Abstract
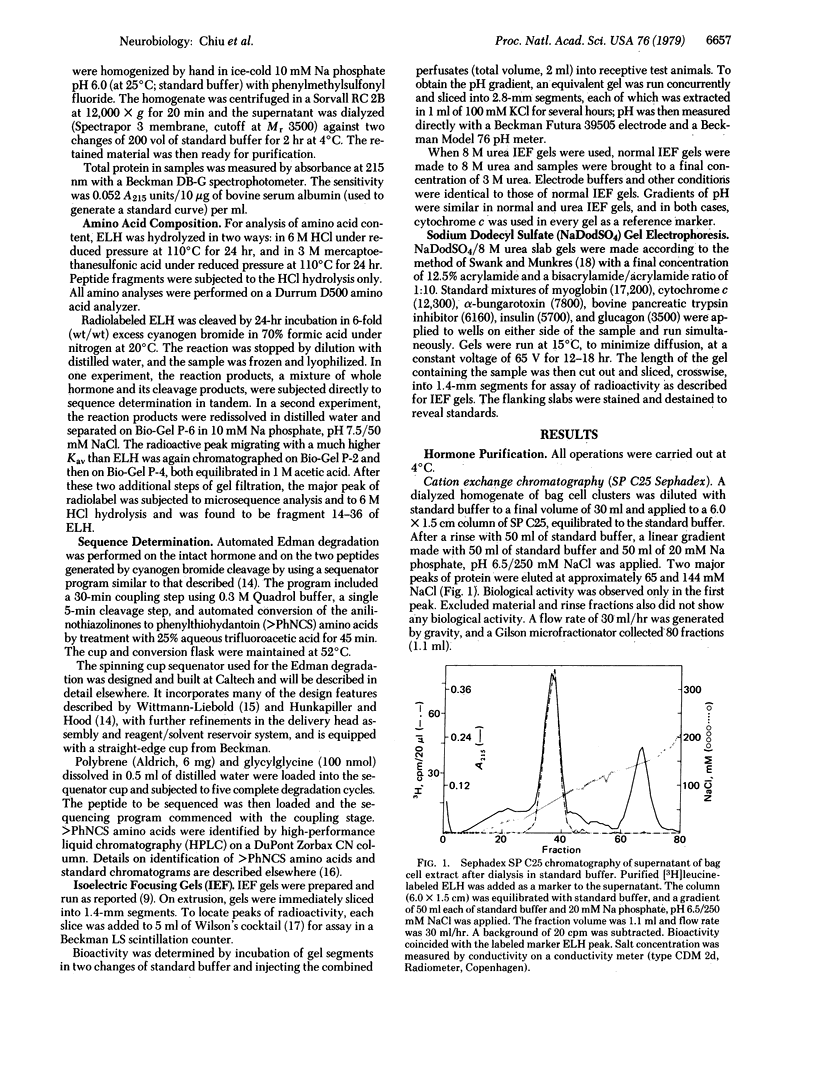
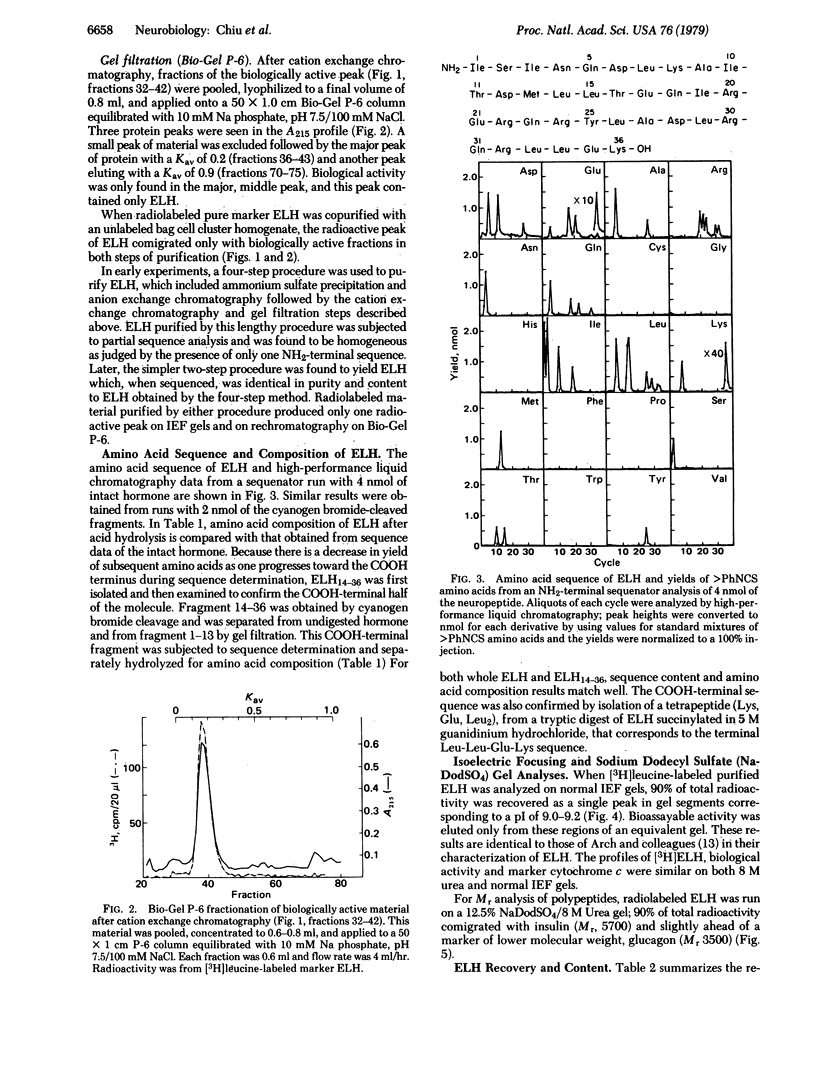
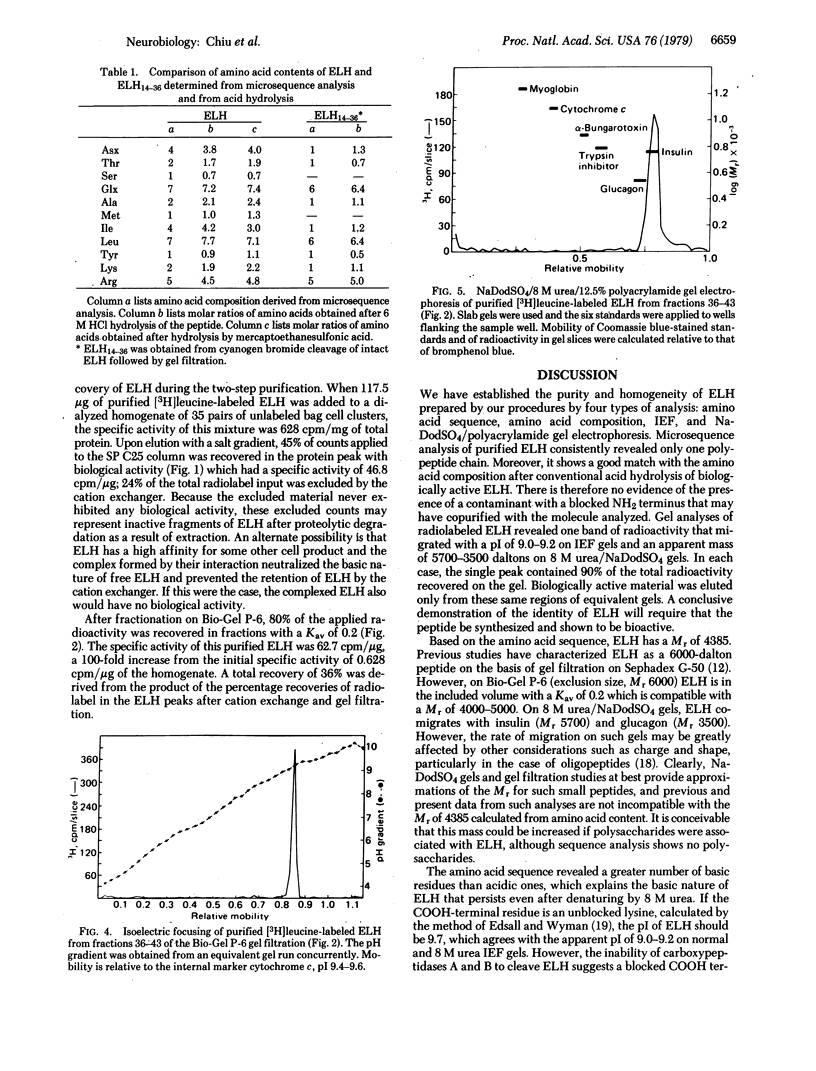
Egg-laying hormone (ELH), a neuropeptide synthesized by the bag cell neurons, induces egg laying and its correlated behavior in Aplysia californica. In the present study, ELH has been purified to homogeneity and its primary structure has been determined. We find this molecule to have 36 amino acid residues with a Mr of 4385 and a calculated isoelectric point of 9.7. Direct microsequence analysis revealed a single amino acid sequence that is in agreement with the amino acid composition determined after acid hydrolysis of ELH: H-Ile-Ser-Ile-Asn-Gln-Asp-Leu-Lys-Ala-Ile-Thr-Asp-Met-Leu-Leu-Thr-Glu-Gln- Ile-Arg-Glu-Arg-Gln-Arg-Tyr-Leu-Ala-Asp-Leu-Arg-Gln-Arg-Leu-Leu-Glu-Lys-OH. Enzyme data indicate that the COOH-terminal lysine may be modified but its exact nature remains to be determined. There is no similarity between the amino acid sequence of ELH and that of presently known vertebrate neuropeptides. The two-step purification procedure, starting with a homogenate of bag cell clusters, consisted of cation exchange chromatography on SP C25 (Sephadex) followed by gel filtration on Bio-Gel P-6. Our purification results in a 100-fold enrichment of ELH from bag cell homogenates and a 36% recovery of purified radiolabeled marker ELH. Analysis of