Abstract
Epsilon toxin (ETX), produced by Clostridium perfringens types B and D, is among the most lethal toxins known. ETX is a potential bioterrorism threat that was listed as a Category B agent by the U.S. Centers for Disease Control until 2012 and it still remains a toxin of interest for several government agencies. We produced a monoclonal antibody (MAb) against ETX (ETX MAb c4D7) in Nicotiana benthamiana and characterized its preventive and therapeutic efficacy in mice. The ETX preparation used was highly lethal for mice (LD50 =1.6 μg/kg) and resulted in a mean time from inoculation to death of 18 and 180 minutes when administered intravenously or intraperitoneally, respectively. High lethal challenge resulted in dramatic increases of a variety of pro-inflammatory cytokines in serum, while lower, but still lethal doses, did not elicit such responses. ETX MAb c4D7 was highly effective prophylactically (ED50 = 0.3 mg/kg; ED100 = 0.8 mg/kg) and also provided protection when delivered 15-30 minutes post-ETX intoxication. These data suggest that ETX MAb c4D7 may have use as a pre- and post-exposure treatment for ETX intoxication.
Keywords: epsilon toxin, monoclonal antibody, Nicotiana benthamiana, preventive, therapeutic
1. Introduction
Epsilon toxin (ETX) is a 33 kDa protein produced by Clostridium perfringens types B and D. ETX is considered the third most potent of all clostridial toxins after botulinum and tetanus toxins (Lonchamp et al., 2010; Robertson et al., 2011; Stiles et al., 2013). Accordingly, ETX has been of concern as a potential bioterrorism agent, which was listed by the U.S. Centers for Disease Control as a Category B agent until 2012 and still remains a toxin of interest to many government agencies throughout the world. Category B agents are considered to be moderately easy to disseminate and would result in significant morbidity if human populations were to be exposed.
C. perfringens type D naturally affects several domestic animal species, including sheep, goats and cattle, causing enterotoxemia, when ETX is produced in the intestine and absorbed into the systemic circulation targeting several internal organs (Garcia et al., 2012). The action of ETX on the gastrointestinal tract is usually minimal, except in goats where it may produce enterocolitis (Garcia et al., 2012).
ETX is produced during the vegetative growth of C. perfringens and secreted as a relatively inactive prototoxin of 32.9 kDa that can be fully activated by removal of 11-13 N-terminal and/or 22-29 C-terminal amino acids (Minami et al., 1997; Miyata et al., 2001). Proteases capable of activating ETX include trypsin, chymotrypsin, C. perfringens lambda toxin and others (Bokori-Brown et al., 2011). ETX appears to form heptameric pores in target cells including endothelial cells; the action of this toxin on the latter causes increased vascular permeability that leads to vasogenic edema mainly in the brain and lungs, that often produces fatal neurologic and respiratory disturbances (Bokori-Brown et al., 2011). ETX may also have a direct effect on neurons by stimulating the release of dopamine from dopaminergic nerve endings and glutamate within the rat and mouse hippocampus (Finnie et al., 1999, Bokori-Brown et al., 2011).
Passive immunization with monoclonal antibodies (MAbs) has been used to neutralize the action of a wide variety of toxins and microorganisms, including ETX (Zeitlin et al., 2000; Chow and Cassadevall, 2012). 4D7 is a murine anti-ETX MAb developed as an ELISA reagent with known neutralizing activity (Hauer and Clough, 1999). To make this MAb more appropriate for potential human use, we chimerized the murine variable regions of 4D7 with human constant regions (ETX MAb c4D7). This antibody was produced in a rapid low-cost Nicotiana benthamiana manufacturing system (magnICON) previously used for production of other MAbs (Pogue et al., 2010) and vaccines (Bendami et al., 2010) under Good Manufacturing Practices (GMP). We present here a study of the preventive and therapeutic use of ETX MAb c4D7 against ETX in mice.
