Abstract
Objective
The site-specificity of endothelial phenotype is attributable to the local hemodynamic forces. The flow regulation of microRNAs (miRNAs) in endothelial cells (ECs) plays a significant role invascular homeostasis and diseases. The objective of this study is to elucidate the molecular mechanism by which the pulsatile shear flow (PS)-induced miR-23b exerts anti-proliferative effects on ECs.
Approach and Results
We used a combination of a cell perfusion system and experimental animals to examine the flow regulation of miR-23b in modulating EC proliferation. Our results demonstrated that PS induces the transcription factor KLF2 to promote miR-23b biosynthesis; the increase in miR-23b then represses cyclin H to impair the activity and integrity of CDK-activating kinase complex (CAK). The inhibitory effect of miR-23b on CAK exerts dual actions to (1) suppress cell cycle progression, and (2) reduce basal transcription by deactivating RNA polymerase II. While PS regulates the miR-23b/CAK pathway to exert anti-proliferative effects on ECs, oscillatory shear flow (OS) has little effect on the miR-23b/CAK pathway and hence does not cause EC growth arrest. Such flow pattern-dependent phenomena are validated with an in vivo model on rat carotid artery: the flow disturbance induced by partial carotid ligation led to a lower expression of miR-23b and a higher EC proliferation in comparison to the pulsatile flow regions of the unligated vessels. Local delivery of miR-23b mitigated the proliferative EC phenotype in partially ligated vessels.
Conclusions
Our findings unveil a novel mechanism by which hemodynamic forces modulate EC proliferative phenotype through the miR-23b/CAK pathway.
Keywords: shear stress, endothelial cell growth, microRNA, CAK
Introduction
Hemodynamic forces play a pivotal role in modulating the physiological and pathological behaviors of vascular endothelial cells (ECs)1. Pulsatile shear flow (PS), which exists in the straight part of arterial tree with a high shear stress and a clear forward direction, is associated with quiescent ECs presenting the athero-protective phenotypes2. In contrast, the disturbed oscillatory shear flow (OS), which exists at vascular curvatures and bifurcations and has no clear flow direction, converts ECs toward a more active state associated with pro-inflammatory phenotypes with high EC turnover and atherogenic gene expressions3. Recent studies have demonstrated that microRNAs (miRNAs) participate in the flow regulation of endothelial functions4, 5, but the feedback regulation of signaling molecules at post-transcriptional level remains to be established.
Comprehensive analyses of miRNA expression profiling in vascular ECs reveal that a series of miRNAs are differentially expressed, both in vitro and in vivo, in response to different patterns of flow4, 6, 7. The transcription factor Krüppel-like Factor 2 (KLF2) is a major mediator for flow-regulation of EC functions8, 9; overexpression of KLF2 leads to the expression of several miRNAs, including miR-23b10. Our previous studies demonstrated that PS up-regulates KLF2,miR-23b, and miR-19a to suppress EC proliferation4, 11, 12, and confer the athero-protective effect of PS. Conversely, other miRNAs, such as miR-21, miR-663, and miR-712 can mediate the pro-inflammatory response of ECs induced by OS5, 13, 14. Recent studies demonstrate the differential miRNAs expression in different locations of the arterial tree, e.g., a higher expression of miR-10a and a lower expression of miR-92a at the descending thoracic aorta with athero-protective flow, in comparison to the internal curvature of aortic arch with athero-prone flow7. These studies support the important roles of miRNAs in regulating endothelial phenotypes under physiological and pathophysiological conditions.
Recent studies, including ours, have shown the important roles of miR-23b in cell growth4, 15, 16, angiogenesis17, and stem cell differentiation18. More than twenty putative targets of miR-23b have been reported; and these have been confirmed in a variety of contexts by the pairing of the seed sequence and mRNA 3’ UTRs. For instance, Zhou et al.17 demonstrated the pro-angiogenic role of miR-23b by targeting sprouty2; Zhang et al.15 reported that miR-23b represses cancer cell proliferation and migration by regulating a cohort of pro-metastatic genes. We previously reported that inhibition of miR-23b reversed the PS-induced E2F1 reduction and Rb hypo-phosphorylation, and attenuated the PS-induced EC growth arrest4. The direct target of miR-23b that mediates these processes, however, remains unclear.
