scholar]
- Costello CE, Contado-Miller JM, Cipollo JF. A glycomics platform for the analysis of permethylated oligosaccharide alditols. J Am Soc Mass Spectrom. 2007;18:1799–1812. doi: 10.1016/j.jasms.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley A, Ramsay SL, Byers S, Hopwood J, Meikle PJ. Monitoring dose response of enzyme replacement therapy in feline mucopolysac-charidosis type VI by tandem mass spectrometry. Pediatr Res. 2004;55:585–591. doi: 10.1203/01.PDR.0000113789.30640.5C. [DOI] [PubMed] [Google Scholar]
- Davies M, Smith KD, Harbin A-M, Hounsell EF. High-performance liquid chromatography of oligosaccharide alditols and glycopeptides on a graphitized carbon column. J Chromatogr. 1992;609:125. doi: 10.1016/0021-9673(92)80155-n. [DOI] [PubMed] [Google Scholar]
- Desaire H, Sirich TL, Leary JA. Evidence of block and randomly sequenced chondroitin polysaccharides: Sequential enzymatic digestion and quantification using ion trap tandem mass spectrometry. Anal Chem. 2001;73:3513–3520. doi: 10.1021/ac010385j. [DOI] [PubMed] [Google Scholar]
- Didraga M, Barroso B, Bischoff R. Recent developments in proteoglycan purification and analysis. Curr Pharm Anal. 2006;2:323–337. [Google Scholar]
- Duteil S, Gareil P, Girault S, Mallet A, Feve C, Siret L. Identification of heparin oligosaccharides by direct coupling of capillary electro-phoresis/ionspray-mass spectrometry. Rapid Commun Mass Spectrom. 1999;13:1889–1898. doi: 10.1002/(SICI)1097-0231(19991015)13:19<1889::AID-RCM719>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- El Rassi Z. Recent progress in reversed-phase and hydrophobic interaction chromatography of carbohydrate species. J Chromatogr. 1996;720:93. [Google Scholar]
- Ernst S, Langer R, Cooney CL, Sasisekharan R. Enzymatic degradation of glycosaminoglycans. Crit Rev Biochem Mol Biol. 1995;30:387–444. doi: 10.3109/10409239509083490. [DOI] [PubMed] [Google Scholar]
- Ernst S, Rhomberg AJ, Biemann K, Sasisekharan R. Direct evidence for a predominantly exolytic processive mechanism for depolymerization of heparin-like glycosaminoglycans by heparinase I. Proc Natl Acad Sci USA. 1998;95:4182–4187. doi: 10.1073/pnas.95.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko JD, Selleck SB. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- Fermas S, Gonnet F, Varenne A, Gareil P, Daniel R. Frontal analysis capillary electrophoresis hyphenated to electrospray ionization mass spectrometry for the characterization of the antithrombin/heparin pentasaccharide complex. Anal Chem. 2007;79:4987–4993. doi: 10.1021/ac070146h. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL. Corneal proteoglycans. In: Iozzo RV, editor. Proteoglycans structure, biology and molecular interactions. Marcel Dekker; New York: 2000. pp. 257–273. [Google Scholar]
- Gallagher JT, Walker A. Molecular distinctions between heparan sulphate and heparin. Analysis of sulphation patterns indicates that heparan sulphate and heparin are separate families of N-sulphated polysaccharides. Biochem J. 1985;230:665–674. doi: 10.1042/bj2300665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MC. The effect of the mobile phase additives on sensitivity in the analysis of peptides and proteins by high-performance liquid chroma-tography-electrospray mass spectrometry. J Chromatogr B. 2005;825:111. doi: 10.1016/j.jchromb.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Gennaro LA, Salas-Solano O, Ma S. Capillary electrophoresis-mass spectrometry as a characterization tool for therapeutic proteins. Anal Biochem. 2006;355:249. doi: 10.1016/j.ab.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Guile GR, Rudd PM, Wing DR, Prime SB, Dwek RA. A rapid high-resolution high-performance liquid chromatographic method for separating glycan mixtures and analyzing oligosaccharide profiles. Anal Biochem. 1996;240:210–226. doi: 10.1006/abio.1996.0351. [DOI] [PubMed] [Google Scholar]
- Gunay NS, Linhardt RJ. Capillary electrophoretic separation of heparin oligosaccharides under conditions amenable to mass spectrometric detection. J Chromatogr A. 2003;1014:225–233. doi: 10.1016/s0021-9673(03)01288-3. [DOI] [PubMed] [Google Scholar]
- Hanisch FG, Chai W, Rosankiewicz JR, Lawson AM, Stoll MS, Feizi T. Core-typing of O-linked glycans from human gastric mucins. Lack of evidence for the occurrence of the core sequence Gal1-6GalNAc. Eur J Biochem. 1993;217:645–655. doi: 10.1111/j.1432-1033.1993.tb18288.x. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. Electrospray mass spectrometry and fragmentation of N-linked carbohydrates derivatized at the reducing terminus. J Am Soc Mass Spectrom. 2000;11:900–915. doi: 10.1016/S1044-0305(00)00156-2. [DOI] [PubMed] [Google Scholar]