2. Material and methods
2.1 Animals, reagents and general experimental procedures
Male and female Balb/C mice (17 to 21 g) housed in a temperature and light cycle controlled room were used. All procedures involving animals were reviewed and approved by the University of California, Davis Committee for Animal Care and Use (Permit 16940). Intravenous (iv) and intraperitoneal (ip) injections were performed by inserting a 0.5-inch, 27-gauge needle into the coccygeal vein or into the caudal part of the abdominal cavity, respectively. Total injection volume (iv or ip) was always 0.5 ml. Trypsin-activated purified ETX was obtained from BEI (ATCC 3626). This toxin preparation was found to be > 95% pure. ETX was diluted in 1% peptone water as needed.
The assay endpoints for each experiment were defined as spontaneous death, development of severe clinical signs necessitating euthanasia, or survival without clinical alterations during a set period of time (see below). Euthanasia was performed by CO2 asphyxiation.
2.2 Determination of the LD50 for ETX
In order to determine the iv and ip lethal dose fifty (LD50) of the ETX preparation used, serial dilutions of this toxin were prepared to obtain concentrations of 2000 ng/ml, 200 ng/ml, 20 ng/ml, 2 ng/ml, 0.2 ng/ml and 0.02 ng/ml. Groups of 8 mice received 0.5 ml iv or ip of each dilution. The LD50 was calculated as previously described (Sayeed et al., 2005). Negative control mice were treated iv with 1% peptone water. Maximum assay duration was 48 hrs.
2.3 Route comparison of the effect of ETX
Two groups (n=8/group) of mice were inoculated with 30 LD50 ip and iv, respectively, and the time from inoculation to death was recorded to compare the effect of ETX administered ip versus iv. Maximum assay duration was 48 hrs.
2.4 Cytokine analysis
Cytokine levels in pooled serum of mice inoculated with variable doses of ETX were assayed using the Milliplex® MAP Mouse Cytokine/Chemokine Polystyrene Bead Panel (#MPXMCYTO-70K, Millipore Corp., Billerica, MA 01821). For this, groups of four mice were inoculated iv with 5, 10, 15, 20, 25, 30, 35, 40, 45 or 50 μg/kg of ETX, The mice were bled by cardiac puncture under anesthesia as soon as possible after onset of clinical signs (2 to 6 hours after inoculation) or, in those that did not show clinical alterations, at periods between 2 and 36 hours after inoculation. The serum samples from all the mice in each group were pooled and stored at −80°C until tested. The protocol established by the manufacturer was used. Briefly, mouse serum samples were thawed, mixed by vortexing, and then clarified through filter spin columns (#UFC30DV00, Millipore Corp.) by room temperature centrifugation at 12,000 × g for 4 min. 25 μl of each standard, control, or undiluted sample were added in duplicate to antibody-conjugated beads and incubated in a 96-well filter plate overnight at 2-8°C with shaking at 650 rpm. After 16-18 hr, wells were washed and 25 μl of detection antibody was added to each well. After one hour of incubation (20°C/650 rpm), 25 μl of Streptavidin-Phycoerythrin was added to each well and incubated for 30 min (20°C/650rpm). Final washes were completed following which 150 μl of sheath fluid was added to each well. The plate was analyzed using a Bio-Plex® 200 Suspension Array System (Bio-Rad Laboratories, Hercules, CA). The instrument settings were as follows: 50 events per bead, 100 μl sample size, and gate settings at 8,000 – 15,000. The software used to perform the assay and analyze data was the Bio-Plex Manager™ Software 6.0, which calculated concentrations in pg/ml based on the respective standard curve for each cytokine. Cytokines measured included GM-CSF, IFNγ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12 (p70), IL-13, MCP-1, and TNFα. Only cytokines at detectable levels were reported for the purposes of this analysis. Dosage groups were combined into two major ‘low’ and ‘high’ (5-20 and 25-50 μg/kg, respectively) groupings for ease of interpretation of data and to compensate for lack of group size to support statistical comparison. Group concentrations for individual cytokines were statistically compared using Wilcoxon Signed rank test; P<0.05 was considered significant.