Herein, we report that the PS-induced miR-23b modulates cell cycle regulatory networks through the posttranscriptional repression of cyclin H (CCNH). Importantly, CCNH repression impairs CDK-activating kinase (CAK) complex to deactivate CDK2, CDK4, and RNA polymerase II (Pol II), thus further attenuating the transcription of cell cycle regulators that lead to EC growth arrest. Moreover, the in vivo studies performed on rat carotid arteries showed that, similar to our in vitro findings, different flow patterns differentially regulate EC proliferation through the miR-23b/CAK pathway. We thus demonstrate that miR-23b is a mechano-sensitive miRNA both in vitro and in vivo. Our findings elucidate the molecular mechanism by which miR-23b modulates mechano-transduction and vascular homeostasis.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
PS induces the expression of miR-23b cluster through KLF2
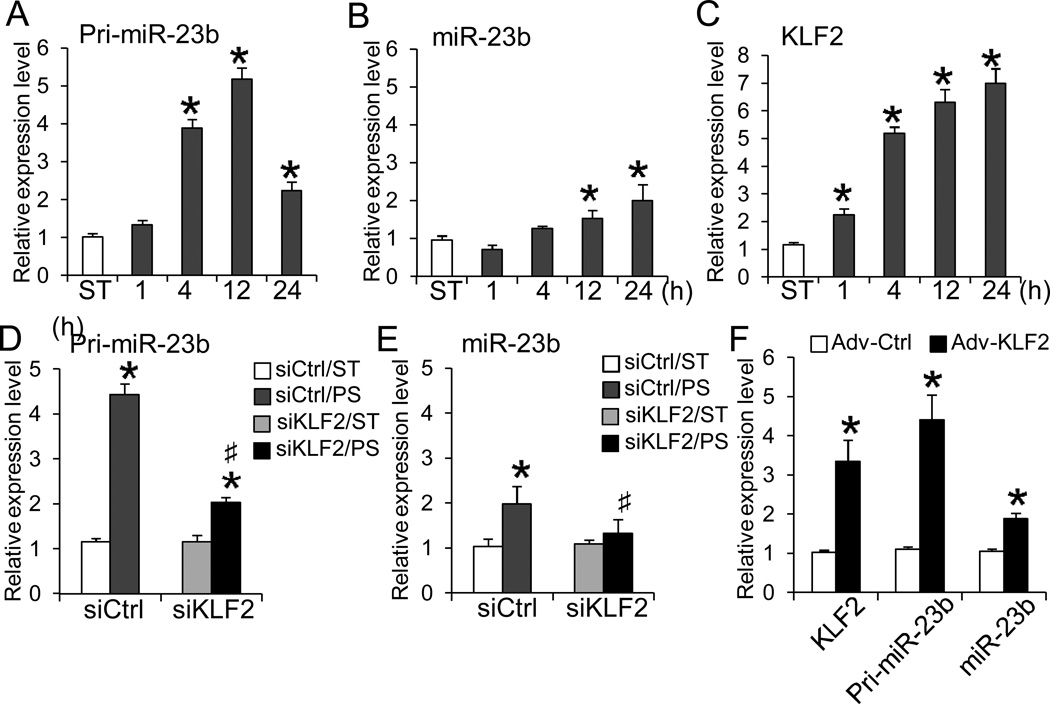
To explore the dynamic regulation of miR-23b, we examined the temporal expression profiles of primary (pri-miR-23b) and mature miR-23b in ECs exposed to PS over 24 h. qRT-PCR analyses revealed that PS increased expressions of both pri-miR-23b and miR-23b over 24 h, with the pri-miR-23b level peaking at 12 h (Fig. 1A) and the mature miR-23b level being sustained over 24 h (Fig. 1B). A KLF2 binding motif (CACCC) was identified in the miR-23b promoter region (Online Fig. IA), suggesting the involvement of this shear-sensitive transcription factor in regulating miR-23b biosynthesis. The temporal expression profile showed that KLF2 was induced by PS within 1 h and sustained over 24 h (Fig. 1C). To verify that KLF2 mediates the PS-induction of miR-23b, KLF2 was knocked down with KLF2-specificsiRNA (siKLF2) transfection in ECs prior to being subjected to PS. The results showed that siKLF2 attenuated the PS-induced increases in pri-miR-23b and mature miR-23b (Figs. 1D and E, tested at their respect 4 and 24 h peaks). Overexpression of KLF2 with adenovirus induced both pri-miR-23b and mature miR-23b (Fig. 1F). Furthermore, the putative promoter region of miR-23b (~700 bp flanking sequence upstream of miR-23b precursor) containing a KLF2 binding motif, as suggested by Yeung et al.19, was subcloned into pGL2 luciferase reporter vector (Pro23b) for promoter activity assay. Overexpression of KLF2 increased the luciferase activity of the Pro23b construct. Mutation of KLF2 binding motif (Pro23bmt) blocked the KLF2-dependent activation (Online Fig. IB). Taken together, these findings strongly indicate that KLF2 is a direct upstream transcription factor for PS-induction of miR-23b in ECs.
Figure 1. PS induces the expressions of miR-23b through KLF2.
ECs were subjected to PS for indicated durations or kept as static control (ST). Temporal expression levels of A. pri-miR-23b, B. miR-23b, and C. KLF2 were determined with qRT-PCR (n=4 each, *P < 0.05 vs. ST). ECs were reversely transfected with siKLF2 or scramble control (siCtrl) for 48 h and then subjected to either 4- or 24-h PS. The relative levels of D. pri-miR-23b (4-h PS) and E. miR-23b (24-h PS) were determined with qRT-PCR (n=4, *P<0.05 vs. Ctrl/ST; #P<0.05 vs. Ctrl/PS). F. ECs were transduced with adenovirus carrying KLF2 (Adv-KLF2) or GFP (Adv-Ctrl) cDNA for 72 h. The expression levels of KLF2, pri-miR-23b, and miR-23b were determined with qRT-PCR (n=4, *P<0.05 vs. Adv-Ctrl).