purified ELH radiolabeled with [35S]methionine or [3H]leucine on isoelectric focusing gels and on 8 M urea/sodium dodecyl sulfate gels showed only a single peak containing 90% of the radiolabel. Radiolabeled ELH migrated with a pI of 9.0-9.2 and an apparent Mr of 3500-5700. ELH retained egg-laying bioactivity when eluted from this segment of the gel. We find that 2.5 nmol of pure ELH consistently induces egg laying at 20°C.
Keywords: bag cells, amino acid sequence, neurosecretory peptide, molluskan neuropeptide, microsequence analysis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch S. Biosynthesis of the egg-laying hormone (ELH) in the bag cell neurons of Aplysia californica. J Gen Physiol. 1972 Jul;60(1):102–119. doi: 10.1085/jgp.60.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch S., Earley P., Smock T. Biochemical isolation and physiological identification of the egg-laying hormone in Aplysia californica. J Gen Physiol. 1976 Aug;68(2):197–210. doi: 10.1085/jgp.68.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek F. E., Cobbs J. S., Pinsker H. M. Bag cell electrical activity underlying spontaneous egg laying in freely behaving Aplysia brasiliana. J Neurophysiol. 1979 May;42(3):804–817. doi: 10.1152/jn.1979.42.3.804. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Kupfermann I., Kandel E. R. Electrophysiological properties and functional interconnections of two symmetrical neurosecretory clusters (bag cells) in abdominal ganglion of Aplysia. J Neurophysiol. 