Abstract
Chronic stress can influence behaviors associated with medial prefrontal cortex (mPFC) function, such as cognition and emotion regulation. Dopamine in the mPFC is responsive to stress and modulates its behavioral effects. The current study tested whether exposure to 10 days of chronic unpredictable stress (CUS) altered the effects of acute elevation stress on dopamine release in the mPFC and on spatial recognition memory. Male rats previously exposed to CUS or non-stressed controls were tested behaviorally, and underwent microdialysis to assess mPFC dopamine or had blood sampled for corticosterone analysis. Dopamine in the mPFC significantly increased in both groups during acute elevation stress compared to baseline levels but was attenuated in CUS rats compared to controls. Control rats exposed to elevation stress immediately prior to the T-maze showed impaired performance, whereas CUS rats did not. No group differences were observed in general motor activity or plasma corticosterone following elevation stress. The present results indicate that prior exposure to this particular CUS procedure reduced dopamine release in the mPFC during acute elevation stress and prevented the impairment of performance on a spatial recognition test following an acute stressor. These findings may contribute to an understanding the complex behavioral consequences of stress.
Keywords: chronic stress, corticosterone, dopamine, microdialysis, spatial memory, rat
INTRODUCTION
The relationship between stress and cognition is thought to be complex, depending upon the characteristics of the stress exposure, the individual organism (e.g. gender, age) and the cognitive task (for reviews see Bowman et al., 2003; Diamond, 2005; Luine et al., 2007; Lupien et al., 2009; Sandi & Pinelo-Nava, 2007; Shors, 2004). Much of the literature supports a curvilinear relationship with moderate levels of stress enhancing cognitive performance, and low or high levels impairing it (Finsterwald & Alberini, 2014). Thus, longer exposures (i.e. 3 weeks or longer) to certain stressors can lead to deficits in performance on spatial and non-spatial tasks in male rats (Beck & Luine, 1999; Conrad et al., 1996; Hains et al., 2009; Hutchinson et al., 2012; Kitraki et al., 2004; Luine et al., 1994; Mizoguchi et al., 2000; Tagliari et al., 2011). However, shorter stress protocols have resulted in improved or no effect on performance on spatial memory tasks (Bartolomucci et al., 2002; Gouirand & Matuszewich, 2005; Isgor et al., 2004; Luine et al, 1996; McFadden et al., 2011; Shors, 2004). For example, our laboratory has found that exposure to 10 days of unpredictable stress enhances the ability of male rats to acquire the location of a hidden platform in the water maze (Gouirand & Matuszewich, 2005). These effects support the hypothesis that while longer or high levels of stress may impair cognitive function, shorter or moderate levels of stress may improve it.
Paradoxically, exposure to a single acute stressor impairs performance on spatial memory tasks when the stressor is given immediately prior to the task. Previous research has found that a brief stressor, such as placing a rat in lit open field box or in restraint for 1 h, reduced performance of male rats on delayed alternation tasks (Conrad et al., 2004; Del Arco et al., 2007A). Further, 30 min exposure to a predator interfered with long-term spatial memory when applied immediately prior to training or memory testing (Diamond et al., 2006; Park et al., 2008). Primates also showed spatial working memory deficits following an acute noise stressor (Arnsten & Goldman-Rakic, 1998). Likewise, humans show working memory deficits following an acute stressor, such as the Trier Social Stress Test or a cortisol challenge (Oei et al, 2006). Acute stress, however, can enhance other forms of learning, such as eye blink or fear conditioning, and its effects appear to depend upon the phase of learning (i.e. acquisition, consolidation, retrieval) (Sandi & Pinelo-Nava, 2007).
One mechanism that may contribute to a stressor disrupting performance on cognitive tasks is the acute increase of dopamine in the medial prefrontal cortex (mPFC). Dopamine neurons in the mPFC are highly sensitive to stress (Finlay & Zigmond, 1997; Herman et al., 2005). Previous studies in rodents have demonstrated an increase in extracellular dopamine in the mPFC during exposure to an acute stressor (for reviews see Flugge et al, 2004; Horger & Roth, 1996). This dopaminergic increase associated with the application of an acute stressor has been observed in rats exposed previously to repeated or chronic stressors, as well as stress-naïve rats. Previous studies have found that exposure to repeated cold stress, social stress, mild stress or neonatal isolation potentiated dopamine increases in the mPFC to a novel acute stressor compared to stress-naïve rats (Cuadra et al., 1999; DiChiara et al., 1999; Gresch et al., 1994; McCormick et al., 2002). Unfortunately, repeated stress exposures that lead to a sensitized increase of dopamine in the mPFC to a novel acute stress have not been tested also in a cognitive task. Thus, it is difficult to determine if the acute potentiated increase in mPFC dopamine contributes to the disruption of performance on a learning/memory task in rodents exposed previously to chronic stress.
Similar to the relationship between stress and cognition, previous research has suggestedt hat the relationship between dopamine in the mPFC and memory is curvilinear (for reviews see Arnsten, 1997; Gamo & Arnsten, 2011; Hains & Arnsten, 2008). A moderate level of dopamine activity and receptor stimulation are thought to be important for a high level of performance on a working memory task, while very high or low levels of dopamine and receptor stimulation in the mPFC are thought to contribute to poor performance on memory tasks (Arnsten & Goldman-Rakic, 1998; Arnsten et al., 1994; Dent et al., 2012; Mizoguchi et al., 2000; Murphy et al., 1996; Vijayraghavan et al., 2007; Zahrt et al., 1997). Dopamine levels in the mPFC in response to an acute stressor may be modulated by prior stress exposure and contribute to the subsequent behavioral performance. Therefore, the current study tested whether exposure to unpredictable stress for 10 days alters the increase in dopamine release in the mPFC to an acute stressor. Furthermore, behavioral studies tested whether unpredictable stress exposure altered spatial memory as assessed by the T-maze, either alone or following an acute stressor. The findings from these studies suggest that rats exposed to the unpredictable stress procedure used in the present study, unlike other stress protocols, have an attenuated dopamine release to an acute stressor compared to non-stressed controls and intact performance in the T-maze following elevation stress, unlike non-stressed control rats. The reduced increase of dopamine in the PFC of CUS rats may contribute to the maintenance of spatial memory during acutely stressful conditions.