2.5 Production of anti-ETX MAb c4D7
Genes containing the variable region sequences of 4D7 were synthesized (Life Technologies; San Diego, CA) and subsequently cloned into plant expression vectors (TMV and PVX, Icon Genetics, GmbH; Giritch et al., 2006) containing codon-optimized human kappa and human IgG1 constant regions followed by transformation into Agrobacterium tumefaciens strain ICF320 (Icon Genetics). Nicotiana benthamiana plants genetically modified to produce mammalian N-glycans (Strasser et al., 2008) were grown for 4 weeks in an enclosed growth room (20-23°C) and used for vacuum infiltration as previously described (Zeitlin et al., 2013). Seven days post-infiltration, the MAb was extracted from the leaf tissue and purified via protein A chromatography (Zeitlin et al., 2013). The purified MAb was aliquoted and stored at −80°C until use. The MAb was fully assembled and of greater than 97% purity, as determined by SDS-PAGE. Endotoxin levels were measured with Endosafe PTS (Charles River; Wilmington, MA) and were < 100 EU/mg.
2.6 Protection of mice from a lethal challenge with ETX using a single high dose of ETX-MAb c4D7
To determine the initial protective value of the ETX-MAb c4D7, 8 mice were injected ip with 30 mg/kg of this antibody, followed 24 h later by an iv injection of 30 LD50 of ETX. A control group received 30 mg/kg of a C. perfringens alpha-toxin (CPA) Mab (N6H71F3; kindly provided by Dr. P. Hauer, NVSL) diluted in 1% of peptone water. Maximum assay duration was 48 hrs after ETX inoculation. The log-rank (Mantel-Cox) test was used to determine statistical significance in all experiments with c4D7.
2.7 Determination of the minimal dose of ETX-MAb c4D7 that protects mice from a lethal challenge with ETX
To determine the minimal protective dose of ETX MAb c4D7 against a lethal challenge with ETX, two fold dilutions of this antibody (between 1.6 to 0.025 mg/kg) were given ip to 7 groups of 8 mice 24 h prior to an iv challenge with 30 LD50 of ETX. A control group received 0.5 ml of saline solution iv. Maximum assay duration was 48 hrs after ETX inoculation.
2.8 Determination of the duration of the protection afforded by variable doses of c4D7 against 30 LD50 of ETX
To evaluate the duration of the protection conferred by ETX MAb c4D7, variable doses of this antibody (between 0.8 to 8 mg/kg), were given ip to 5 groups of 4 mice each at 1, 5, 10, 15 and 20 days prior to iv challenge with 30 LD50 of ETX. A control group received 0.5 ml of saline solution iv. Maximum assay duration was 48 hrs after ETX inoculation.
2.9 Therapeutic effect of c4D7 after challenge with 30 LD50 of ETX
To evaluate the therapeutic efficacy of ETX MAb c4D7 post-ETX exposure, 5 groups of 8 mice each were injected ip with 30 LD50 of ETX after which 10× the minimal 100% protective dose of ETX MAb c4D7 (determined above: 8 mg/kg) was given iv at 15, 30, 45 or 60 minutes to each group. The control group received only 0.5 ml of saline solution iv 15 min post ETX injection. Maximum assay duration was 48 hrs after ETX MAB c4D7 injection.