PS-Induced miR-23b modulates the expressions and activities of cell cycle regulatory proteins, as well as cell cycle progression
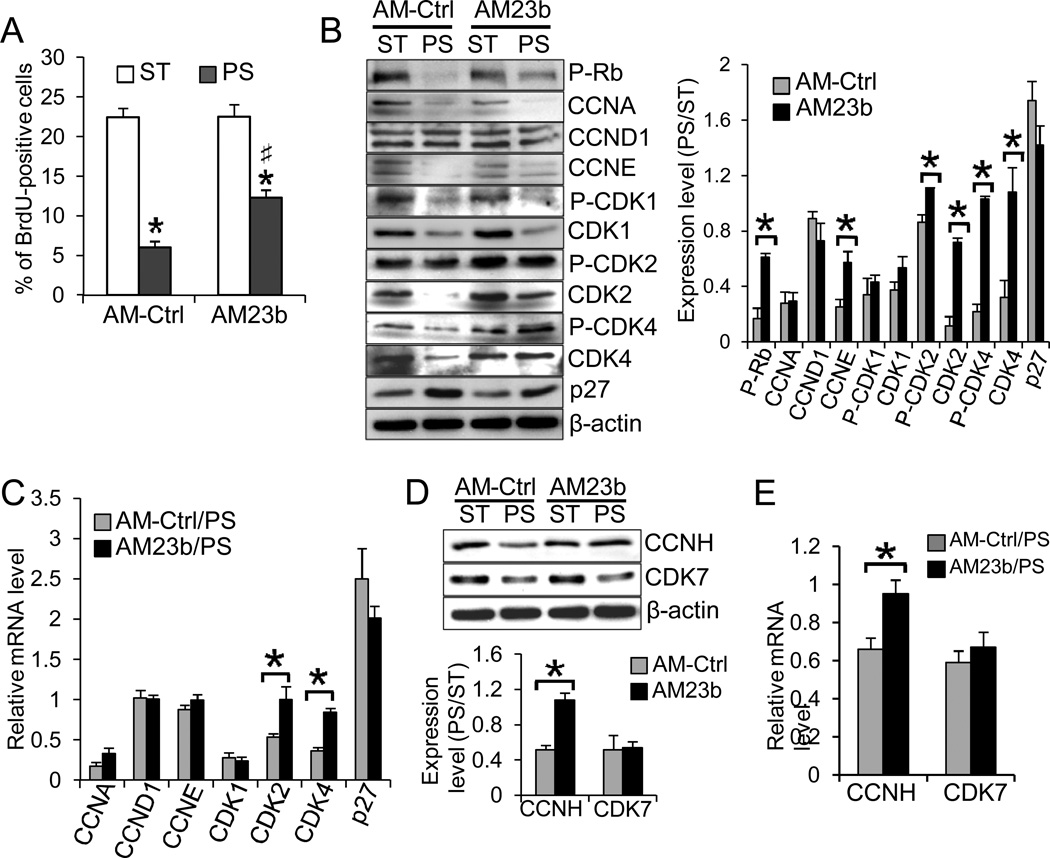
To examine the temporal effect of PS on EC proliferation, we performed the BrdU-incorporation assay with ECs subjected to various durations of PS. As shown in Online Fig. IIA, PS transiently increased the number of BrdU-positive cells over the ST control at 4 h, but caused the ECs to enter a quiescent state later (12 and 24 h). In addition, PS regulated the expressions of cell cycle regulators as shown by Western blot analyses. The exposure of ECs to 24-h PS resulted in the hypo-phosphorylation state of Rb and downregulations of CCNA, CCNE, CDK1, CDK2, and CDK4, and up-regulation of the CDK inhibitor p27, compared with the ST control (Online Fig. IIB). The temporal changes of these G1/S-related cyclins and CDKs agreed with the BrdU-incorporation assay (Online Fig. IIA). In comparison to the control group, knockdown of miR-23b with Anti-miR-23b (AM23b) attenuated the PS-induced EC growth arrest (Fig. 2A). Transfection of AM23b partially reversed the PS-suppressions of P-Rb, CCNE, P-CDK2, P-CDK4, CDK2 and CDK4 (Fig. 2B), which may in turn promote EC entry into the synthetic phase of cell cycle. The effect of AM23b on expression levels of CDK2 and CDK4,but not CCNE, was confirmed at the mRNA level with qRT-PCR; AM23b had no significant effects on other cell cycle regulators (CCNA, CCND1, CDK1, and p27) (Fig. 2C). However, based on the miRNA target prediction20, neither CDK2 nor CDK4 mRNAs contain miR-23b binding sequence in their 3’UTRs. Since AM23b transfection attenuated the PS-reductions of CDK2 and CDK4activities, we examined the miR-23b targets among the upstream regulators of CDK2 and CDK4, and found the miR-23b binding sequence in the 3’UTR of CCNH mRNA, which is highly conserved among human, chimpanzee, mouse, rat, and dog (Online Fig.III). CCNH has been demonstrated to form the CDK-activating kinase complex (CAK) with CDK7 to phosphorylate CDK1, CDK2, and CDK421. We found that PS indeed downregulated CCNH and that transfecting ECs with AM23b attenuated the PS-downregulation of CCNH, but did not have significant effects on CDK7 (Figs.2D–E). These results indicate that CCNH may play a role in themiR-23b-mediated reductions of expressions and activities of CDK2 and CDK4, and hence the cell cycle progression under PS.
Figure 2. MiR-23b regulates PS-induced EC growth arrest through modulating the expressions and activities of cell cycle regulatory proteins.
ECs were reversely transfected with AM-Ctrl or AM23b and then subjected to 24-h PS or kept as ST. A. BrdU was added into the media during the last 4 h of the periods. ECs were stained with anti-BrdU and subjected to flow cytometry analysis. Knockdown of miR-23b significantly attenuated the PS-induced EC growth arrest (n=4, *P<0.05 vs. Ctrl/ST; #P<0.05 vs. Ctrl/PS). B–E. Effects of miR-23b knockdown on the expression of cell cycle regulators were determined by Western blot (B and D, representatives of triplicate experiments with similar results) and quantified by qRT-PCR analyses (C and E). The bar graphs in B and D are the results of densitometry analyses of the Western blot experiments normalized to internal protein levels and presented as the ratio of PS/ST (n=4 each, *P<0.05 vs. AM-Ctrl).