METHODS
Subjects and Housing
Male Sprague-Dawley (Charles River-derived) adult rats from Northern Illinois University Psychology’s animal colony were used for all experiments. The rats (250–400 g) were maintained on a 12/12 h light/dark cycle (lights on at 6:00 and off at 18:00) in a temperature controlled room (22±2 ºC). All rats were pair-housed for the entire experimental procedure or until intracranial surgery, at which time each rat was housed singly in a standard Plexiglas cage (46 x 25 x 21cm). All procedures were in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition; National Research Council, 2011) and approved by the local institutional animal care and use committee.
Rats were randomly assigned either to a non-stressed control group or to chronic unpredictable stress group (CUS). All rats were weighed daily (08:00) to monitor their overall health. Rats in the CUS group received various stressors for 10 days as described in Table 1 (Gouirand & Matuszewich, 2005). Rats were assigned to the microdialysis study (n = 17), the behavioral study (n = 46) or plasma collection for corticosterone levels (n = 33). Adrenal glands were dissected and weighed for all rats assigned to behavioral experiment.
Table 1.
Chronic Unpredictable Stress Schedule.
| DAY | TIME | STRESSOR | DURATION |
|---|---|---|---|
| Day 1 | 13:00 | Wet bedding (400 ml tap water in home cage) | 4 h |
| 18:00 | Lights on | 12 h | |
| Day 2 | 11:00 | Cold room isolation (4°C) | 1 h |
| 12:00 | Cage rotation | 50 min | |
| Day 3 | 11:00 | Cage rotation | 50 min |
| 18:00 | Food and water deprivation | 14 h | |
| Day 4 | 10:00 | Lights off | 3 h |
| 15:00 | Restraint in Plexiglas restrainers | 1 h | |
| Day 5 | 15:00 | Cold room isolation * | 15 min |
| 16:00 | Isolation housing | 14 h | |
| Day 6 | 11:00 | Cage rotation | 20 min |
| 15:00 | Lights off | 2 h | |
| Day 7 | 11:00 | Wet bedding | 4 h |
| 14:00 | Cage rotation | 20 min | |
| Day 8 | 15:00 | Cold room isolation | 15 min |
| 15:15 | Restraint in home cage | 45 min | |
| Day 9 | 10:00 | Cage rotation | 20 min |
| 18:00 | Food and water deprivation | 14 h | |
| Day 10 | 10:00 | Lights off | 3 h |
| 18:00 | Lights on | 12 h |
Rats exposed to CUS and used in the microdialysis study received a 21 gauge stainless steel guide cannula at 10:00 on Day 5. They did not receive any additional stressors on Day 5. On Day 10, a microdialysis probe was inserted at 14:00 and the last stressor was conducted in the room for microdialysis. Non-stressed control rats received surgery and a microdialysis probe at the same time points (ie. surgery 6 days prior to and a probe 19 h before microdialysis).
Surgery and Microdialysis Experiment
For the microdialysis experiment, rats were anesthetized with a combination of xylazine (6 mg/kg) and ketamine (70 mg/kg) and placed into a Kopf stereotaxic frame. A 21-gauge stainless steel guide cannula (11 mm in length, Small Parts, Inc., Miami Lakes FL USA) was positioned above the mPFC (+3.2 mm anterior and ±0.7 mm medial to bregma) (Paxinos & Watson, 1998). The cannula and a metal male connector were secured to the skull with 3 stainless steel screws and cranioplastic cement. An obturator fashioned from 27-gauge stainless steel wire was inserted into the cannula.
Microdialysis probes were constructed in the laboratory as previously described (Matuszewich & Yamamoto, 2004). Briefly, a 26-gauge thin wall stainless steel tube was fitted with a dialysis membrane (13,000 dalton cut off, 210 μm o.d.; Spectrum Laboratories, Inc., Rancho Domingues CA USA) and a 5 cm piece of polyethylene 20 tubing (Fisher Scientific, Inc., Pittsburg PA USA), which served as the inlet for the perfusion medium. The dialysis membrane was 4.4 mm x 210 μm diameter with 0.4 mm inactivated with epoxy at the tip. Four cm of capillary tubing (125 μm o.d., 50 μm i.d.; Polymicro Technologies, Phoenix AZ USA) served as the outlet from the dialysis membrane. The exposed portion of the dialysis membrane extended beyond the guide cannula −5.8 mm ventral to the skull. The in vitro rate of recovery for microdialysis probes ranged from 12–18 % for dopamine when measured at room temperature with Dulbecco’s phosphate buffered medium (138 mM NaCl, 2.1 mM KCl, 0.5 mM MgCl2, 1.5 mM KH2PO4, 8.1 mM NaH2PO4, 1.2 mM CaCl2, and 5 mM d-glucose, pH 7.4).
Five days following surgery, the obturator was removed from the guide cannula and replaced with a microdialysis probe. The rat was returned to its Plexiglas cage and attached to a tether and swivel (Instech Laboratories, Inc., Plymouth Meeting PA USA). The following morning (09:00h), Dulbecco’s phosphate-buffered saline medium was perfused at a rate of 1.0 μl/min through the microdialysis probe using a KD Scientific syringe infusion pump (Fisher Scientific, Inc., Pittsburg PA USA). After a 3 h equilibration period, the following 25 min samples were collected: 3 baseline samples, 1 sample during elevation stress and 3 post-stress samples. For the elevation stress, each rat was gently moved from its home cage and placed onto a plastic tray (35 x 45 cm), which was balanced 41 cm above the table on a wooden apparatus. During elevation stress, the rat could investigate the plastic tray and was monitored by an observer to make certain that it remained on the tray. After 25 min of elevation, the rat was returned to the home cage for 3, 25 min post-stress samples.
Following the microdialysis experiment, rats were overdosed with chloral hydrate (250 mg/ml). Blue McCormicks’ food coloring was perfused through the micrdialysis probe to dye the active surface of the membrane. Once completely anesthetized, the rat was decapitated and the brain quickly removed from the skull and frozen in a cryostat. Twenty micron coronal sections were taken from +4.7mm to +1.6mm and mounted on slides. The slides were examined under an Olympus BH-2 microscope (Fisher Scientific, Inc., Pittsburg PA USA) to assess probe placement. Slides were then stained with cresyl violet, cover slipped and examined again for accurate probe location. Only data from rats with probes located in the mPFC with the ventral tip of the probe through the infralimbic region (−5.8mm) were used for statistical analysis (Paxinos & Watson, 1998).