- Hemström P, Irgum K. Hydrophilic interaction chromatography. J Sep Sci. 2006;29:1784–1821. doi: 10.1002/jssc.200600199. [DOI] [PubMed] [Google Scholar]
- Henriksen J, Ringborg LH, Roepstorrf P. On-line size-exclusion chromatography/mass spectrometry of low molecular mass heparin. J Mass Spectrom. 2004;39:1305–1312. doi: 10.1002/jms.723. [DOI] [PubMed] [Google Scholar]
- Henriksen J, Roepstorff P, Ringborg LH. Ion-pairing reversed-phased chromatography/mass spectrometry of heparin. Carbohydr Res. 2006;341:382–387. doi: 10.1016/j.carres.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Herndon ME, Stipp CS, Lander AD. Interactions of neural glycosaminoglycans and proteoglycans with protein ligands: Assessment of selectivity, heterogeneity and the participation of core proteins in binding. Glycobiology. 1999;9:143–155. doi: 10.1093/glycob/9.2.143. [DOI] [PubMed] [Google Scholar]
- Hitchcock AM, Costello CE, Zaia J. Glycoform quantification of chondroitin/dermatan sulfate using an LC/MS/MS platform. Biochemistry. 2006;45:2350–2361. doi: 10.1021/bi052100t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock AM, Yates KE, Shortkroff S, Costello CE, Zaia J. Optimized extraction of glycosaminoglycans from normal and osteoarthritic cartilage for glycomics profiling. Glycobiology. 2006;17:25–35. doi: 10.1093/glycob/cwl046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock A, Costello C, Zaia J. Comparative glycomics of connective tissue glycosaminoglycans proteomics. Proteomics. 2008;8:1384–1397. doi: 10.1002/pmic.200700787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjerpe A, Antonopoulos CA, Engfeldt B. Determination of sulphated disaccharides from chondroitin sulphates by high-performance liquid chromatography. J Chromatogr. 1979;171:339. doi: 10.1016/s0021-9673(01)95313-0. [DOI] [PubMed] [Google Scholar]
- Huckerby TN, Tai GH, Nieduszynski IA. Oligosaccharides derived by endo-beta-galactosidase digestion of bovine corneal keratan sulphate— Characterisation of tetrasaccharides with incomplete sulphation and containing unsulphated N-acetylglucosamine residues. Eur J Biochem. 1998;253:499–506. doi: 10.1046/j.1432-1327.1998.2530499.x. [DOI] [PubMed] [Google Scholar]
- Iozzo R. Proteoglycan protocols. Humana Press; Totowa: 2001. [Google Scholar]
- Itoh S, Kawasaki N, Ohta M, Hayakawa T. Structural analysis of a glycoprotein by liquid chromatography-mass spectrometry and liquid chromatography with tandem mass spectrometry—Application to recombinant human thrombomodulin. J Chromatogr. 2002;978:141–152. doi: 10.1016/s0021-9673(02)01423-1. [DOI] [PubMed] [Google Scholar]
- Juhasz P, Biemann K. Mass spectrometric molecular-weight determination of highly acidic compounds of biological significance via their complexes with basic polypeptides. Proc Natl Acad Sci USA. 1994;91:4333–4337. doi: 10.1073/pnas.91.10.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz P, Biemann K. Utility of non-covalent complexes in the matrix-assisted laser desorption ionization mass spectrometry of heparinderived oligosaccharides. Carbohydr Res. 1995;270:131–147. doi: 10.1016/0008-6215(94)00012-5. [DOI] [PubMed] [Google Scholar]
- Kakehi K, Kinoshita M, Yasueda S. Hyaluronic acid: Separation and biological implications. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;797:347–355. doi: 10.1016/s1570-0232(03)00479-3. [DOI] [PubMed] [Google Scholar]
- Kakizaki I, Takahashi R, Ibori N, Kojima K, Takahashi T, Yamaguchi M, Kon A, Takagaki K. Diversity in the degree of sulfation and chain length of the glycosaminoglycan moiety of urinary trypsin inhibitor isomers. Biochim Biophys Acta. 2007;1770:171–177. doi: 10.1016/j.bbagen.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Karlsson NG, Wilson NL, Wirth HJ, Dawes P, Joshi H, Packer NH. Negative ion graphitised carbon nano-liquid chromatography/mass spectrometry increases sensitivity for glycoprotein oligosaccharide analysis. Rapid Commun Mass Spectrom. 2004;18:2282–2292. doi: 10.1002/rcm.1626. [DOI] [PubMed] [Google Scholar]