1970 Nov;33(6):865–876. doi: 10.1152/jn.1970.33.6.865. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Stimulation of egg laying: possible neuroendocrine function of bag cells of abdominal ganglion of Aplysia californica. Nature. 1967 Nov 25;216(5117):814–815. doi: 10.1038/216814a0. [DOI] [PubMed] [Google Scholar]
- Pinsker H. M., Dudek F. E. Bag cell control of egg laying in freely behaving aplysia. Science. 1977 Jul 29;197(4302):490–493. doi: 10.1126/science.197.4302.490. [DOI] [PubMed] [Google Scholar]
- Price D. A., Greenberg M. J. Structure of a molluscan cardioexcitatory neuropeptide. Science. 1977 Aug 12;197(4304):670–671. doi: 10.1126/science.877582. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Ward S., Wilson D. L., Gilliam J. J. Methods for fractionation and scintillation counting of radioisotope-labeled polyacrylamide gels. Anal Biochem. 1970 Nov;38(1):90–97. doi: 10.1016/0003-2697(70)90158-2. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B. Amino acid sequence studies on ten ribosomal proteins of Escherichia coli with an improved sequenator equipped with an automatic conversion device. Hoppe Seylers Z Physiol Chem. 1973 Oct-Nov;354(10-11):1415–1431. doi: 10.1515/bchm2.1973.354.2.1415. [DOI] [PubMed] [Google Scholar]


