Abstract
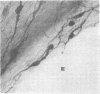
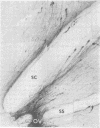
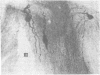
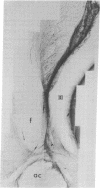
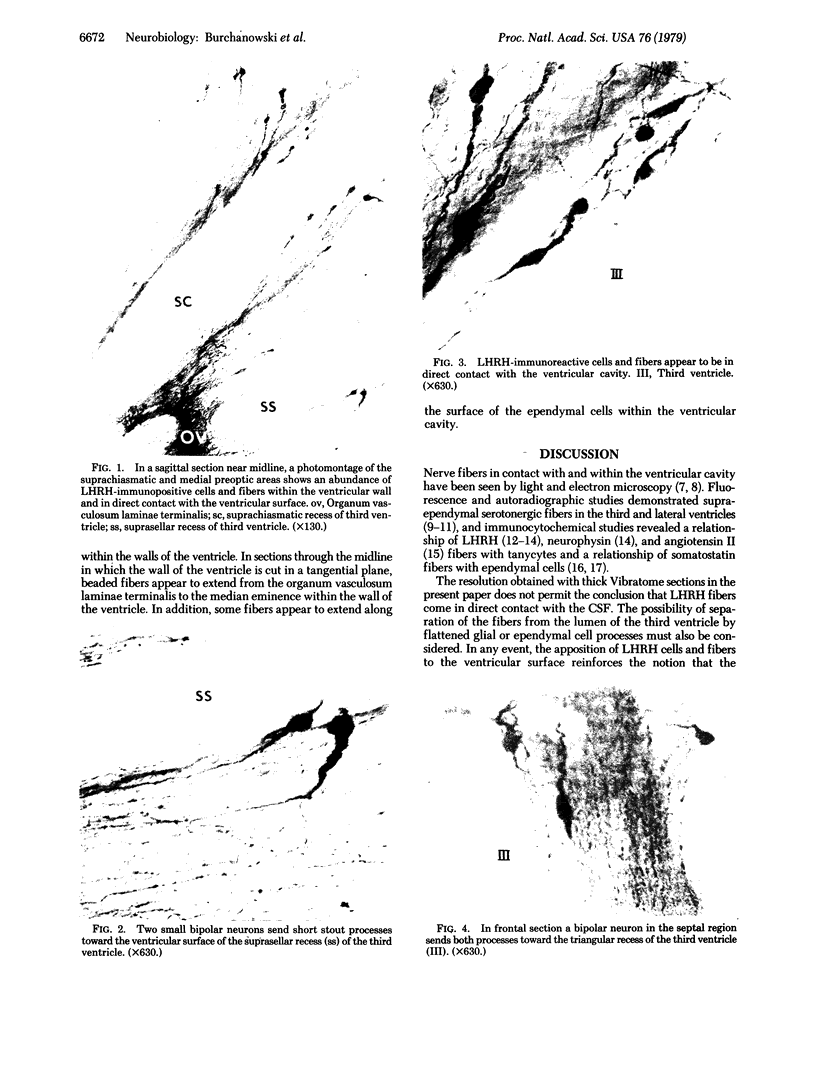
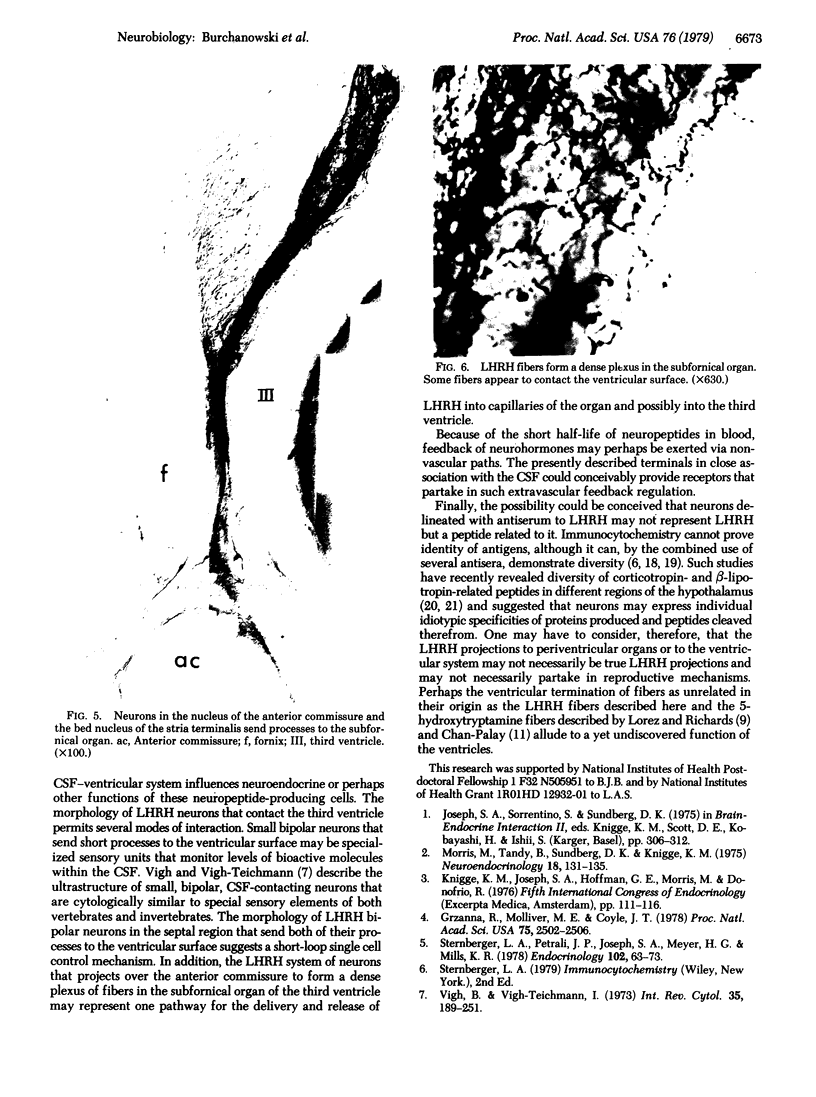
Immunospecific staining in 100-micron-thick Vibratome section by the unlabeled antibody method resembles Golgi staining and reveals an abundance of luliberin- (luteinizing hormone releasing hormone, LHRH) positive cells and fibers in close contact with the surface of the third ventricle. The polarity of LHRH cells can be seen and processes can be traced for several millimeters. In the medial preoptic and suprachiasmatic areas bipolar LHRH neurons send short stout processes to the ventricular surface and long processes toward the organum vasculosum laminae terminalis. These cells resemble the receptor cells contacting the cerebrospinal fluid that have been described by Vigh and Vigh-Teichmann [Vigh, B. & Vigh-Teichmann, I. (1973) Int. Rev. Cytol. 35, 189-251]. In the septal region some bipolar neurons send both of their processes towards the ventricular surface. LHRH neurons in the nucleus of the anterior commissure and the bed nucleus of the stria terminalis project over the anterior commissure to form a dense plexus of fibers in the subfornical organ. The proximity of LHRH perikarya and fibers to the ventricular surface supports the hypothesis [Knigge, K.M., Joseph, S.A., Scott, D.E. & Jacobs, J.J. (1971) in The Neuroendocrinology of Human Reproduction, eds. Mack, H.C. & Sherman, A.J., (Thomas, Springfield, IL), pp. 6-22.] that the cerebrospinal fluid functions in neuroendocrine control mechanisms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Gallager D. W. Raphe origin of serotonergic nerves terminating in the cerebral ventricles. Brain Res. 1975 May 2;88(2):221–231. doi: 10.1016/0006-8993(75)90386-8. [DOI] [PubMed] [Google Scholar]