- Karlsson NG, Schulz BL, Packer NH, Whitelock JM. Use of graphitised carbon negative ion LC-MS to analyse enzymatically digested glycosaminoglycans. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;824:139–147. doi: 10.1016/j.jchromb.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Karousou EG, Militsopoulou M, Porta G, De Luca G, Hascall VC, Passi A. Polyacrylamide gel electrophoresis of fluorophore-labeled hyaluronan and chondroitin sulfate disaccharides: Application to the analysis in cells and tissues. Electrophoresis. 2004;25:2919–2925. doi: 10.1002/elps.200406034. [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Ohta M, Hyuga S, Hashimoto O, Hayakawa T. Analysis of carbohydrate heterogeneity in a glycoprotein using liquid chromatography/mass spectrometry and liquid chromatography with tandem mass spectrometry. Anal Biochem. 1999;269:297. doi: 10.1006/abio.1999.4026. [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Ohta M, Hyuga S, Hyuga M, Hayakawa T. Application of liquid chromatography/mass spectrometry and liquid chromatography with tandem mass spectrometry to the analysis of the site- specific carbohydrate heterogeneity in erythropoietin. Anal Biochem. 2000;285:82. doi: 10.1006/abio.2000.4739. [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Haishima Y, Ohta M, Itoh S, Hyuga M, Hyuga S, Hayakawa T. Structural analysis of sulfated N-linked oligosaccharides in erythropoietin. Glycobiology. 2001;11:1043–1049. doi: 10.1093/glycob/11.12.1043. [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Itoh S, Ohta M, Hayakawa T. Microanalysis of N-linked oligosaccharides in a glycoprotein by capillary liquid chromatography/ mass spectrometry and liquid chromatography/tandem mass spectrometry. Anal Biochem. 2003;316:15–22. doi: 10.1016/s0003-2697(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Kim YS, Ahn MY, Wu SJ, Kim DH, Toida T, Teesch LM, Park Y, Yu G, Lin J, Linhardt RJ. Determination of the structure of oligosaccharides prepared from acharan sulfate. Glycobiology. 1998;8:869–877. doi: 10.1093/glycob/8.9.869. [DOI] [PubMed] [Google Scholar]
- King B, Savas P, Fuller M, Hopwood J, Hemsley K. Validation of a heparan sulfate-derived disaccharide as a marker of accumulation in murine mucopolysaccharidosis type IIIA. Mol Genet Metab. 2006;87:107–112. doi: 10.1016/j.ymgme.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Sugahara K. Microanalysis of glycosaminoglycan- derived oligosaccharides labeled with a fluorophore 2-aminobenzamide by high-performance liquid chromatography: Application to disaccharide composition analysis and exosequencing of oligosaccharides. Anal Biochem. 1999;269:367–378. doi: 10.1006/abio.1999.4027. [DOI] [PubMed] [Google Scholar]
- Kjellen L, Lindahl U. Proteoglycans: Structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Koizumi K. High-performance liquid chromatographic separation of carbohydrates on graphitized carbon columns. J Chromatogr A. 1996;720:119–126. doi: 10.1016/0021-9673(94)01274-1. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Okada Y, Fukuda M. High-performance liquid chromatography of mono- and oligo-saccharides on a graphitized carbon column. Carbohydr Res. 1991;215:67. [Google Scholar]
- Kon A, Takagaki K, Kawasaki H, Nakamura T, Endo M. Application of 2-aminopyridine fluorescence labeling to glycosaminoglycans. J Biochem (Tokyo) 1991;110:132–135. doi: 10.1093/oxfordjournals.jbchem.a123531. [DOI] [PubMed] [Google Scholar]
- Kuberan B, Lech M, Zhang L, Wu ZL, Beeler DL, Rosenberg R. Analysis of heparan sulfate oligosaccharides with ion pair-reverse phase capillary high performance liquid chromatography-microelectrospray ionization time-of-flight mass spectrometry. J Am Chem Soc. 2002;124:8707–8718. doi: 10.1021/ja0178867. [DOI] [PubMed] [Google Scholar]
- Kuhn AV, Ruttinger HH, Neubert RH, Raith K. Identification of hyaluronic acid oligosaccharides by direct coupling of capillary electrophoresis with electrospray ion trap mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:576–582. doi: 10.1002/rcm.950. [DOI] [PubMed] [Google Scholar]
- Kuraya N, Hase S. Analysis of pyridylaminated O-linked sugar chains by two-dimensional sugar mapping. Anal Biochem. 1996;233:205–211. doi: 10.1006/abio.1996.0029. [DOI] [PubMed] [Google Scholar]
- Lamari FN, Militsopoulou M, Mitropoulou TN, Hjerpe A, Karamanos NK. Analysis of glycosaminoglycan-derived disaccharides in bio-logic: Samples by capillary electrophoresis and protocol for sequencing glycosaminoglycans. Biomed Chromatogr. 2002;16:95–102. doi: 10.1002/bmc.144. [DOI] [PubMed] [Google Scholar]
- Lander AD. Proteoglycans: Master regulators of molecular encounter? Matrix Biol. 1998;17:465–472. doi: 10.1016/s0945-053x(98)90093-2. [DOI] [PubMed] [Google Scholar]