High Performance Liquid Chromatography
Microdialysis samples were analyzed for dopamine with high performance liquid chromatography using electrochemical detection (HPLC-EC). A Rheodyne injector (Cotati CA USA) with a 20 μl loop delivered the dialysis sample onto a reverse phase Synergi 4 μ C18 column 150 x 2 mm (Phenomenex, Torrance CA USA). A Shimadzu 10ADVP solvent delivery system continuously pumped mobile phase (32 mM citric acid, 54.3 mM sodium acetate, 0.074 mM ethylenediaminetetraacetic acid, 0.32mM octyl sodium sulfate and 6% acetonitrile) at a flow rate of 0.21 ml/min. Compounds were detected with an LC-4B amperometric detector (Bioanalytical Systems, West Lafayette IN USA), with a 3 mm glassy carbon working electrode maintained at a potential of +0.5 V relative to an Ag/AgCl reference electrode. Given the above conditions, the limit of detection for dopamine was 0.1 pg/20 μl. Data were collected using ChromPerfect Spirit Software (Justice Innovations, Inc., Denville NJ USA).
Behavioral Experiments
Rats designated for the behavioral study were assessed in 2 behavioral tests: 1) the T-maze to assess spatial recognition (Conrad et al., 1996; Wright & Conrad, 2008); and 2) the open field to assess habituation to novelty and general locomotion. A subset of the rats were placed in the elevated plus maze immediately prior to T-maze testing to approximate the acute stressor of elevation in the microdialysis experiment.
T-maze
The procedure for testing in the T-maze was modified from Conrad et al. (1996) to assess spatial recognition memory. The T-maze consisted of 3 wooden arms (each 49×16×32 cm), painted red that formed the shape of a “T”. A red divider, made of same material as the maze, was then inserted into the maze to block the entry and view of the left or right arm. The maze was located in a small testing room with multiple cues (e.g. posters, tables, shelves) available outside of the maze.
For the first trial, each rat was placed individually at the end of the stem arm of the T-maze (“home” arm), facing the wall. Either the left or right arm was blocked during the first trial, so the animal could only explore the home arm and the unblocked arm designated as the “other” arm. The animal was allowed to explore the home and other arms of the T-maze for 15 min. After the exploring for 15 min, the animal was promptly removed from the maze and placed into a standard Plexiglas cage with bedding for 1 min before starting the second trial. During the 1 min period, the divider was removed and the maze was wiped clean with a disinfectant solution. The rat was placed back into the end of the home arm facing the wall and allowed to explore all three arms for 5 min.
Testing in the T-maze was recorded with a DVD-recorder attached to an overhead bullet camera with 3.6mm lens (Spyville.com). These recordings later were entered into a computer using Noldus EthoVision 3.0 tracking software and were analyzed for the following measures: frequencies of entrances into each arm, distance traveled in each arm, total time in each arm, and total distance traveled during the second trial. The frequencies of entrances, distance traveled, and time in each arm were then used to calculate the proportion of entrances, distance, and time in each arm.
Elevated Plus Maze
To expose rats to acute elevation stress, a subset of the control and CUS rats were placed in the elevated plus maze (EPM). The apparatus consisted of 4 arms, 2 open arms (11×50 cm) with 0.5 cm ledges and two enclosed arms of the same size with 50 cm high walls. The arms were attached to a central square (10 cm2) and shaped a plus sign. The entire apparatus was elevated 48 cm above the floor. The testing room was dimly illuminated with red light.
For testing, each rat was placed individually on the apparatus with half of their body in a closed arm facing the central square. The rat was allowed to explore the maze for 5 min and then placed in a home cage with bedding and immediately moved to the T-maze testing room (Matuszewich et al., 2007) regardless of performance on the T-maze. Between rats, the maze was cleaned with a disinfectant solution. The test was digitally recorded and the following behaviors were scored manually for the 5 min trial: latency to enter the open arm, time spent with all four paws in the open arms, the frequency of entries into the open arms and the total number of arms entered.
Open Field
To test motor behavior, a large plywood box (75×75×29 cm) painted grey was used for open field testing. The rat was placed in the open field along the center of the southern wall and was allowed to explore for 15 min. The apparatus was cleaned thoroughly between rats with a disinfectant solution. All testing sessions were recorded using a DVD-recorder attached to an overhead bullet camera for further analysis. The total horizontal distance traveled and velocity during each testing session was calculated using the Noldus EthoVision 3.0 tracking software system. The measures were assessed in 3–5 min blocks (total of 15 min) to assess habituation to the open field.
Corticosterone Measures
To measure corticosterone, trunk blood was collected from non-stressed and CUS rats. All rats were rapidly decapitated between 10:00–11:00 a.m. (4–5 hours into light). The blood was collected into a 15 ml vial with 0.3 ml heparin sodium sulfate (1000 U/ml), centrifuged for 15 min (2500 x g) and the plasma frozen until assayed.
Plasma corticosterone was measured using radioimmunoassay as previously described (Frye et al., 1996). Corticosterone was extracted from plasma by heating at 60°C for 30 min. Samples were incubated for 60 min at room temperature with 3[H] Corticosterone (NET 182: specific activity = 48.2 ci/mmol; Perkin Elmer, Waltham, Massachusetts USA) and a 1:20,000 dilution of antibody (Endocrine Sciences, Inc., Agoura, CA USA). Bound and free corticosterone were separated with the addition of dextran-coated charcoal following 15-min incubation on ice and centrifugation at 3000 x g for 10 min. Unknowns were interpolated from the standard curve using Assay Zap. The minimum level of detection with the assay is 15 pg/tube and the inter- and intra-assay reliability co-efficients were 0.05 and 0.08, respectively.
Statistical Analyses
Independent t-tests compared weight gain differences (weight in grams on Day 11-weight on Day 1) and the mean basal microdialysate concentrations of dopamine for control and CUS rats. The basal microdialysate concentration for each rat was defined as the mean of the 3 samples prior to elevation stress. The final baseline sample and the 4 samples following the onset of the elevation stress were converted to percent of the average baseline and compared with a 2-way, repeated-measures analysis of variance (ANOVA). Body weights over 11 days of stress also were compared over time by group with a 2-way repeated-measures ANOVA. A 2-way ANOVA compared corticosterone plasma levels and adrenal gland weights in control and CUS rats in basal and elevation-stimulated conditions.