3. Results
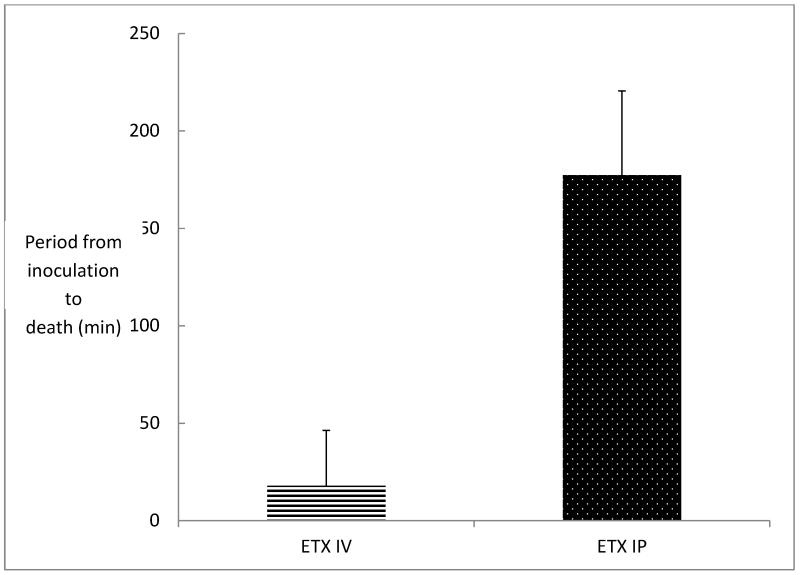
The iv and ip LD50 of the ETX preparation used for this experiment was determined to be 1.6 μg per kg for both injection routes. When groups of mice (n=8) were inoculated iv or ip with 30 LD50 of ETX, lethality (100%) and clinical symptoms (seizures, convulsions, hyperexcitability, rotation, circling and/or depression) were similar for both groups of animals. However, the average time from inoculation to assay end point in mice injected iv with 30 LD50 of ETX was 18 ± 6 minutes, while the average time from inoculation to assay end point in mice injected ip with the same dose of ETX was 177 ± 60 minutes (2.95 h) (Fig. 1).
Figure 1.
Comparison of the period between inoculation and death in mice challenged with 30 LD50 of ETX intravenously versus mice challenged with 30 LD50 of ETX intraperitoneally. Groups of 8 mice were inoculated. The lethality was 100% in both groups. Vertical bars represent SD.
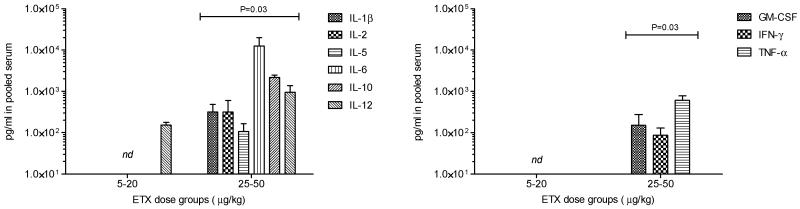
Analysis of the cytokine response in serum taken from mice treated with increasing doses of toxin (Fig. 2) indicated that with smaller lethal doses (5-20 μg/kg; ~ 3-12 LD50), minimal changes to pro-inflammatory cytokines were detected. However, with higher doses (25-50 μg/kg; ~15-30 LD50) of toxin, dramatic increases to a variety of cytokines were observed.
Figure 2.
Cytokine production in pooled serum from animals exposed to either 5-20 or 25-50 μg/kg ETX. Bars represent group averages from animals exposed at (5, 10, 15, or 20 and 25, 30, 35, 40, 45, 50 μg/kg ETX); error bars represent standard deviation. Statistical comparison was performed for differences between the two major groups (P<0.5 was considered significant).
In a pilot passive immunization study, mice (n=8) were injected ip with a single high dose (30 mg/kg) of ETX MAb c4D7 or CPA-MAb and challenged with 30 LD50 of ETX iv. No clinical illness or death was observed in any of the ETX MAb c4D7 treated mice (P < 0.001) while all mice injected with CPA-MAb showed clinical signs similar to those described above and died within one hour of inoculation.