CCNH is a direct target of miR-23b in regulating EC proliferation under PS
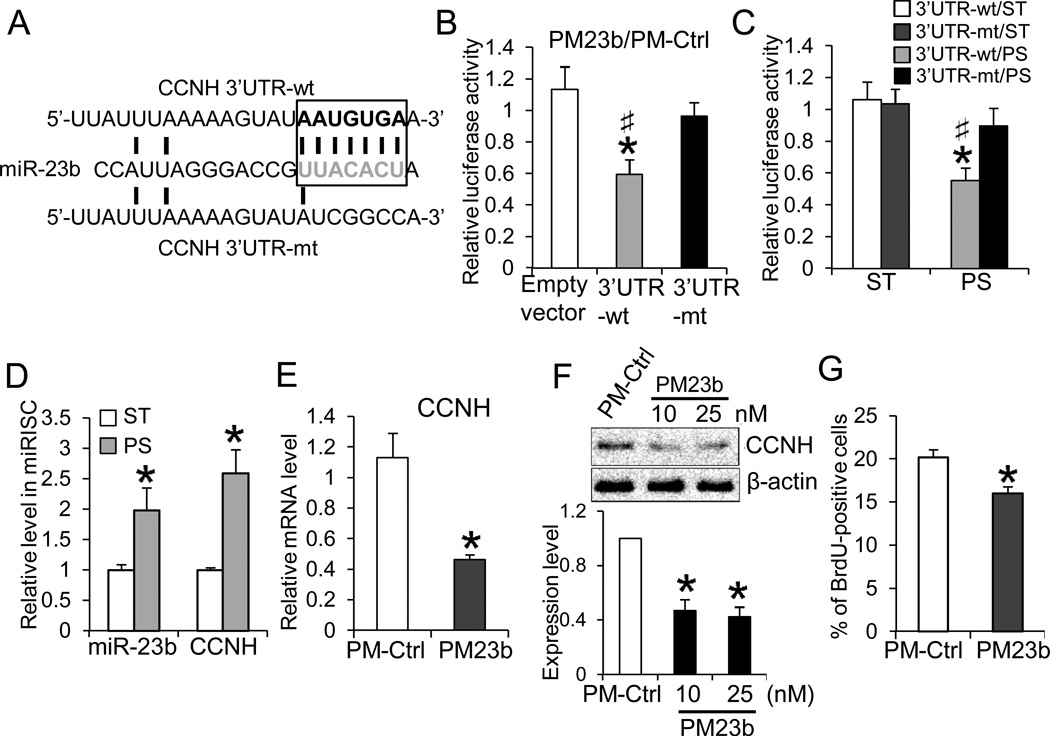
To determine whether CCNH is a direct target of miR-23b in ECs, we constructed reporters containing the luciferase cDNA fused to either the wild-type or mutant 3’UTR of CCNH mRNA (Fig. 3A, CCNH-3’UTR-wt and -mt, respectively) and tested their activities in ECs. Transfection of Pre-miR-23b (PM23b) repressed the luciferase activity of CCNH-3’UTR-wt; the repression was not observed with empty vector or CCNH-3’UTR-mt (Fig. 3B). In comparison with the ST condition, PS reduced the reporter activity of CCNH-3’UTR-wt, but did not have significant effect on the mutant (Fig.3C). To verify the association of miR-23b and CCNH mRNA in the miRISC complex, we performed RNA immunoprecipitation22, using the anti-pan-argonaute (AGO) antibody. The miR-23b and CCNH transcripts in miRISC were found to increase under PS in comparison to ST (Fig.3D). These results indicate that PS induced the association of CCNH and miR-23b in miRISC and that the CCNH modulation by PS involves a miRNA-dependent mechanism. In addition, overexpression of miR-23b with PM23b in ECs decreased CCNH expression (at both protein and mRNA levels, as shown in Figs.3E and 3F), and reduced the number of BrdU-positive cells (Fig. 3G). To further assess the role of CCNH in regulating EC proliferation, we performed gain- or loss-of-function experiments of CCNH in EC proliferation. As expected, knockdown of CCNH decreased the number of BrdU-positive cells (Online Fig.IVA), while overexpression of CCNH attenuated the PS-induced EC growth arrest (Online Fig. IVB).
Figure 3. CCNH is a direct target of miR-23b.A.
Luciferase reporters containing the CCNH 3’UTR (3’UTR-wt) or the mutated CCNH 3’UTR (3’UTR-mt) sequences were constructed. B. ECs were transfected with the reporter constructs together with CMV-β-gal, and PM23b or PM-Ctrl. The transfection of PM23b attenuated CCNH3’UTR-wt luciferase activity under ST condition. The luciferase activities are normalized to β-gal and presented as the ratio between PM23b and PM-Ctrl (n=5, *P < 0.05 vs. empty vector; #P<0.05 vs. 3’UTR-mt). C. 24-h PS reduced luciferase activities of 3’UTR-wt, but not 3’UTR-mt (n=4, *P < 0.05 vs. ST; #P<0.05 vs. 3’UTR-mt/PS). D. 24-h PS increased miR-23b and CCNH in miRISC. miRISCs were immuno-precipitated with anti-pan-AGO, and the levels of miR-23b and CCNH mRNA were determined with qRT-PCR (n=4, *P < 0.05 vs. ST). E–G. PM23b transfection decreased CCNH mRNA (E) and protein (F), as well as the percentage of BrdU-positive cells (G) (n=4, *P<0.05 vs. PM-Ctrl). The Western blot results in F. are representative of triplicate experiments with similar results, and the bar graph is the results of the densitometry analyses normalized to internal protein levels.