For the T-maze, 3 rats did not leave the home arm during the second trial and were therefore not included in the data analysis. Arm preferences in the T-maze were analyzed according to Conrad and colleagues (2007). Preference for the novel arm versus the familiar arm was analyzed using Wilcoxon tests for each group. The total distance traveled in the T-maze was analyzed using a 2-way ANOVA comparing control and CUS rats exposed to the EPM or not exposed to the EPM. Similarly, EPM and open field data were analyzed by using 2-way ANOVAs (group x EPM). Open field activity was also analyzed by using a repeated measures ANOVA (group x time) to assess habituation in 5 min time periods. The behavioral data were analyzed using SAS 9.1 software (Cary, NC USA). Post hoc Tukey’s pairwise tests were used to further analyze any significant treatment differences and significance was fixed at p < 0.05 for all tests.
RESULTS
Dopamine in the mPFC
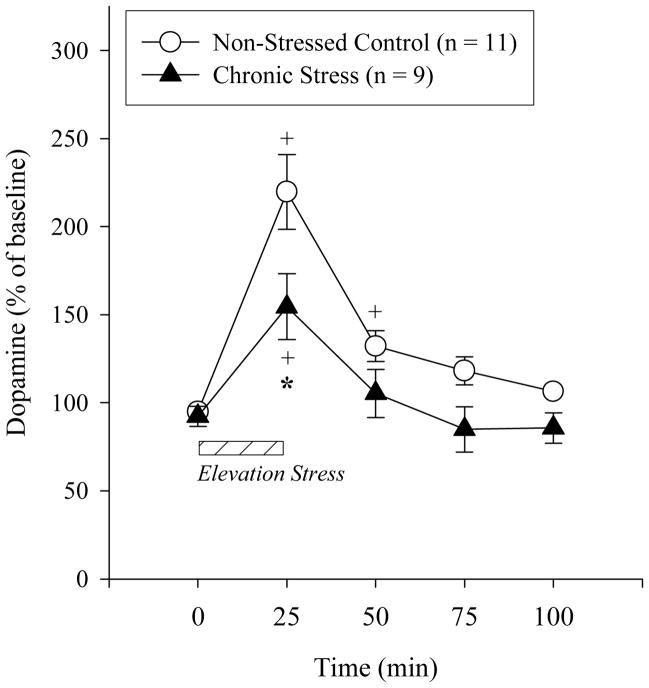
Dopamine was assessed in the mPFC of rats exposed to CUS or non-stressed control rats prior, during and following 25 min elevation stress. In the mPFC, basal microdialysate concentrations of dopamine did not differ significantly between groups (control 0.65 ± 0.09; CUS 0.56 ± 0.08 pg/20 μl; t(15)=0.15, NS). However, given the individual differences of baseline levels, the dopamine data was converted into a percent of the average baseline for each rat and compared across samples. In both groups, there was a significant increase in dopamine concentrations during elevation stress (F(4,88)=29.44, p< 0.001; Figure 1). Control rats also had greater dopamine levels in the 1st post-stress sample (50 min), while CUS exposed rats did not. The magnitude of the dopamine increase during elevation stress was greater in the non-stressed control rats compared to the CUS rats as indicated by a significant Time x Group interaction (F(4,88)=3.80, p< 0.01). The groups differed during the elevation stress sample according to Tukey's post hoc comparisons.
Figure 1.
During elevation stress (indicated by the striped bar
 ), dopamine significantly increased in non-stress control and CUS rats compared to pre-stress levels (+ p< .05 with Tukey post hoc from basal microdialysate levels). The increase in dopamine was significantly attenuated during elevation stress (25 min) in CUS rats compared to controls (* p<.05 with Tukey post hoc from control rats).
), dopamine significantly increased in non-stress control and CUS rats compared to pre-stress levels (+ p< .05 with Tukey post hoc from basal microdialysate levels). The increase in dopamine was significantly attenuated during elevation stress (25 min) in CUS rats compared to controls (* p<.05 with Tukey post hoc from control rats).
Spatial Memory Performance
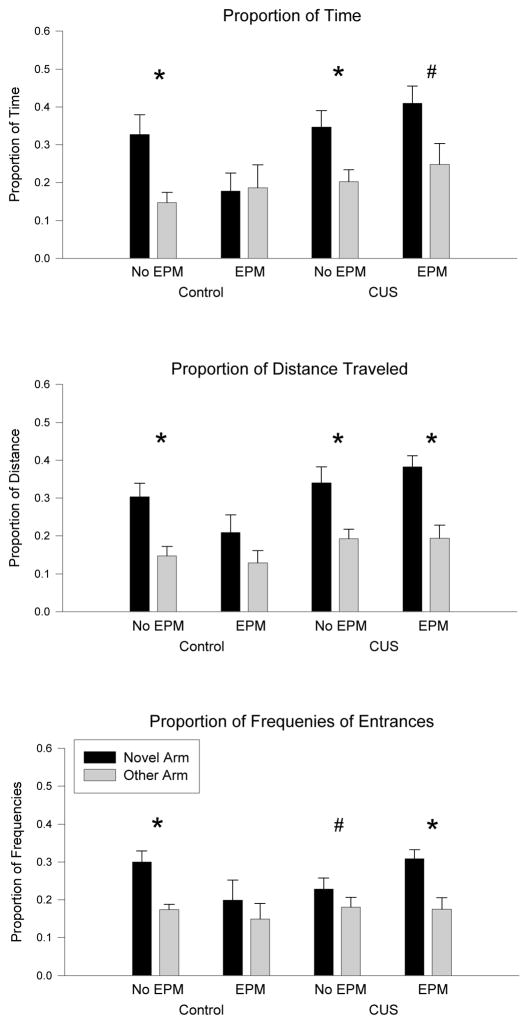
Both control and CUS rats showed preference for the novel arm in the T-maze following the 15 min habituation trial (Figure 2). Control rats, not exposed to the EPM, showed a significant preference for the novel arm (Proportion of Time: WS =165.00, p<0.05; Proportion of Distance: WS =172.00, p<0.01; Proportion of Frequencies of Entrances: WS =175.00, p<0.01) compared to the other arm. Likewise, CUS rats not exposed to EPM also showed a significant preference for the novel arm compared to the other arm (Proportion of Time: WS =166.50, p<0.01; Proportion of Distance: WS =172.50, p<0.01; Proportion of Frequencies of Entrances: WS =151.50, p=0.10).