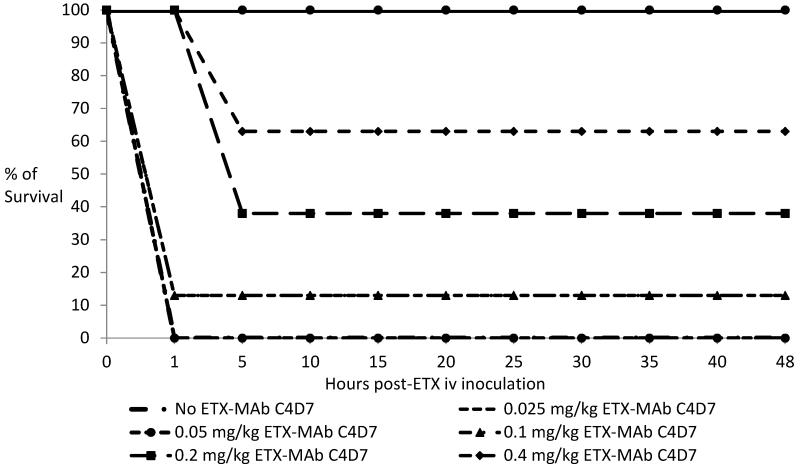
When experiments were performed to determine the minimal prophylactic protective dose of ETX MAb c4D7 administered ip 24 h prior to inoculation against a 30 LD50 iv challenge of ETX, groups of mice (n=8) were completely protected against clinical signs and death (P < 0.001) with 0.8 mg/kg or 1.6 mg/kg of MAb (Fig. 3). Animals passively immunized with doses of 0.4 mg/kg or less, experienced decreasing levels of protection (Fig. 3). In these experiments death occurred between 1 and 5 hours post-ETX inoculation.
Figure 3.
Kaplan-Meier-curves of protection of mice passively immunized with different doses of ETX MAb c4D7 ip and challenged 24 h later with 30 LD50 of ETX iv. Mice injected with 0.8 g/kg or more of this antibody were completely protected against clinical signs and death.
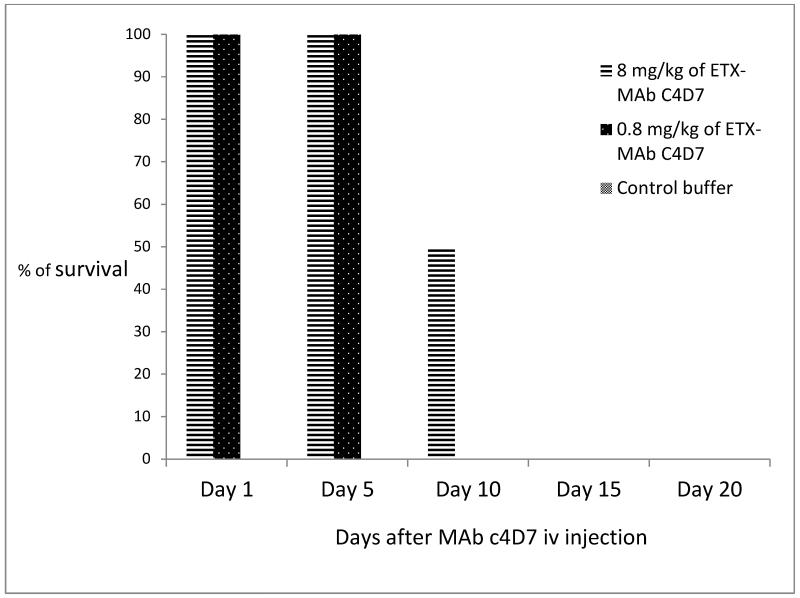
To establish the duration of passive immunity conferred to mice, groups of four mice were treated with 0.8 or 8 mg/kg of ETX MAb c4D7 ip (1× or 10× of the minimal 100% protective dose from the experiment described above) and challenged with 30 LD50 of ETX iv. Mice treated with either 0.8 or 8 mg/kg were completely protected against clinical signs and death (P < 0.01) for up to 5 days after antibody injection and 8 mg/kg afforded 50% protection for 10 days. All animals that developed clinical signs died between 2 and 3 hours post-ETX inoculation (Fig. 4).
Figure 4.
Duration of the immunity in mice passively immunized with ETX MAb c4D7 iv at different intervals and challenged with 30 LD50 of ETX ip.
