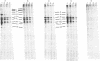Abstract
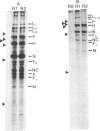
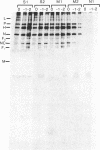
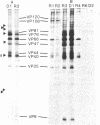
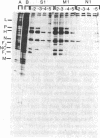
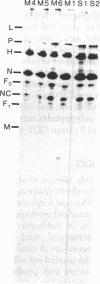
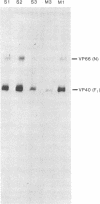
Precipitation with hyperimmune rabbit sera, sera from patients convalescing from measles, and sera from patients with subacute sclerosing panencephalitis, followed by electrophoresis, enabled antigenic relationships between the individual polypeptides of measles and canine distemper viruses to be examined. Virus isolates from patients with acute measles or subacute sclerosing panencephalitis showed no antigenic differences. With rabbit hyperimmune sera, antigenic crossreactivity was present between all polypeptides of measles and canine distemper viruses except H. The N polypeptides showed the highest degree of crossreactivity and were interpreted as group-specific antigens. Both convalescent measles sera and sera from subacute sclerosing panencephalitis showed high antibody titers to all measles polypeptides except L and M. However, these sera contained only low activities to the N and F1 polypeptides from canine distemper virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breese S. S., Jr, De Boer C. J. Ferritin-tagged antibody cross-reactions among rinderpest, canine distemper, and measles viruses. J Gen Virol. 1973 Jul;20(1):121–125. doi: 10.1099/0022-1317-20-1-121. [DOI] [PubMed] [Google Scholar]
- Cretescu L., Beare A. S., Schild G. C. Formation of antibody to matrix protein in experimental human influenza A virus infections. Infect Immun. 1978 Nov;22(2):322–327. doi: 10.1128/iai.22.2.322-327.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs E. P., Taylor W. P., Lawman M. J., Bryant J. Classification of peste des petits ruminants virus as the fourth member of the genus Morbillivirus. Intervirology. 1979;11(5):268–274. doi: 10.1159/000149044. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Silver S. M., Choppin P. W. Measles virus polypeptides synthesis in infected cells. Virology. 1978 May 1;86(1):254–263. doi: 10.1016/0042-6822(78)90025-9. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Kiessling W., ter Meulen V. Membrane proteins of subacute sclerosing panencephalitis and measles viruses. Nature. 1978 Mar 30;272(5652):460–462. doi: 10.1038/272460a0. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Choppin P. W. A comparison of the polypeptides of four measles virus strains. Virology. 1977 May 15;78(2):463–474. doi: 10.1016/0042-6822(77)90123-4. [DOI] [PubMed] [Google Scholar]
- Orvell C., Norrby E. Further studies on the immunologic relationships among measles, distemper, and rinderpest viruses. J Immunol. 1974 Dec;113(6):1850–1858. [PubMed] [Google Scholar]
- Paucha E., Harvey R., Smith A. E. Cell-free synthesis of simian virus 40 T-antigens. J Virol. 1978 Oct;28(1):154–170. doi: 10.1128/jvi.28.1.154-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott R., Becht H., Orlich Antigenic relationship between the surface antigens of avian and equine influenze viruses. Med Microbiol Immunol. 1975 Sep 19;161(4):253–261. doi: 10.1007/BF02122713. [DOI] [PubMed] [Google Scholar]
- Rott R. Molecular basis of infectivity and pathogenicity of myxovirus. Brief review. Arch Virol. 1979;59(4):285–298. doi: 10.1007/BF01317469. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Hay A. J., Skehel J. J. Characterization of virus-specific messenger RNAs from avian fibroblasts infected with fowl plague virus. J Gen Virol. 1977 Aug;36(2):237–248. doi: 10.1099/0022-1317-36-2-237. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. L., Norrby E. Structural polypeptides of measles virus. J Gen Virol. 1978 May;39(2):219–229. doi: 10.1099/0022-1317-39-2-219. [DOI] [PubMed] [Google Scholar]
- Waters D. J., Bussell R. H. Polypeptide composition of measles and canine distemper viruses. Virology. 1973 Oct;55(2):554–557. doi: 10.1016/0042-6822(73)90202-x. [DOI] [PubMed] [Google Scholar]
- Yamanouchi K., Egashira Y., Uchida N., Kodama H., Kobune F. Giant cell formation in lymphoid tissues of monkeys inoculated with various strains of measles virus. Jpn J Med Sci Biol. 1970 Jun;23(3):131–145. doi: 10.7883/yoken1952.23.131. [DOI] [PubMed] [Google Scholar]