Figure 2.
Control rats were impaired in their performance in the T-maze after exposure to elevation stress on the elevated plus maze (EPM), whereas rats exposed to CUS maintained their preference for the novel arm. CUS rats showed a preference for the novel arm, regardless of condition, as indicated by greater proportion of time spent (A), distance traveled (B), and entrances (C) into the novel arm compared to the arm with which they had previous experienced (Other Arm). Control rats showed impaired performance after exposure to EPM by spending similar amounts of time (Upper Panel), traveling similar distances (Middle Panel) and entering (Lower Panel) both arms equally during the 5 min test. (*p<.05 and # p<.10 for novel v. other arm Wilcoxon analysis.)
Following acute elevation stress in the elevated plus maze immediately prior to testing, rats exposed to CUS continued to show preference for the novel arm, but control rats did not. The control rats exposed to the EPM did not show a significant preference for the novel arm compared to the other arm in any measure (Proportion of Time (B): WS =206.00, NS; Proportion of Distance (C): WS =227.00, NS; Proportion of Frequencies of Entrances (A): WS=217.50, NS). Rats exposed to CUS and exposed to the acute elevation stress of EPM had a significant preference for the novel arm compared to the other arm (Proportion of Time: WS =85.00, p<0.10; Proportion of Distance: WS =100.00, p<0.01; Proportion of Frequencies of Entrances: WS =94.00, p<0.01). Overall, only control rats exposed to elevation stress in the EPM prior to T-maze testing had impaired performance in the T-maze.
Differences in the total exploration of the maze on the second trial were also found between groups. Control rats explored the maze less than the rats exposed to CUS as measured by total distance traveled during the second trial (F(1,40)=5.35, p<0.05; Table 2). However, no significant difference in the total distance traveled in the T-maze was found between those rats exposed to the EPM versus those who were not (F(1,40)=0.70, NS). The interaction between CUS and EPM exposure also did not reach significance (F(1,40)=2.92, NS).
Table 2.
Behavioral and Physiological Measures.
| Measure | Control | CUS | ||
|---|---|---|---|---|
| No Elevation Stress | Elevation Stress | No Elevation Stress | Elevation Stress | |
| Total Distance Traveled in T-Maze(cm) * | 1323.57 (59.37) | 1081.21 (134.03) | 1379.84 (76.75) | 1498.62 (86.38) |
| Total Distance Traveled in Open Field (cm) | 5764.02 (262.30) | 5138.56 (382.86) | 5717.38 (502.02) | 6001.53 (325.90) |
| Adrenal Weight per 100 g of Body Weight # | 8.30 (0.49) | 8.59 (0.63) | 10.02 (0.74) | 8.91 (0.61) |
| Corticosterone (ug/dl) | 2.88 (0.55) | 27.12 (6.35) ^ | 3.56 (0.41) | 22.25 (3.69) ^ |
Numbers represent the mean (SEM);
CUS>Control p<0.05;
CUS> Control, p<0.10;
Elevation Stress > No Elevation Stress, p <0.05
Elevated Plus Maze
There were no significant differences between the control and CUS groups in the elevated plus maze (Table 3). The groups did not differ in the latency to enter into the open arms (F(1,8)=0.89, NS), the total time spent in the open arms (F(1,8)=0.36, NS), or the number of entries into the open arms (F(1,8)=0.03, NS). Importantly, the control and CUS rats did not differ in the total number of arms entered suggesting that motor activity was similar between groups (F(1,8)=0.39, NS). Lastly, no significant differences where found between groups in the proportion of rats that did not enter into the open arms (χ²(1)=0.10, NS). These findings suggest that CUS and control rats displayed similar amounts of anxious behavior when assessed in the EPM.
Table 3.
Behavioral Measures in Elevated Plus Maze.
| Measure | Control | CUS |
|---|---|---|
| Latency to open arm (s) | 37.33 (13.67) | 20.75 (5.15) |
| Time in open arms (s) | 46.83 (12.32) | 57.50 (11.03) |
| Open arm entries | 2.50 (1.12) | 2.75 (0.75) |
| Total arm entries | 8.33 (1.80) | 9.75 (0.25) |
| Percent of rats that did not enter the open arm | 57.14% | 50% |
Numbers represent the mean (SEM) unless otherwise specified
Motor Activity to a Novel Environment
Overall, locomotor activity decreased over time for both control rat and rats exposed to 10 days of CUS (distance: F(2,84)=60.19, p<.001; velocity F(2,84)=60.12, p<.001). However, no significant differences between groups were found in the distance traveled in the open field (Table 2). Control and CUS rats traveled similar distances (F(1,42)=1.03, NS) and velocities (F(1,42)=1.02, NS) in the open field. Similar distances and velocities were also found between those rats exposed to the EPM and those rats who where not exposed to the EPM (distance: F(1,42)=0.36, NS; F(1,42)=0.18, NS). Furthermore, there was no significant interaction between CUS exposure and EPM exposure (distance: F(1, 42)=1.28, NS; velocity: F(1,42)=1.28, NS). These data support previous findings and suggest that there were no significant differences in motor behavior among groups.
Physiological Measures
Rats exposed to the 10 day stress protocol showed lower total body weight gain compared to non-stressed controls across all studies (t(95)=8.06, p<.001). Overall, control rats gained 42.34±2.29 g over the 11 days prior to testing, while CUS rats gained 14.48±2.60 g. When compared daily, rats exposed to CUS also showed less weight gain than control rats as indicated by a significant group x day interaction (F(1,94=12.24, p<.05).
Elevation stress significantly increased corticosterone levels in all rats (F(1,29)=36.08, p< 0.001; Table 2), but there was no difference between control and CUS rats in their basal or elevation-stimulated corticosterone levels. The adrenal gland weights of rats exposed to CUS were greater than those of control rats when measured as a proportion of body weight although this only reached marginal significance (F(1,43)=3.08, p<0.10; Table 2). There was no difference in the adrenal gland weights in those rats exposed to the EPM compared to those who were not (F(1,43)=0.20, NS).