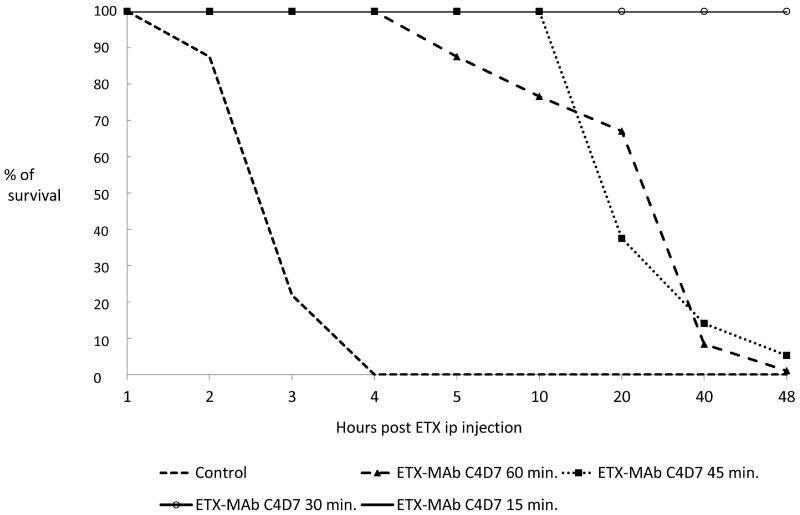
Although the potential post-exposure window is narrow due to the quick lethality of ETX, ETX MAb c4D7 was evaluated to determine whether post-exposure intervention with this antibody could rescue mice (Fig. 5). Groups of mice (n=8) treated iv with 8 mg/kg of c4D7 15 or 30 min after ip inoculation with 30 LD50 ETX were completely protected from clinical signs of intoxication and death (P < 0.001). Injection of ETX MAb c4D7 at 45 and 60 minutes after ETX challenge resulted in a delay in the appearance of the clinical signs of intoxication and time to death but did not result in any survivors (Fig. 5).
Figure 5.
Therapeutic use of ETX MAb c4D7 in mice challenged with 30 LD50 of ETX ip. 10× the ED100 protective dose of ETX MAb c4D7 was given to mice (n=8) at 15, 30, 45 or 60 minutes after ETX challenge.
4. Discussion
Lethality from iv ETX exposure occurred very quickly (starting a few minutes after inoculation with a mean time between inoculation and death of 18 min). Although the time to death was approximately 10 times longer for ip exposed mice, the LD50 of our ETX preparation was the same for both routes (1.6 g per kg). This is higher than the 0.1 g per kg of mouse previously reported by Sakurai et al (1995). Variations in LD50 of several ETX preparations considered to be of high purity have been reported (Bokori et al., 2011; Minami et al., 1997). These variations are probably due to different protocols used to purify and evaluate purity of the toxin. The LD50 was initially calculated in our preparations rather than using previously reported LD50. Therefore we were able to relate results of protection with the real LD50 of this preparation. The longer time between inoculation and death observed in mice challenged ip than in those challenged iv was surprising as it is usually assumed that the pharmacokinetics of drugs given iv is almost identical to that of drugs given ip. This should be kept into consideration at the time of selecting inoculation routes for ETX experiments.
Although the lethality of ETX is relatively well-characterized in mice, little is known about the physiological responses to intoxication. We therefore decided to investigate the response of the main pro-inflammatory cytokines in mice intoxicated with low or high lethal doses of ETX. We found a strong pro-inflammatory cytokine response in animals inoculated with high ETX doses (~15-30 LD50) but not in mice receiving a lower, yet lethal, challenge (~3-12 LD50). Activation of early cytokines such as IL-1β, GM-CSF, and TNF-α were apparent in the dosage groups receiving over 25 μg/kg of ETX, and the concentrations may have been much higher closer to administration of the ETX because of the proinflammatory nature of these molecules. Inflammatory mediators important to the further activation of circulating T-cells (IL-5) and overall signaling of the inflammatory cascade such as IL-2, IL-6, and IFN-γ were also detected in high levels in the serum in the higher dosage groups. IL-12 was detected in all dosage groups which indicates antigenic stimulation in macrophages in animals receiving ETX doses as low as 5 μg/kg. The presence of IL-10, a chief mediator of modulating inflammatory response in the presence of apparent infection, was also detected in the dosage groups receiving more than 25 μg/kg ETX. The presence of detectable cytokines in animals receiving doses of ETX at and above 25 μg/kg suggests that the inflammatory cascade consistent with a high-dose challenge was initiated upon exposure to ETX; the animals receiving lower amounts of toxin may have also produced cytokines but at levels that may have not been detectable by current methods. Because a highly purified ETX preparation which was free of endotoxins, was used in these series of experiments, it is reasonable to assume that the cytokine response observed was due to ETX and not to a contaminant.