PS-induced miR-23b suppresses basal transcription by targeting CAK
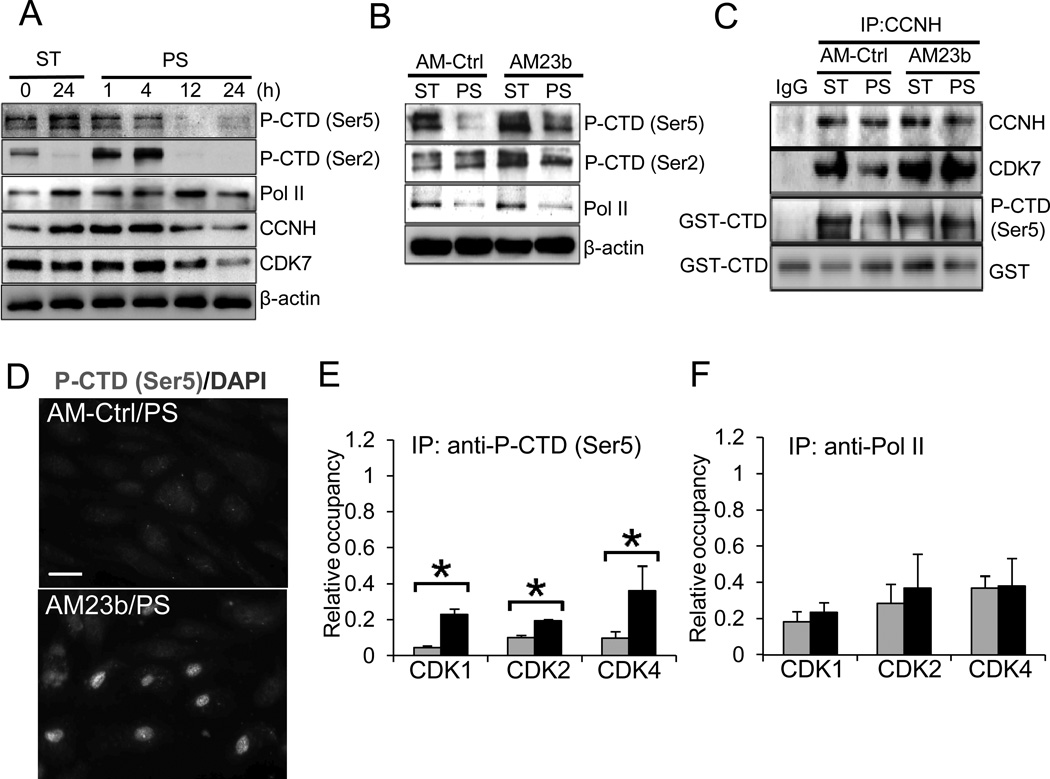
The CAK complex (CCNH-CDK7) regulates basal transcription by phosphorylating the C-terminal domain (CTD) of Pol II23, in addition to regulating the activities of CDKs. The CTD consists of 52 repeats of the highly conserved sequence Tyr-Ser-Pro-Thr-Ser-Pro-Ser, and the Ser residues at positions 2 and 5 are known targets for CAK phosphorylation. Western blot analyses showed that the CTD was highly phosphorylated at Ser5 in ECs kept under ST condition for 24 h, and that prolonged PS (12 and 24 h) led to dephosphorylation at Ser5 below the ST level (Fig. 4A). Interestingly, in contrast to Ser5, the phosphorylation level of Ser2 was very low in static ECs at 24 h; after showing transient increases at 1 and 4 h under PS, the Ser2 phosphorylation decreased to the low ST level. These results indicate that the reduction of phosphorylation of Ser5, rather than Ser2, is a consequence of prolonged PS. Together with the results that the prolonged PS decreased expression levels of CCNH/CDK7(Figs. 2E–F and 4A), these findings indicate that PS downregulates the CAK pathway to modulate Pol II activities. To further confirm the effect of PS on the CTD phosphorylation, we performed immunofluorescence staining with the antibody recognizing the Ser5 phosphorylation, and the results agreed with Western blot analyses (Online Fig. V).
Figure 4. PS-induced miR-23b modulates the integrity and activity of CAK complex, which mediates basal transcription of CDK2 and CDK4.
ECs were subjected to PS or kept under ST for indicated times. A. PS-regulation of the phosphorylation of CTD (P-CTD) at Ser5 and Ser2 residues, and the expressions of total Pol II, CCNH, CDK7, and β-actin. B–C. AM23b reversed the PS-reduction of Ser5 and Ser2 phosphorylation of CTD (B), and CAK integrity and activities (C). CAK complex was immunoprecipitated with CCNH antibody, and the CAK activities was determined by kinase assays using GST-CTD as the substrate; the CAK integrity was determined by the presence of CCNH and CDK7 in the complex. D. AM23b reversed PS-reduction of phospho-Ser5 was confirmed by immunofluorescence staining. Scale bar, 20 um. E–F. Effects of AM23b on PS-regulation on Pol II recruitment to the TSS of CDKs. Chromatin Immunoprecipitations (ChIP) were performed with antibodies against the phospho-Ser5 CTD (E) or total Pol II (F), and the levels of enriched TSS of CDK1, CDK2, and CDK4 were determined with qRT-PCR. Results were normalized to corresponding inputs and presented as the ratio of PS/ST (n=5, *P<0.05 vs. AM-Ctrl/PS). Results in A–D are representative images of four experiments with similar results.