DISCUSSION
The current study found that exposure to 10 days of unpredictable stress attenuated dopamine efflux in the mPFC during acute elevation stress. Both CUS and non-stressed control rats showed elevated dopamine microdialysate levels during the acute stressor compared to baseline levels, but the dopamine increase in the mPFC of CUS rats was significantly lower than the increase of control rats. In the T-maze, the application of a similar acute stressor immediately prior to the spatial memory test impaired performance in control rats but not in rats exposed to CUS. No differences were observed between control and CUS rats in measures of general motor activity or habituation to novelty that would account for the discrepancy in performance on the spatial memory test or the microdialysis data. To our knowledge, this is the first report of a chronic unpredictable stress procedure attenuating the increase in mPFC dopamine during an acute stressor and preventing stress-induced impairment on a spatial memory task.
In both the microdialysis and behavioral experiments, the responses of the non-stressed control rats were consistent with previous studies. Similar to published research, control rats showed an increase in PFC dopamine levels in the microdialysis samples collected during and immediately following an acute stressor (Figure 1; Abercrombie et al., 1989; Butts et al., 2013; Butts et al., 2011; Del Arco et al., 2007B; Finlay et al., 1995; Gresch et al., 1994; Pehek et al., 2006). Of clinical relevance, emerging imaging studies suggest dopamine is also released in prefrontal cortical regions under stressed conditions in healthy subjects (Lataster et al., 2011; Nagano-Saito et al., 2014). Likewise, the application of an acute stressor has been shown to impair performance on spatial learning tasks, such as the water maze or Y-maze in rodents (Conrad et al., 2004; Del Arco et al., 2007B; Diamond et al., 2006; Park et al., 2008; Segovia et al., 2008). In the current study, exposure to the elevated plus maze immediately prior to the T-maze reduced the preference for the novel arm of control rats (Figure 2). Similar impairments in performance on memory tasks have been associated with increases in dopamine in the PFC and the integrity of the dopaminergic system (Arnsten, 1997; Arnsten & Goldman-Rakic, 1998; Conrad et al., 2004; Dent et al., 2012; Mizoguchi et al., 2000; Murphy et al., 1996; Sorg & Kalivas, 1993; Vijayraghavan et al., 2007; Zahrt et al., 1997). The increase of mPFC dopamine during acute elevation stress in control rats may contribute to the disrupted performance on the T-maze in the current study.
Rats exposed to 10 days of CUS, on the other hand, had an attenuated dopamine increase in the PFC and normal performance in the T-maze following an acute stressor. Rats exposed to CUS still showed an increase in dopamine levels in the microdialysis sample collected during elevation stress, but it was significantly reduced compared to control rats. Moreover, acute elevation stress in the EPM did not disrupt performance of the CUS rats in the T-maze as demonstrated by a significant preference for the novel arm. The attenuated dopamine response in the PFC to elevation stress may contribute to improved performance on the T-maze by staying within an optimal range for dopamine activity in the PFC, which has been suggested to contribute to working memory processes (Arnsten, 1997; Arnsten & Goldman-Rakic, 1998; Dent et al., 2012). Izaki and colleagues (1998) suggested that a 40% increase in dopamine levels in the mPFC may be optimal for acquisition of a new task; and in the current study, dopamine levels in the mPFC increased ~50% in rats exposed to CUS. The reduced dopamine increases in CUS rats may contribute to adequate memory function, not reaching a level of dopamine that would disrupt spatial memory processes.
Although consistent with the current behavioral data, the attenuated increase in mPFC dopamine differs from the effects of other chronic stress protocols. Previous research reported that exposure to repeated stress results in an increased dopamine response (Di Chiara et al., 1999; Gresch et al., 1994) or a decreased dopamine response in the mPFC to a novel, acute stressor (McCormick et al., 2002; Mokler et al., 2007). Dopamine metabolism also shows sensitized responses to a novel stressor when the rat has been previously exposed to predictable stress (Anisman & Zacharko, 1990; Beck & Luine, 1999; Imperato et al., 1992; Richardson, 1984; Sorg & Kalivas, 1993; Thierry et al, 1968). The augmented response of dopamine in the mPFC to a novel acute stressor following chronic stress exposure observed by other research groups has been suggested to contribute to mental illness and an inability to successfully cope with a novel life stressor (Finlay & Zigmond, 1997; Hains & Arnsten, 2008). However, attenuated DA release following a novel stressor in rats exposed to CUS may facilitate coping to the novel stressor and result in no impairments or improved performance during behavioral tasks (Gouirand & Matuszewich, 2005; Vyas et al., 2004).
The type of chronic stress exposure may be critical for the impact of a novel acute stressor on the pattern of dopamine release in the mPFC and subsequent behavioral changes. The protocol for unpredictable stress used for the current study applies 2 moderate stressors per day for 10 consecutive days (Haile et al., 2001; Ortiz et al., 1996). Other unpredictable stress procedures have applied fewer types of moderate stressors or milder stressors for a longer time period (e.g. 14–31 days)(Cuadra et al, 2001; Cuadra et al., 1999; DiChiara et al., 1999; Willner, 2005). Supporting the distinction between the unpredictable stress procedures, exposure to the current CUS procedure has not been shown to increase behaviors associated with anxiety as measured in the elevated plus maze or light/dark box (Matuszewich et al., 2007; Vyas & Chattarji, 2004; Table 2) or anhedonia as measured by sucrose consumption in male rats (Gouirand & Matuszewich, 2005). The behavioral “depressive profile” associated with many of the mild stress procedures (e.g. Di Chiara et al., 1999; Willner, 1984; Willner et al., 1992; Willner, 2005; Zurita et al., 2000) encompasses a pattern of neurobiological markers consistent with human affective disorder, including decreased dopamine activity in the nucleus accumbens (Willner, 2005) and greater activation of medial PFC activity with more self-reported anhedonia (reviewed in Willner et al., 2013; Keedwell, 2011). Both the behavioral and neurochemical results in the current study suggest that exposing rats to the present CUS protocol appears to be distinct from other unpredictable stress protocols, resulting not in pathology but potentially successful coping behaviors.