The marked cytokine responses detected as early as 2 hours after inoculation in some mice, suggest that at least some of the clinical signs and lesions observed in animals intoxicated with ETX, might be due to the sudden release of pro-inflammatory cytokines. If this is the case, this could help explain the differences in clinical signs and lesions observed in different animal species, as cytokine response can show significant variations between animal species. The dose response experiments demonstrated that c4D7 can provide potent prophylactic protection against lethal challenge with an ED50 of 0.3 mg/kg and an ED100 of 0.8 mg/kg. For comparison, the only FDA approved anti-toxin MAb (anti-anthrax; Raxibacumab) is dosed at 40-80 mg/kg. Although the serum half-life of chimeric MAbs in mice (5-7 d) is significantly shorter than in humans (~21 d) we found that a single dose of ETX MAb c4D7 protected against lethal challenge for up to 10 days. Finally, although the post-exposure window was extremely limited due to the rapid lethality of ETX, ETX MAb c4D7 could rescue animals up to 30 minutes post-exposure. In total, the results suggest c4D7 may have utility for pre- and post-exposure prophylaxis of intoxication by ETX.
5. Conclusion
This study revealed that ETX MAb c4D7 produced in N. benthamiana can be used for prevention and potentially post-exposure therapeutic treatment of exposure to C. perfringens type D ETX. High doses of ETX elicit an early muti-cytokine response within a few minutes of inoculation.
Supplementary Material
Highlights.
-
*
MAb c4D7 produced in N. benthamiana prevents mice from disease by ETX.
-
*
MAb c4D7 produced in N. benthamiana are therapeutic for mice against ETX.
-
*
ETX elicits an early multi-cytokine response in mice.
Acknowledgements
The authors thank Drs. Victor Klimyuk and Yuri Gleba for providing access to the magnICON system, and Dr. Herta Steinkellner the glycosylation-modified plants. This work was supported by the National Institutes of Health (U01AI082276 to L.Z.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Drs. Whaley and Zeitlin are co-owners of Mapp Biopharmaceutical. All co-authors reports grants from National Institutes of Health (through MAPP Biopharmaceutical) during the conduct of the study. All authors express that there are no other conflicts of interest that could bias the work presented in this manuscript.