To investigate whether miR-23b mediates the PS-regulation of CAK activity and the CTD phosphorylation, ECs were transfected with either AM23b or AM-Ctrl and then subjected to 24-h PS or kept as ST. As shown in Fig. 4B, AM23b transfection reversed the PS-induced Ser5 dephosphorylation, but not Ser2 phosphorylation nor the level of Pol II. In ECs under PS. AM23b transfection also restored the integrity and activity of the CAK complex as indicated by the increased presence of CDK7 in the immunocomplex with CCNH and the increases of GST-CTD phosphorylation, respectively (Fig. 4C). Immunostaining (Fig. 4D) also showed that AM23b transfection increased Ser5 phosphorylation of the CTD in EC nuclei under PS. Furthermore, our results revealed that overexpression of CCNH reversed the PS-suppression of CTD phosphorylation, and that knocking down CCNH decreased the CTD phosphorylation (Online Fig. VI). Taken together, these data indicate that miR-23b mediated the PS-induced downregulation of CCNH expression and hence the decrease in CAK activity, which in turn reduced Ser5 phosphorylation of the CTD.
To examine the effects of altered the CTD phosphorylation on transcription controls of the cell cycle regulatory proteins (CDK1, CDK2, and CDK4), chromatin-immunoprecipitation (ChIP) assays were performed using antibodies againstSer5phosphorylation of the CTD and total Pol II. The immunocomplexes were analyzed using qRT-PCR with primers specific to the proximal regions of the transcription start sites (TSS) of CDK1, CDK2, and CDK4. PS significantly decreased both the phospho-CTD (Ser5) and total Pol II occupancy in the TSS of these genes (Fig. 4E & 4F), consistent with the PS reductions of CTD phosphorylation and Pol II expression. As expected, AM23b transfection attenuated the reductions of phospho-CTD occupancy in the TSS of these genes, but had no significant effects on the total Pol II binding; these findings are consistent with the findings that miR-23b is involved in Ser5 phosphorylation of the CTD but not Pol II expression (Fig. 4B). Notably, although AM23b enhanced the phospho-CTD binding at CDK1 TSS (Fig. 4E), its mRNA level was not affected (Fig. 2E), indicating that the increase of initiation complex at TSS may not be sufficient to progress through the subsequent transcription events.
Differential regulation of miR-23b by flow patterns
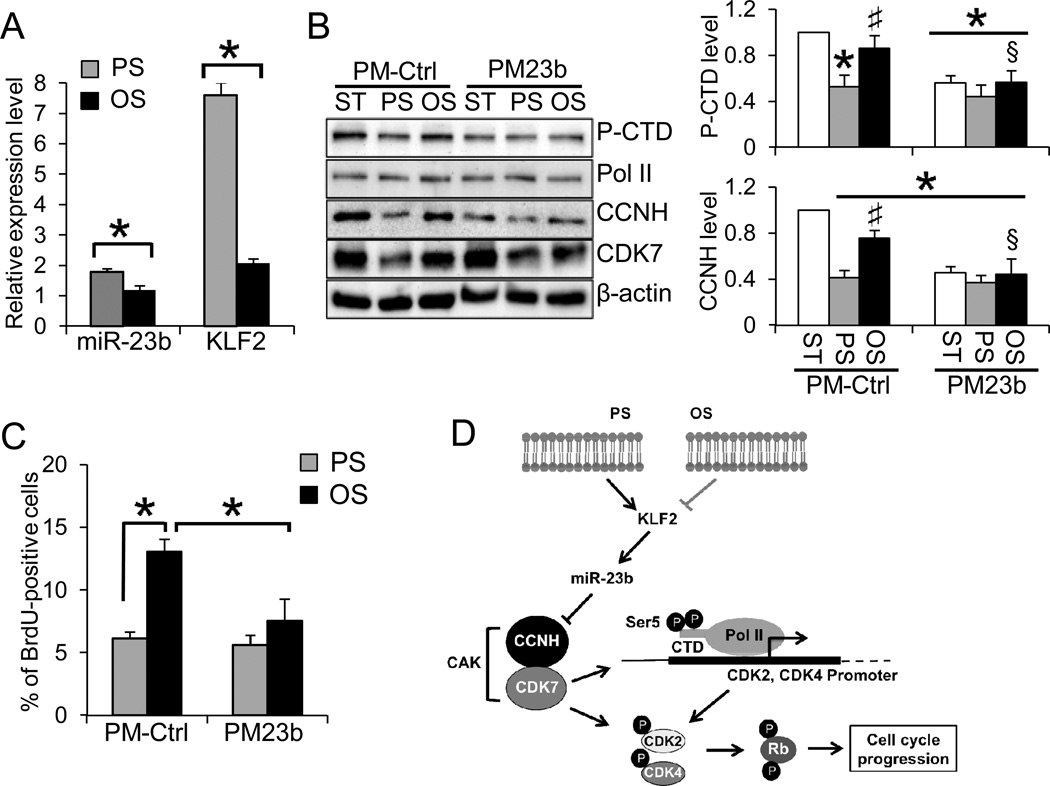
PS and OS differentially regulate EC gene expression and functions. As shown in Fig. 5A, PS significantly increasedKLF2 and miR-23b expressions in comparison with the levels under OS and static conditions. The fluorescence in situ hybridization (FISH) with miR-23b-LNA probe showed that miR-23b staining in the nuclear and peri-nuclear regions was much stronger under PS than OS (Online Fig. VII), which is consistent with the qRT-PCR results (Fig. 5A). Furthermore, in comparison to PS, Ser5 phosphorylation of the CTD and expressions of CAK components were significantly higher under static and OS conditions (Fig. 5B). Under ST and OS conditions, overexpression of miR-23b decreased the CCNH expression and CAK complexes to levels similar to those under PS (Fig. 5B). Along the same line, the number of BrdU-positive cells under OS was significantly reduced, and that miR-23b overexpression significantly attenuated the EC proliferation under OS (Fig. 5C). These findings indicate that PS but not OS exerts strong anti-proliferative effect on ECs through the miR-23b/CAK pathway (Fig. 5D).