In the current study, acute changes in plasma corticosterone levels do not appear to directly contribute to the magnitude of dopamine increase in the mPFC during elevation stress or performance in the T-maze. Both control and CUS rats had similar increases in plasma corticosterone following elevation stress, but the groups differed in the magnitude of the increases in mPFC dopamine and disruption of performance on a spatial memory test. Our finding is consistent with a previous microdialysis study that also reported no correlation between dopamine release in the mPFC and plasma corticosterone levels in response to an acute stressor (Imperato et al., 1991 but see Sullivan, 2004). Dopamine release in the mPFC also has been shown not to respond to systemic administration of corticosterone (Imperato et al., 1991). However, the repeated increase in glucocorticoids with the application of each individual stressor during the 10 day CUS protocol may be important for both the neurocemical and behavioral changes. Reducing corticosterone levels through adrenalectomy decreased basal and potassium stimulated levels of dopamine in the mPFC, suggesting that the presence of glucocorticoids helps to maintain mPFC dopamine function (Mizoguchi et al., 2004). Basal and stimulated dopamine release in other forebrain regions is also sensitive to disruption of corticosterone secretion following injections of the corticosterone synthesis inhibitor, metyrapone (Piazza et al., 1996; Rouge-Pont et al., 1995). In the current study, the daily increases in corticosterone with each stressor application may be important for the long-term neurochemical changes in the mPFC associated with CUS, but not for the immediate response to elevation stress.
In conclusion, prior exposure to CUS reduced dopamine release in the mPFC during acute elevation stress and prevented impaired performance on a spatial recognition test following an acute stressor. One proposed function of the stress-induced mPFC dopamine increase is to moderate appropriate coping behaviors (Berridge et al., 2003; Sullivan, 2004). The attenuated dopamine response in the mPFC of CUS rats during an acute stressor may increase the probability of appropriate coping behavior in a particular environment. It has been proposed that a moderate level of dopamine release and receptor stimulation in the mPFC is necessary for appropriate coping behavior, in particular for optimal cognitive function (Arnsten, 1997; Dent et al., 2012; Gamo et al., 2011; Vijayraghavan et al., 2007). Further examination of the effects of chronic stress on dopamine function in the mPFC may provide a greater understanding of the pathological and adaptive consequences of stress.
Acknowledgments
Source of funding: This research was supported through a grant from the National Institutes of Health DA016947-02 to L. Matuszewich and by the Psychology Department of Northern Illinois University. This research was supported in part by grants from the National Center for the Research Resources (5P20RR016466) and the National Institute of General Medical Sciences (8P20GM103395-12), from the National Institutes of Health to C.A. Frye. Its contents are the sole responsibility of the authors and do not necessarily represent the official view of NIGMS, NCRR or NIH.
Footnotes
Conflicts of interest: No conflicts of interest declared
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–8. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zacharko RM. Multiple neurochemical and behavioral consequences of stressors: implications for depression. Pharmacol Ther. 1990;46:119–36. doi: 10.1016/0163-7258(90)90039-5. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–62. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–8. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116:143–51. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, de Biurrun G, Czéh B, van Kampen M, Fuchs E. Selective enhancement of spatial learning under chronic psychosocial stress. Eur J Neurosci. 2002;15:1863–6. doi: 10.1046/j.1460-9568.2002.02043.x. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Espana RA, Stalnaker TA. Stress and coping: Asymmetry of dopamine efferents within the prefrontal cortex. In: Hugdahl K, Davidson RJ, editors. The Asymmetrical Brain. Cambridge, MA: MIT Press; 2003. pp. 69–103. [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Butts KA, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate dopamine efflux to stress via descending glutamatergic feedback to the ventral tegmental area. Int J Neuropsychopharmacol. 2013;16:1799–1807. doi: 10.1017/S1461145713000187. [DOI] [PubMed] [Google Scholar]
- Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc Natl Acad Sci U S A. 2011;108:18459–64. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–34. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol Biochem Behav. 2004;78:569–79. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27:8278–85. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra G, Zurita A, Lacerra C, Molina V. Chronic stress sensitizes frontal cortex dopamine release in response to a subsequent novel stressor: reversal by naloxone. Brain Res Bull. 1999;48:303–8. doi: 10.1016/s0361-9230(98)00179-8. [DOI] [PubMed] [Google Scholar]
- Cuadra G, Zurita A, Gioino G, Molina V. Influence of different antidepressant drugs on the effect of chronic variable stress on restraint-induced dopamine release in frontal cortex. Neuropsychopharmacology. 2001;25:384–94. doi: 10.1016/S0893-133X(01)00234-2. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, García-Verdugo JM, Mora F. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm. 2007a;114:43–8. doi: 10.1007/s00702-006-0565-8. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Garrido P, de Blas M, Mora F. Stress, prefrontal cortex and environmental enrichment: studies on dopamine and acetylcholine release and working memory performance in rats. Behav Brain Res. 2007b;176:267–73. doi: 10.1016/j.bbr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Dent MF, Neill DB. Dose-dependent effects of prefrontal dopamine on behavioral state in rats. Behav Neurosci. 2012;126:620–39. doi: 10.1037/a0029640. [DOI] [PubMed] [Google Scholar]
- Diamond DM. Cognitive, endocrine and mechanistic perspectives on non-linear relationships between arousal and brain function. Nonlinearity Biol Toxicol Med. 2005;3:1–7. doi: 10.2201/nonlin.003.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–6. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999;46:1624–33. doi: 10.1016/s0006-3223(99)00236-x. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ. The effects of stress on central dopaminergic neurons: possible clinical implications. Neurochem Res. 1997;22:1387–94. doi: 10.1023/a:1022075324164. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–28. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol Learn Mem. 2014 doi: 10.1016/j.nlm.2013.09.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge G, Van Kampen M, Mijnster MJ. Perturbations in brain monoamine systems during stress. Cell Tissue Res. 2004;315:1–14. doi: 10.1007/s00441-003-0807-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, McCormick CM, Coopersmith C, Erskine MS. Effects of paced and non-paced mating stimulation on plasma progesterone, 3 alpha-diol and corticosterone. Psychoneuroendocrinology. 1996;21:431–9. doi: 10.1016/0306-4530(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Arnsten AF. Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci. 2011;125:282–96. doi: 10.1037/a0023165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouirand AM, Matuszewich L. The effects of chronic unpredictable stress on male rats in the water maze. Physiol Behav. 2005;86:21–31. doi: 10.1016/j.physbeh.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem. 1994;63:575–83. doi: 10.1046/j.1471-4159.1994.63020575.x. [DOI] [PubMed] [Google Scholar]