References
- Bendandi M, Marillonnet S, Kandzia R, Thieme F, Nickstadt A, Herz S, Fröde R, Inogés S, Lòpez-Dìaz de Cerio A, Soria E, Villanueva H, Vancanneyt G, McCormick A, Tusé D, Lenz J, Butler-Ransohoff JE, Klimyuk V, Gleba Y. Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin’s lymphoma. Ann Oncol. 2010;21:2420–2427. doi: 10.1093/annonc/mdq256. [DOI] [PubMed] [Google Scholar]
- Bokori-Brown M, Savva CG, Fernandez da Costa SP, Naylor CE, Basak AK, Titball RW. Molecular basis of toxicity of Clostridium perfringens epsilon toxin. FEBS J. 2011;278:4589–4601. doi: 10.1111/j.1742-4658.2011.08140.x. [DOI] [PubMed] [Google Scholar]
- Chow SK, Casadevall A. Monoclonal antibodies and toxins--a perspective on function and isotype. Toxins (Basel) 2012;4:430–454. doi: 10.3390/toxins4060430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnie JW, Blumbergs PC, Manavis J. Neuronal damage produced in rat brains by Clostridium perfringens type D epsilon toxin. J Comp Pathol. 1999;120:415–420. doi: 10.1053/jcpa.1998.0289. [DOI] [PubMed] [Google Scholar]
- Garcia JP, Beingesser J, Fisher DJ, Sayeed S, McClane BA, Posthaus H, Uzal FA. The effect of Clostridium perfringens type C strain CN3685 and its beta toxin mutant in goats. Vet Microbiol. 2012;15:412–419. doi: 10.1016/j.vetmic.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, Gleba Y. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci U S A. 2006;103:14645–14646. doi: 10.1073/pnas.0606631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer PJ, Clough NE. Development of monoclonal antibodies suitable for use in antigen quantification potency tests for clostridial veterinary vaccines. Dev Biol Stand. 1999;101:85–94. [PubMed] [Google Scholar]
- Minami J, Katayama S, Matsushita C, Okabe A. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol Immunol. 1997;41:527–535. doi: 10.1111/j.1348-0421.1997.tb01888.x. [DOI] [PubMed] [Google Scholar]
- Miyata S, Matsushita O, Minami J, Katayama S, Shimamoto S, Okabe A. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens epsilon-toxin in the synaptosomal membrane. J Biol Chem. 2001;276:13778–13783. doi: 10.1074/jbc.M011527200. [DOI] [PubMed] [Google Scholar]
- Lonchamp E, Dupont J, Wioland L, Courjaret R, Mbebi-Liegeois C, Jover E, Doussau F, Popoff MR, Bossu JL, Barry J, Poulain B. Clostridium perfringens epsilon toxin targets granule cells in the mouse cerebellum and stimulates glutamate release. PLoS ONE. 2010;5:e13046. doi: 10.1371/journal.pone.0013046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue GP, Vojdani F, et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotech J. 2010;8:638–654. doi: 10.1111/j.1467-7652.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- Robertson SL, Jihong L, Uzal FA, McClane BA. Evidence for a Prepore Stage in the action of Clostridium perfringens Epsilon Toxin. PLoS ONE. 2011;6:e22053. doi: 10.1371/journal.pone.0022053. doi:10.1371/journal.pone.0022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed S, Fernandez-Miyakawa ME, Fisher DJ, Adams V, Poon R, Rood JI, Uzal FA, McClane BA. Epsilon-toxin is required for most Clostridium perfringens Type D vegetative culture supernatants to cause lethality in the mouse intravemous injection model. Infect Immun. 2005;73:7413–7421. doi: 10.1128/IAI.73.11.7413-7421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai J, Kobayashi K. Lethal and dermonecrotic activities of Clostridium perfringens iota toxin: biological activities induced by cooperation of two nonlinked component. Microbiol Immunol. 1995;39:249–253. doi: 10.1111/j.1348-0421.1995.tb02197.x. [DOI] [PubMed] [Google Scholar]
- Stiles BG, Barth G, barth H, Popoff MR. Clostridium perfringens epsilon toxin: a malevolent molecule for animals and man. Toxins. 2013;5:2138–2160. doi: 10.3390/toxins5112138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R, Stadlmann J, Schahs M, Stiegler G, Quendler H, Mach L, Glossl J, Weterings K, Pabst M, Steinkellner H. Generation of glycol-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan strkucture. Plant Biotech J. 2008;6:392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- Zeitlin L, Cone RA, Moench TR, Whaley KJ. Preventing infectious disease with passive immunization. Microbes Infect. 2000;2:701–708. doi: 10.1016/s1286-4579(00)00355-5. [DOI] [PubMed] [Google Scholar]
- Zeitlin L, Bohorov O, Bohorova N, Hiatt A, Kim do H, Pauly MH, Velasco J, Whaley KJ, Barnard DL, Bates JT, Crowe JE, Jr, Piedra PA, Gilbert BE. Prophylactic and therapeutic testing of Nicotiana-derived RSV-neutralizing human monoclonal antibodies in the cotton rat model. MAbs. 2013;5:263–269. doi: 10.4161/mabs.23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.