- Laremore TN, Murugesan S, Park TJ, Avci FY, Zagorevski DV, Linhardt RJ. Matrix-assisted laser desorption/ionization mass spectrometric analysis of uncomplexed highly sulfated oligosaccharides using ionic liquid matrices. Anal Chem. 2006;78:1774–1779. doi: 10.1021/ac051121q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder RM, Huckerby TN, Nieduszynski IA, Plaas AH. Age-related changes in the structure of the keratan sulphate chains attached to fibromodulin isolated from articular cartilage. Biochem J. 1998;330:753–757. doi: 10.1042/bj3300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Tieckelmann H. High-performance liquid chromatographic determinations of disaccharides resulting from enzymatic degradation of isomeric chondroitin sulfates. Anal Biochem. 1979;94:231–236. doi: 10.1016/0003-2697(79)90814-5. [DOI] [PubMed] [Google Scholar]
- Lee GJ-L, Tieckelmann H. High-performance liquid chromatographic separation of unsaturated disaccharides derived from heparan sulfate and heparin. J Chromatogr. 1980;195:402. [Google Scholar]
- Mahoney DJ, Aplin RT, Calabro A, Hascall VC, Day AJ. Novel methods for the preparation and characterization of hyaluronan oligosaccharides of defined length. Glycobiology. 2001;11:1025–1033. doi: 10.1093/glycob/11.12.1025. [DOI] [PubMed] [Google Scholar]
- Makino Y, Kuraya N, Omichi K, Hase S. Classification of sugar chains of glycoproteins by analyzing reducing end oligosaccharides obtained by partial acid hydrolysis. Anal Biochem. 1996;238:54–59. doi: 10.1006/abio.1996.0250. [DOI] [PubMed] [Google Scholar]
- Mao WJ, Thanawiroon C, Linhardt RJ. Capillary electrophoresis for the analysis of glycosaminoglycans and glycosaminoglycan-derived oligosaccharides. Biomed Chromatogr. 2002;16:77–94. doi: 10.1002/bmc.153. [DOI] [PubMed] [Google Scholar]
- Mason KE, Meikle PJ, Hopwood JJ, Fuller M. Characterization of sulfated oligosaccharides in mucopolysaccharidosis type IIIA by electrospray ionization mass spectrometry. Anal Chem. 2006;78:4534–4542. doi: 10.1021/ac052083d. [DOI] [PubMed] [Google Scholar]
- Mattu TS, Royle L, Langridge J, Wormald MR, Van den Steen PE, Van Damme J, Opdenakker G, Harvey DJ, Dwek RA, Rudd PM. O-glycan analysis of natural human neutrophil gelatinase B using a combination of normal phase-HPLC and online tandem mass spectrometry: Implications for the domain organization of the enzyme. Biochemistry. 2000;39:15695–15704. doi: 10.1021/bi001367j. [DOI] [PubMed] [Google Scholar]
- McFarland MA, Marshall AG, Hendrickson CL, Nilsson CL, Fredman P, Mansson JE. Structural characterization of the GM1 ganglioside by infrared multiphoton dissociation/electron capture dissociation, and electron detachment dissociation electrospray ionization FT-ICR MS/ MS. J Am Soc Mass Spectrom. 2005;16:752–762. doi: 10.1016/j.jasms.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Mehmet H, Scudder P, Tang PW, Hounsell EF, Caterson B, Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem. 1986;157:385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- Militsopoulou M, Lamari FN, Hjerpe A, Karamanos NK. Determination of twelve heparin- and heparan sulfate-derived disaccharides as 2-aminoacridone derivatives by capillary zone electrophoresis using ultraviolet and laser-induced fluorescence detection. Electrophoresis. 2002;23:1104–1109. doi: 10.1002/1522-2683(200204)23:7/8<1104::AID-ELPS1104>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Militsopoulou M, Lamari F, Karamanos NK. Capillary electrophoresis: A tool for studying interactions of glycans/proteoglycans with growth factors. J Pharm Biomed Anal. 2003;32:823–828. doi: 10.1016/s0731-7085(03)00185-7. [DOI] [PubMed] [Google Scholar]
- Minamisawa T, Hirabayashi J. Acquisition of structural information on glycosaminoglycans by using mass spectrometry. Trends Glycosci Glycotechnol. 2006;18:293–312. [Google Scholar]
- Monton MR, Soga T. Metabolome analysis by capillary electro- phoresis-mass spectrometry. J Chromatogr A. 2007;1168:237–246. doi: 10.1016/j.chroma.2007.02.065. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Zhang F, Aljada A, Chaturvedi S, Takieddin M, Zhang H, Chi L, Castelli MC, Friedman K, Goldberg MM, Linhardt RJ. Pharmacokinetics and pharmacodynamics of oral heparin solid dosage form in healthy human subjects. J Clin Pharmacol. 2007;47:1508–1520. doi: 10.1177/0091270007307242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggar EF, Costello CE, Zaia J. Competing fragmentation processes in tandem mass spectra of heparin-like glycosaminoglycans. J Am Soc Mass Spectrom. 2004;15:1534–1544. doi: 10.1016/j.jasms.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Naimy H, Leymarie N, Bowman M, Zaia J. Characterization of heparin oligosaccharides binding specifically to antithrombin III using mass spectrometry. Biochemistry. 2008;47:3155–3161. doi: 10.1021/bi702043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Kawamura Y, Kato K, Shimada I, Arata Y, Takahashi N. Identification of neutral and sialyl N-linked oligosaccharide structures from human serum glycoproteins using three kinds of high-performance liquid chromatography. Anal Biochem. 1995;226:130–138. doi: 10.1006/abio.1995.1200. [DOI] [PubMed] [Google Scholar]