- Haile CN, GrandPre T, Kosten TA. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology (Berl) 2001;154:213–20. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illness. Learn Mem. 2008;15:551–64. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci USA. 2009;106:17957–62. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Horger BA, Roth RH. The role of mesoprefrontal dopamine neurons in stress. Crit Rev Neurobiol. 1996;10:395–418. doi: 10.1615/critrevneurobiol.v10.i3-4.60. [DOI] [PubMed] [Google Scholar]
- Hutchinson KM, McLaughlin KJ, Wright RL, Bryce Ortiz J, Anouti DP, Mika A, et al. Environmental enrichment protects against the effects of chronic stress on cognitive and morphological measures of hippocampal integrity. Neurobiol Learn Mem. 2012;97:250–60. doi: 10.1016/j.nlm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Puglisi-Allegra S, Casolini P, Angelucci L. Changes in brain dopamine and acetylcholine release during and following stress are independent of the pituitary-adrenocortical axis. Brain Res. 1991;538:111–7. doi: 10.1016/0006-8993(91)90384-8. [DOI] [PubMed] [Google Scholar]
- Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992;577:194–99. doi: 10.1016/0006-8993(92)90274-d. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–48. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Izaki Y, Hori K, Nomura M. Dopamine and acetylcholine elevation on lever-press acquisition in rat prefrontal cortex. Neurosci Lett. 1998;258:33–6. doi: 10.1016/s0304-3940(98)00841-6. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58(11):843–53. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Ktraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Lataster J, Collip D, Ceccarini J, Haas D, Booij L, van Os J, et al. Psychosocial stress is associated with in vivo dopamine release in human ventromedial prefrontal cortex: a positron emission tomography study using [¹ 8 F]fallypride. Neuroimage. 2011;58:1081–9. doi: 10.1016/j.neuroimage.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–70. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magariños AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiol Behav. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19:743–51. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK. Chronic stress augments the long-term and acute effects of methamphetamine. Neuroscience. 2004;124:637–46. doi: 10.1016/j.neuroscience.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Matuszewich L, Karney JJ, Carter SR, Janasik SP, O'Brien JL, Friedman RD. The delayed effects of chronic unpredictable stress on anxiety measures. Physiol Behav. 2007;90:674–81. doi: 10.1016/j.physbeh.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Kehoe P, Mallinson K, Cecchi L, Frye CA. Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacol Biochem Behav. 2002;73:77–85. doi: 10.1016/s0091-3057(02)00758-x. [DOI] [PubMed] [Google Scholar]
- McFadden LM, Paris JJ, Mitzelfelt MS, McDonough S, Frye CA, Matuszewich L. Sex- dependent effects of chronic unpredictable stress in the water maze. Physiol Behav. 2011;102:266–75. doi: 10.1016/j.physbeh.2010.10.022. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–74. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Takeda S, Aburada M, Tabira T. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J Neurosci. 2004;24:5492–9. doi: 10.1523/JNEUROSCI.0086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokler DJ, Torres OI, Galler JR, Morgane PJ. Stress-induced changes in extracellular dopamine and serotonin in the medial prefrontal cortex and dorsal hippocampus of prenatally malnourished rats. Brain Res. 2007;1148:226–33. doi: 10.1016/j.brainres.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996;93:1325–9. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A, Dagher A, Booij L, Gravel P, Welfeld K, Casey KF, Leyton M, Benkelfat C. Stress-induced dopamine release in human medial prefrontal cortex-18 F-Fallypride/PET study in healthy volunteers. Synapse. 2014 doi: 10.1002/syn.21700. In Press. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. Washington: The National Academies Press; 2011. [Google Scholar]
- Oei NY, Everaerd WT, Elzinga BM, van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress. 2006;9:133–41. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–52. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn Mem. 2008;15:271–80. doi: 10.1101/lm.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology. 2006;31:265–77. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Barrot M, Rougé-Pont F, Marinelli M, Maccari S, Abrous DN, et al. Suppression of glucocorticoid secretion and antipsychotic drugs have similar effects on the mesolimbic dopaminergic transmission. Proc Natl Acad Sci USA. 1996;93:15445–50. doi: 10.1073/pnas.93.26.15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JS. Brain part monoamines in the neuroendocrine mechanisms activated by immobilization stress in the rat. Int J Neurosci. 1984;23:57–67. doi: 10.3109/00207458408985345. [DOI] [PubMed] [Google Scholar]
- Rougé-Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. II. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7189–95. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, de Blas M, Garrido P, Mora F. Effects of an enriched environment on the release of dopamine in the prefrontal cortex produced by stress and on working memory during aging in the awake rat. Behav Brain Res. 2008;187:304–11. doi: 10.1016/j.bbr.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Learning during stressful times. Learn Mem. 2004;11:137–44. doi: 10.1101/lm.66604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the medial prefrontal cortex. Neuroscience. 1993;53:695–703. doi: 10.1016/0306-4522(93)90617-o. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7:131–43. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- Tagliari B, Scherer EB, Machado FR, Ferreira AG, Dalmaz C, Wyse AT. Antioxidants prevent memory deficits provoked by chronic variable stress in rats. Neurochem Res. 2011;36:2373–80. doi: 10.1007/s11064-011-0563-6. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Javoy F, Glowinski J, Kety SS. Effects of stress on the metabolism of norepinephrine, dopamine and serotonin in the central nervous system of the rat. I. Modifications of norepinephrine turnover. J Pharmacol Exp Ther. 1968;163:163–71. [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–84. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–4. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–73. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacology (Berl) 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural- neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–34. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Willner P, Scheel-Kruger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav R. 2013;37:2331–71. doi: 10.1016/j.neubiorev.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behav Brain Res. 2008;187:41–7. doi: 10.1016/j.bbr.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–35. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita A, Martijena I, Cuadra G, Brandão ML, Molina V. Early exposure to chronic variable stress facilitates the occurrence of anhedonia and enhanced emotional reactions to novel stressors: reversal by naltrexone pretreatment. Behav Brain Res. 2000;117:163–71. doi: 10.1016/s0166-4328(00)00302-8. [DOI] [PubMed] [Google Scholar]