Figure 5. Flow patterns differentially regulate miR-23b/CAK pathway and EC proliferation.
A. Differential regulation of miR-23b and KLF2 under 24-h PS and OS. The expression levels were normalized to the internal controls and presented as the ratio of PS/ST or OS/ST (n=4, *P<0.05 vs. PS). B. PM23b decreased the CCNH expression and CTD phosphorylation (Ser5) under ST (Lanes 1 vs. 4) and OS (Lanes 3 vs. 6), but not PS (Lanes 2 vs. 5). Images represent three experiments with similar results, and the bar graph is the densitometry analyses of Western blot (n=3, *P<0.05 vs. PM-Ctrl/ST; #P<0.05 vs. PM-Ctrl/PS; §P<0.05 vs. PM-Ctrl/OS).C. PM23 further reduced the percentage of BrdU-positive cells under OS (black bars), but had little effects on PS condition (grey bars). The results were quantified by flow cytometry analyses (n=5, *P<0.05).D. Schematic representation of the proposed mechanism by which miR-23b mediates the flow regulation of EC cell cycle progression.
Flow disturbance reduces miR-23b expression and promotes EC proliferation in partial carotid ligation in vivo
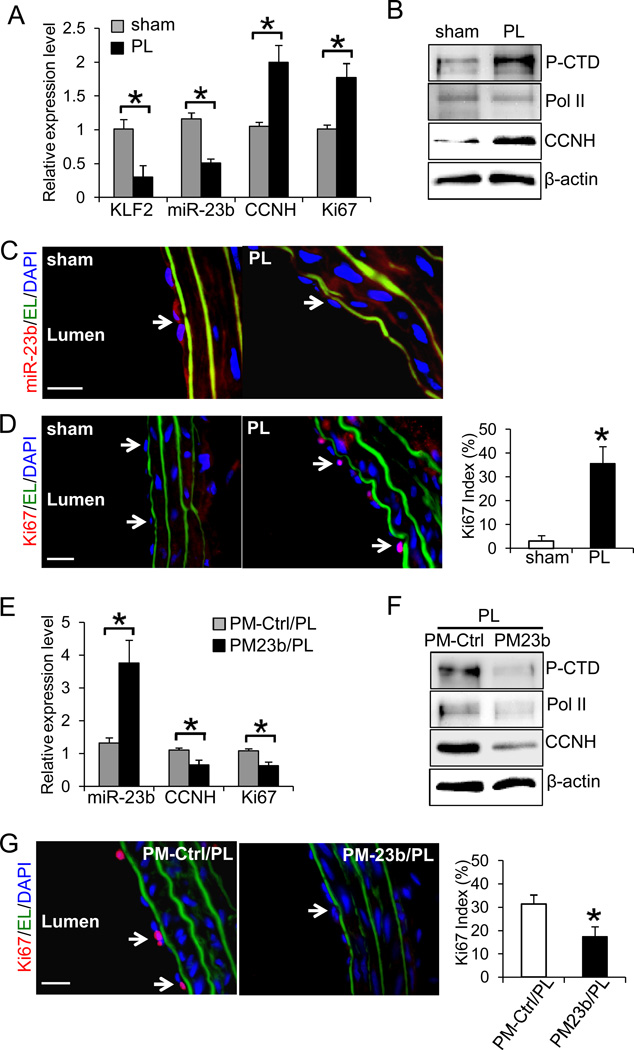
To validate our in vitro findings, the flow-regulation of miR-23b and EC proliferation was studied in the rat carotid partial ligation model24. Three branches of the left carotid artery (LCA) were surgically ligated (PL) or left intact (sham). Ultrasonographic study confirmed that the partial carotid ligation created a disturbed flow with low shear stress in PL, while the flow in the sham group was maintained as pulsatile flow with high shear stress (Online Fig VIII). The effects of blood flow disturbance on the expression levels of miR-23b were examined one-week post-operation. Intima RNA was extracted from the segments of LCAs by perfusion with TRIzol and subjected to qRT-PCR analyses. In agreement with our in vitro studies, the expressions of KLF2 and miR-23b in PL were significantly lower than those in sham, and the levels of CCNH and proliferation marker Ki67 in PL were higher than those in sham (Fig. 6A). Western blot analyses of intima proteins extracted from LCAs showed that Ser5 phosphorylation of the CTD and the expression of CCNH were higher in PL than sham (Fig. 6B). Furthermore, the immunofluorescence staining of the cross-sections of LCA segments revealed that PL group had a reduced level of miR-23b (Fig. 6C) and a strong proliferative phenotype (Fig. 6D) in endothelium (as indicated by detection of vWF-positive and CD45-negative cells in Online Fig. IX), in comparison to sham group. To further determine the effect of miR-23b on EC proliferative phenotype in response to flow disturbance, we locally introduced miR-23b into the segments of LCAs with PM23b-loaded pluronic F127 thermo-gel immediately after partial ligation of LCAs. As shown in Figs. 6E–H, local delivery of miR-23b attenuated PL-inductions of CCNH, Ki67, as well as Ser5 phosphorylation of the CTD, in comparison with those of PL receiving control RNA. These outcomes are consistent with our in vitro results and strongly support that miR-23b is critical in regulating endothelial proliferation in response to flow disturbance.