- Ninñonuevo M, An H, Yin H, Killeen K, Grimm R, Ward R, German B, Lebrilla C. Nanoliquid chromatography-mass spectrometry of oligosaccharides employing graphitized carbon chromatography on microchip with a high-accuracy mass analyzer. Electrophoresis. 2005;26:3641–3649. doi: 10.1002/elps.200500246. [DOI] [PubMed] [Google Scholar]
- Oguma T, Toyoda H, Toida T, Imanari T. Analytical method for keratan sulfates by high-performance liquid chromatography/turboionspray tandem mass spectrometry. Anal Biochem. 2001a;290:68–73. doi: 10.1006/abio.2000.4940. [DOI] [PubMed] [Google Scholar]
- Oguma T, Toyoda H, Toida T, Imanari T. Analytical method of heparan sulfates using high-performance liquid chromatography turboionspray ionization tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001b;754:153–159. doi: 10.1016/s0378-4347(00)00601-0. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Bernfield M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 2000;404:725–728. doi: 10.1038/35008000. [DOI] [PubMed] [Google Scholar]
- Pervin A, al-Hakim A, Linhardt RJ. Separation of glycosaminoglycan-derived oligosaccharides by capillary electrophoresis using reverse polarity. Anal Biochem. 1994;221:182–188. doi: 10.1006/abio.1994.1395. [DOI] [PubMed] [Google Scholar]
- Plaas AH, West LA, Wong-Palms S, Nelson FR. Glycosaminoglycan sulfation in human osteoarthritis. Disease-related alterations at the non-reducing termini of chondroitin and dermatan sulfate. J Biol Chem. 1998;273:12642–12649. doi: 10.1074/jbc.273.20.12642. [DOI] [PubMed] [Google Scholar]
- Pye DA, Vives RR, Turnbull JE, Hyde P, Gallagher JT. Heparan sulfate oligosaccharides require 6-O-sulfation for promotion of basic fibroblast growth factor mitogenic activity. J Biol Chem. 1998;273:22936–22942. doi: 10.1074/jbc.273.36.22936. [DOI] [PubMed] [Google Scholar]
- Ramsay SL, Meikle PJ, Hopwood JJ. Determination of monosaccharides and disaccharides in mucopolysaccharidoses patients by electrospray ionisation mass spectrometry. Mol Genet Metab. 2003;78:193–204. doi: 10.1016/s1096-7192(03)00018-0. [DOI] [PubMed] [Google Scholar]
- Rhomberg AJ, Ernst S, Sasisekharan R, Biemann K. Mass spectrometric and capillary electrophoretic investigation of the enzymatic degradation of heparin-like glycosaminoglycans. Proc Natl Acad Sci USA. 1998a;95:4176–4181. doi: 10.1073/pnas.95.8.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhomberg AJ, Shriver Z, Biemann K, Sasisekharan R. Mass spectrometric evidence for the enzymatic mechanism of the depolymerization of heparin-like glycosaminoglycans by heparinase II. Proc Natl Acad Sci USA. 1998b;95:12232–12237. doi: 10.1073/pnas.95.21.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roboz J, Deng L, Ma L, Silides D. Monitoring the stepwise decomposition of hyaluronan by hyaluronidase in pleural/peritoneal effusions from patients with malignant mesothelioma using negative electrospray mass spectrometry; Proc 48th ASMS Conf Mass Spectrom Allied Topics.2000. [Google Scholar]
- Royle L, Mattu TS, Hart E, Langridge JI, Merry AH, Murphy N, Harvey DJ, Dwek RA, Rudd PM. An analytical and structural database provides a strategy for sequencing O-glycans from microgram quantities of glycoproteins. Anal Biochem. 2002;304:70–90. doi: 10.1006/abio.2002.5619. [DOI] [PubMed] [Google Scholar]
- Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan el RM, Wilson IA, Daha MR, Dwek RA, Rudd PM. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278:20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- Ruiz-Calero V, Moyano E, Puignou L, Galceran MT. Pressure-assisted capillary electrophoresis-electrospray ion trap mass spectrometry for the analysis of heparin depolymerised disaccharides. J Chromatogr A. 2001;914:277–291. doi: 10.1016/s0021-9673(00)01181-x. [DOI] [PubMed] [Google Scholar]
- Saad OM, Leary JA. Compositional analysis and quantification of heparin and heparan sulfate by electrospray ionization ion trap mass spectrometry. Anal Chem. 2003;75:2985–2995. doi: 10.1021/ac0340455. [DOI] [PubMed] [Google Scholar]
- Saad OM, Leary JA. Delineating mechanisms of dissociation for isomeric heparin disaccharides using isotope labeling and ion trap tandem mass spectrometry. J Am Soc Mass Spectrom. 2004;15:1274–1286. doi: 10.1016/j.jasms.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Saad OM, Ebel H, Uchimura K, Rosen SD, Bertozzi CR, Leary JA. Compositional profiling of heparin/heparan sulfate using mass spectrometry: Assay for specificity of a novel extracellular human endosulfatase. Glycobiology. 2005;15:818–826. doi: 10.1093/glycob/cwi064. [DOI] [PubMed] [Google Scholar]