- Baker B. L., Yu Y. Distribution of growth hormone-release-inhibiting hormone (somatostatin) in the rat brain as observed with immunocytochemistry. Anat Rec. 1976 Nov;186(3):343–355. doi: 10.1002/ar.1091860302. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Indoleamine neurons and their processes in the normal rat brain and in chronic diet-induced thiamine deficiency demonstrated by uptake of 3H-serotonin. J Comp Neurol. 1977 Dec 15;176(4):467–493. doi: 10.1002/cne.901760402. [DOI] [PubMed] [Google Scholar]
- Changaris D. G., Severs W. B., Keil L. C. Localization of angiotensin in rat brain. J Histochem Cytochem. 1978 Jul;26(7):593–607. doi: 10.1177/26.7.357644. [DOI] [PubMed] [Google Scholar]
- Grzanna R., Molliver M. E., Coyle J. T. Visualization of central noradrenergic neurons in thick sections by the unlabeled antibody method: a transmitter-specific Golgi image. Proc Natl Acad Sci U S A. 1978 May;75(5):2502–2506. doi: 10.1073/pnas.75.5.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. A., Sternberger L. A. The unlabeled antibody method. Contrasting color staining of beta-lipotropin and ACTH-associated hypothalamic peptides without antibody removal. J Histochem Cytochem. 1979 Nov;27(11):1430–1437. doi: 10.1177/27.11.92499. [DOI] [PubMed] [Google Scholar]
- Knigge K. M., Joseph S. A., Hoffman G. E. Organization of LRF-and SRIF-neurons in the endocrine hypothalamus. Res Publ Assoc Res Nerv Ment Dis. 1978;56:49–67. [PubMed] [Google Scholar]
- Larsson L. I., Polak J. M., Buffa R., Sundler F., Solcia E. On the immunocytochemical localization of the vasoactive intestinal polypeptide. J Histochem Cytochem. 1979 May;27(5):936–938. doi: 10.1177/27.5.479555. [DOI] [PubMed] [Google Scholar]
- Lorez H. P., Richards J. G. Distribution of indolealkylamine nerve terminals in the ventricles of the rat brain. Z Zellforsch Mikrosk Anat. 1973 Nov 15;144(4):511–522. doi: 10.1007/BF00307377. [DOI] [PubMed] [Google Scholar]