- Saitoh H, Takagaki K, Majima M, Nakamura T, Matsuki A, Kasai M, Narita H, Endo M. Enzymic reconstruction of glycosaminoglycan oligosaccharide chains using the transglycosylation reaction of bovine testicular hyaluronidase. J Biol Chem. 1995;270:3741–3747. doi: 10.1074/jbc.270.8.3741. [DOI] [PubMed] [Google Scholar]
- Sakai S, Hirano K, Toyoda H, Linhardt RJ, Toida T. Matrix assisted laser desorption ionization-time of flight mass spectrometry analysis of hyaluronan oligosaccharides. Anal Chim Acta. 2007;593:207–213. doi: 10.1016/j.aca.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz BL, Packer NH, Karlsson NG. Small-scale analysis of O-linked oligosaccharides from glycoproteins and mucins separated by gel electrophoresis. Anal Chem. 2002;74:6088–6097. doi: 10.1021/ac025890a. [DOI] [PubMed] [Google Scholar]
- Seidler DG, Peter-Katalinic J, Zamfir AD. Galactosaminoglycan function and oligosaccharide structure determination. Sci World J. 2007;7:233–241. doi: 10.1100/tsw.2007.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyrek E, Dubin PL, Henriksen J. Nonspecific electrostatic binding characteristics of the heparin-antithrombin interaction. Biopolymers. 2007;86:249–259. doi: 10.1002/bip.20731. [DOI] [PubMed] [Google Scholar]
- Simpson RC, Fenselau CC, Hardy MR, Townsend RR, Lee YC, Cotter RJ. Adaptation of a thermospray liquid chromatography/mass spectrometry interface for use with alkaline anion exchange liquid chromatography of carbohydrates. Anal Chem. 1990;62:248–252. doi: 10.1021/ac00202a005. [DOI] [PubMed] [Google Scholar]
- Stuhlsatz HW, Hirtzel F, Keller R, Cosma S, Greiling H. Studies on the polydispersity and heterogeneity of proteokeratan sulfate from calf and porcine cornea. Z Physiol Chem. 1981;362:841–852. doi: 10.1515/bchm2.1981.362.2.841. [DOI] [PubMed] [Google Scholar]
- Tai GH, Huckerby TN, Nieduszynski IA. Multiple non-reducing chain termini isolated from bovine corneal keratan sulfates. J Biol Chem. 1996;271:23535–23546. doi: 10.1074/jbc.271.38.23535. [DOI] [PubMed] [Google Scholar]
- Tai GH, Nieduszynski IA, Fullwood NJ, Huckerby TN. Human corneal keratan sulfates. J Biol Chem. 1997;272:28227–28231. doi: 10.1074/jbc.272.45.28227. [DOI] [PubMed] [Google Scholar]
- Takagaki K, Ishido K. Synthesis of chondroitin sulfate oligosaccharides using enzymatic reconstruction. Trends Glycosci Glycotechnol. 2000;12:295–306. [Google Scholar]
- Takagaki K, Kojima K, Majima M, Nakamura T, Kato I, Endo M. Ion-spray mass spectrometric analysis of glycosaminoglycan oligosaccharides. Glycoconj J. 1992;9:174–179. doi: 10.1007/BF00731162. [DOI] [PubMed] [Google Scholar]
- Takagaki K, Nakamura T, Izumi J, Saitoh H, Endo M, Kojima K, Kato I, Majima M. Characterization of hydrolysis and transglycosylation by testicular hyaluronidase using ion-spray mass spectrometry. Biochemistry. 1994;33:6503–6507. doi: 10.1021/bi00187a017. [DOI] [PubMed] [Google Scholar]
- Takagaki K, Munakata H, Majima M, Endo M. Enzymatic reconstruction of a hybrid glycosaminoglycan containing 6- sulfated, 4-sulfated, and unsulfated N-acetylgalactosamine. Biochem Biophys Res Commun. 1999;258:741–744. doi: 10.1006/bbrc.1999.0697. [DOI] [PubMed] [Google Scholar]
- Takagaki K, Munakata H, Kakizaki I, Majima M, Endo M. Enzymatic reconstruction of dermatan sulfate. Biochem Biophys Res Commun. 2000a;270:588–593. doi: 10.1006/bbrc.2000.2452. [DOI] [PubMed] [Google Scholar]
- Takagaki K, Munakata H, Majima M, Kakizaki I, Endo M. Chimeric glycosaminoglycan oligosaccharides synthesized by enzymatic reconstruction and their use in substrate specificity determination of Streptococcus hyaluronidase. J Biochem (Tokyo) 2000b;127:695–702. doi: 10.1093/oxfordjournals.jbchem.a022659. [DOI] [PubMed] [Google Scholar]
- Takagaki K, Munakata H, Kakizaki I, Iwafune M, Itabashi T, Endo M. Domain structure of chondroitin sulfate E octasaccharides binding to type V collagen. J Biol Chem. 2002;277:8882–8889. doi: 10.1074/jbc.M106479200. [DOI] [PubMed] [Google Scholar]
- Tang PW, Scudder P, Mehmet H, Hounsell EF, Feizi T. Sulphate groups are involved in the antigenicity of keratan sulphate and mask in antigen expression on their poly-N-acetyllactosamine backbones. An immu-nochemical and chromatographic study of keratan sulphate oligosac-charides after desulphation or nitrosation. Eur J Biochem. 1986;160:537–545. doi: 10.1111/j.1432-1033.1986.tb10072.x. [DOI] [PubMed] [Google Scholar]