- Morris M., Tandy B., Sundberg D. K., Knigge K. M. Modification of brain and CSF LH-RH following deafferentation. Neuroendocrinology. 1975;18(1):131–135. doi: 10.1159/000122391. [DOI] [PubMed] [Google Scholar]
- Rodríguez E. M. The cerebrospinal fluid as a pathway in neuroendocrine integration. J Endocrinol. 1976 Dec;71(3):407–443. doi: 10.1677/joe.0.0710407. [DOI] [PubMed] [Google Scholar]
- Silverman A. J. Distribution of luteinizing hormone-releasing hormone (LHRH) in the guinea pig brain. Endocrinology. 1976 Jul;99(1):30–41. doi: 10.1210/endo-99-1-30. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Joseph S. A. The unlabeled antibody method. Contrasting color staining of paired pituitary hormones without antibody removal. J Histochem Cytochem. 1979 Nov;27(11):1424–1429. doi: 10.1177/27.11.92498. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Petrali J. P., Joseph S. A., Meyer H. G., Mills K. R. Specificity of the immunocytochemical luteinizing hormone-releasing hormone receptor reaction. Endocrinology. 1978 Jan;102(1):63–73. doi: 10.1210/endo-102-1-63. [DOI] [PubMed] [Google Scholar]
- Sundler F., Alumets J., Fahrenkrug J., Håkanson R., Schaffalitzky de Muckadell O. B. Cellular localization and ontogeny of immunoreactive vasoactive intestinal polypeptide (VIP) in the chicken gut. Cell Tissue Res. 1979 Feb 15;196(2):193–201. doi: 10.1007/BF00240095. [DOI] [PubMed] [Google Scholar]
- Vigh B., Vigh-Teichmann I. Comparative ultrastructure of the cerebrospinal fluid-contacting neurons. Int Rev Cytol. 1973;35:189–251. doi: 10.1016/s0074-7696(08)60355-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman E. A., Hsu K. C., Ferin M., Kozlowski G. P. Localization of gonadotropin-releasing hormone (Gn-RH) in the hypothalamus of the mouse by immunoperoxidase technique. Endocrinology. 1974 Jul;95(1):1–8. doi: 10.1210/endo-95-1-1. [DOI] [PubMed] [Google Scholar]