- Thanawiroon C, Rice KG, Toida T, Linhardt RJ. LC/MS sequencing approach for highly sulfated heparin-derived oligosaccharides. J Biol Chem. 2004;279:2608–2615. doi: 10.1074/jbc.M304772200. [DOI] [PubMed] [Google Scholar]
- Thomsson KA, Karlsson NG, Hansson GC. Liquid chromatography-electrospray mass spectrometry as a tool for the analysis of sulfated oligosaccharides from mucin glycoproteins. J Chromatogr A. 1999;854:131–139. doi: 10.1016/s0021-9673(99)00625-1. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan. In: Iozzo RV, editor. Proteoglycans structure, biology and molecular interactions. Marcel Dekker, Inc.; New York: 2000. pp. 61–92. [Google Scholar]
- Toyoda H, Motoki K, Tanikawa M, Shinomiya K, Akiyama H, Imanari T. Determination of human urinary hyaluronic acid, chondroitin sulphate and dermatan sulphate as their unsaturated disaccharides by high-performance liquid chromatography. J Chromatogr. 1991;565:141–148. doi: 10.1016/0378-4347(91)80378-p. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Kinoshita-Toyoda A, Fox B, Selleck SB. Structural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless, and tout-velu. Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymes. J Biol Chem. 2000;275:21856–21861. doi: 10.1074/jbc.M003540200. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Kinoshita-Toyoda A, Selleck SB. Structural analysis of glycosaminoglycans in Drosophila and Caenorhabditis elegans and demonstration that tout-velu, a Drosophila gene related to EXT tumor suppressors, affects heparan sulfate in vivo. J Biol Chem. 2000;275:2269–2275. doi: 10.1074/jbc.275.4.2269. [DOI] [PubMed] [Google Scholar]
- Turnbull JE, Hopwood JJ, Gallagher JT. A strategy for rapid sequencing of heparan sulfate and heparin saccharides. Proc Natl Acad Sci USA. 1999;96:2698–2703. doi: 10.1073/pnas.96.6.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu R, Furukawa J-i, Nakagawa H, Shinohara Y, Deguchi K, Monde K, Nishimura S-I. High throughput quantitative glycomics and glycoform-focused proteomics of murine dermis and epidermis. Mol Cell Proteomics. 2005;4:1977–1989. doi: 10.1074/mcp.M500203-MCP200. [DOI] [PubMed] [Google Scholar]
- Varki A, Cummings R, Esko J, Freeze H, Hart G, Marth G. Essentials of glycobiology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1999. [PubMed] [Google Scholar]
- Venkataraman G, Shriver Z, Raman R, Sasisekharan R. Sequencing complex polysaccharides. Science. 1999;286:537–542. doi: 10.1126/science.286.5439.537. [DOI] [PubMed] [Google Scholar]
- Vives RR, Goodger S, Pye DA. Combined strong anion-exchange HPLC and PAGE approach for the purification of heparan sulphate oligosaccharides. Biochem J. 2001;354:141–147. doi: 10.1042/0264-6021:3540141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi N. Advances in chondroitin sulfate analysis: Application in physiological and pathological States of connective tissue and during pharmacological treatment of osteoarthritis. Curr Pharm Des. 2006;12:639–658. doi: 10.2174/138161206775474350. [DOI] [PubMed] [Google Scholar]
- Volpi N, Maccari F. Electrophoretic approaches to the analysis of complex polysaccharides. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;834:1–13. doi: 10.1016/j.jchromb.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Vongchan P, Warda M, Toyoda H, Toida T, Marks RM, Linhardt RJ. Structural characterization of human liver heparan sulfate. Biochim Biophys Acta. 2005;1721:1–8. doi: 10.1016/j.bbagen.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Vynios DH, Karamanos NK, Tsiganos CP. Advances in analysis of glycosaminoglycans: Its application for the assessment of physiological and pathological states of connective tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:21–38. doi: 10.1016/s1570-0232(02)00498-1. [DOI] [PubMed] [Google Scholar]
- West LA, Roughley P, Nelson FR, Plaas AH. Sulphation heterogeneity in the trisaccharide (GalNAcSbeta1, 4GlcAbeta1,3GalNAcS) isolated from the non-reducing terminal of human aggrecan chondroitin sulphate. Biochem J. 1999;342:223–229. [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Amster IJ, Chi L, Linhardt RJ. Electron detachment dissociation of glycosaminoglycan tetrasaccharides. J Am Soc Mass Spectrom. 2007a;18:234–244. doi: 10.1016/j.jasms.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Chi L, Linhardt RJ, Amster IJ. Distinguishing glucuronic from iduronic acid in glycosaminoglycan tetrasaccharides by using electron detachment dissociation. Anal Chem. 2007b;79:2015–2022. doi: 10.1021/ac061636x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M, Koeleman CAM, Deelder AM, Hokke CN. Normal-phase nanoscale liquid chromatography–Mass spectrometry of underivatized oligosaccharides at low-femtomole sensitivity. Anal Chem. 2004;76:833–838. doi: 10.1021/ac034936c. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Deelder AM, Hokke CH. Protein glycosylation analysis by liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;825:124–133. doi: 10.1016/j.jchromb.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Koeleman CA, Hokke CH, Deelder AM. Protein glycosylation analyzed by normal-phase nano-liquid chromatogra- phy–mass spectrometry of glycopeptides. Anal Chem. 2005;77:886–894. doi: 10.1021/ac048619x. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Hase S, Fukuda S, Sano O, Ikenaka T. Structures of the sugar chains of interferon-gamma produced by human myelomonocyte cell line HBL-38. J Biochem (Tokyo) 1989;105:547–555. doi: 10.1093/oxfordjournals.jbchem.a122703. [DOI] [PubMed] [Google Scholar]
- Yang B, Yang BL, Savani RC, Turley EA. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994;13:286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Miyauchi S, Kikuchi H, Tawada A, Tokuyasu K. Analysis of unsaturated disaccharides from glycosaminoglycuronan by high-performance liquid-chromatography. Anal Biochem. 1989;177:327–332. doi: 10.1016/0003-2697(89)90061-4. [DOI] [PubMed] [Google Scholar]
- Zaia J. Mass spectrometry of oligosaccharides. Mass Spectrom Rev. 2004;23:161–227. doi: 10.1002/mas.10073. [DOI] [PubMed] [Google Scholar]
- Zaia J. Principles of mass spectrometry of glycosaminoglycans. J Biomacromol Mass Spectrom. 2005;1:3–36. [Google Scholar]
- Zaia J. Mass spectrometric ionization of carbohydrates. In: Gross ML, Caprioli RM, editors. Encyclopedia of mass spectrometry. Vol. 6. Elsevier; Amsterdam: 2006. pp. 889–903. Ionization methods. [Google Scholar]
- Zaia J, Costello CE. Compositional analysis of glycosaminoglycans by electrospray mass spectrometry. Anal Chem. 2001;73:233–239. doi: 10.1021/ac000777a. [DOI] [PubMed] [Google Scholar]
- Zaia J, McClellan JE, Costello CE. Tandem mass spectrometric determination of the 4S/6S sulfation sequence in chondroitin sulfate oligosaccharides. Anal Chem. 2001;73:6030–6039. doi: 10.1021/ac015577t. [DOI] [PubMed] [Google Scholar]
- Zamfir A, Peter-Katalinicć J. Glycoscreening by on-line sheathless capillary electrophoresis/electrospray ionization-quadrupole time of flight-tandem mass spectrometry. Electrophoresis. 2001;22:2448–2457. doi: 10.1002/1522-2683(200107)22:12<2448::AID-ELPS2448>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Zamfir A, Peter-Katalinicć J. Capillary electrophoresis-mass spectrometry for glycoscreening in biomedical research. Electrophoresis. 2004;25:1949–1963. doi: 10.1002/elps.200405825. [DOI] [PubMed] [Google Scholar]
- Zamfir A, Seidler DG, Kresse H, Peter-Katalinc J. Structural characterization of chondroitin/dermatan sulfate oligosaccharides from bovine aorta by capillary electrophoresis and electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun Mass Spectrom. 2002;16:2015–2024. doi: 10.1002/rcm.820. [DOI] [PubMed] [Google Scholar]
- Zamfir A, Seidler DG, Schonherr E, Kresse H, Peter-Katalinicć J. Online sheathless capillary electrophoresis/nanoelectrospray ionizationtandem mass spectrometry for the analysis of glycosaminoglycan oligosaccharides. Electrophoresis. 2004;25:2010–2016. doi: 10.1002/elps.200405925. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Conrad AH, Tasheva ES, An K, Corpuz LM, Kariya Y, Suzuki K, Conrad GW. Detection and quantification of sulfated dis-accharides from keratan sulfate and chondroitin/dermatan sulfate during chick corneal development by ESI-MS/MS. Invest Ophthalmol Visual Sci. 2005a;46:1604–1614. doi: 10.1167/iovs.04-1453. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kariya Y, Conrad AH, Tasheva ES, Conrad GW. Analysis of keratan sulfate oligosaccharides by electrospray ionization tandem mass spectrometry. Anal Chem. 2005b;77:902–910. doi: 10.1021/ac040074j. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xie J, Liu J, Linhardt RJ. Tandem MS can distinguish hyaluronic acid from N-acetylheparosan. J Am Soc Mass Spectrom. 2008;19:82–90. doi: 10.1016/j.jasms.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A, Zaia J. Separation of heparin oligosaccharides by size-exclusion chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;837:76–86. doi: 10.1016/j.jchromb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Zubarev RA. Reactions of polypeptide ions with electrons in the gas phase. Mass Spectrom Rev. 2003;22:57–77. doi: 10.1002/mas.10042. [DOI] [PubMed] [Google Scholar]