Figure 6. Flow disturbance reduces miR-23b expression and promotes EC proliferation in vivo.
A. The expression levels of miR-23b, KLF2, CCNH, and Ki67 in rat left carotid arteries (LCAs) with partial ligation (PL) or sham surgery (sham) were determined with qRT-PCR. Endothelial RNA samples were extracted from LCA by perfusing TRIzol through the carotid intima (n=4 each, *P<0.05: PL vs. sham). B. Total intima proteins were extracted from LCAs of PL and sham, and the levels of CTD phosphorylation, Pol II and CCNH were determined by Western blot. C. The decrease in miR-23b in PL was confirmed by FISH staining (red) in frozen sections. D. The increase in Ki67 in PL was demonstrated by immunofluorescence staining of Ki67 (red) in frozen sections. The Ki67 index: the number of Ki67-positive ECs / the number of ECs (n=4 each, 10 sections per sample, *P<0.05 vs. sham). Effects of PM23b treatment on E. the expression levels of miR-23b, CCNH, and Ki67 in intimal RNA extracted from PL LCAs with local delivery of PM-Ctrl (PM-Ctrl/PL) or PM23b (PM23b/PL) (n=4 each, *P<0.05: PM-Ctrl/PL vs. PM23b/PL; and F. the levels of CTD phosphorylation, Pol II, and CCNH in PM-Ctrl/PL and PM23b/PL were determined by Western blot. G. PM23b treatment reduced Ki67 staining in PL. EL: elastic lamina (green), DAPI: nuclear staining (blue). Ki67 index (n=4 each, 10 sections per sample, *P<0.05 vs. PM-Ctrl/PL). White arrows indicate the positive staining of miR-23b or Ki67 in endothelium. Results in B–D and F–G are representative images from four animals with similar results. Scale bar, 20 um.
Discussion
MiR-23b has been shown to play important roles in tumor and cardiovascular biology. Recent studies demonstrated that miR-23b is down-regulated in various tumor types when compared to the normal tissues and that it functions as a tumor suppressor to repress malignant growth of the cell lines tested15, 16, 25. Our previous report4 and present findings also support the anti-proliferative role of miR-23b in vascular ECs responding to hemodynamic force with a clear direction. As summarized in Fig. 5D, we have demonstrated a novel mechanism by which flow patterns modulate EC phenotypes through miR-23b. Our data suggest that PS-induction of miR-23b is dependent on flow-sensitive transcription factor KLF2. The KLF2-dependent elevation of miR-23b in response to PS leads to the decrease of CCNH, which impairs the integrity and activity of the CAK complex to lead to dual actions: 1) the reductions of CDK2 and CDK4 activities, and 2) the attenuation of CTD phosphorylation of Pol II, which reduces CDK2/4 transcription. These actions result in Rb hypo-phosphorylation and hence the anti-proliferative/quiescent EC phenotype under prolonged PS. Our findings also demonstrate that exposure of ECs to disturbed flow leads to down-regulation of miR-23b and the augment of CAK pathway and proliferative phenotype and that overexpression of miR-23b attenuated these effects caused by disturbed flow, both in vitro and in vivo.These findings establish the roles of a flow-sensitive miRNA in regulating endothelial phenotypes and vascular pathophysiology involving local hemodynamics.
KLF2 has been shown as an athero-protective transcription factor in ECs that modulates ~70% of the genes that are responsive to shear stress8. The flow induction of KLF2 is believed to regulate many endothelial functions, including EC proliferation8, 9. Indeed, our results demonstrate that KLF2 overexpression is sufficient to reduce the BrdU-incorporation of ECs, and that knocking down KLF2 significantly increases the percentage of BrdU-positive ECs under both ST and PS conditions, in comparison to control transfection (Online Fig. X). However, there was a lack of a clear understanding of the mechanism that mediates this anti-proliferative effect of KLF2 on ECs. Recent studies reported that KLF2 itself is regulated at the posttranscriptional level26 and that endothelial overexpression of KLF2 induces the expression and secretion of miRNAs10. Our data demonstrate that KLF2 overexpression leads to inductions of primary and mature miR-23b and that knocking down KLF2 blocks the PS-induction of miR-23b. Thus, our present findings provide a consistent line of evidence that not only confirms the anti-proliferative role of KLF2, but also identifiesmiR-23b as a novel modulator forKLF2-regualted EC phenotype.
We further examined the molecular mechanism by which miR-23b modulates cell cycle regulatory proteins. Although miR-23b mediates PS suppressions of CDK2 and CDK4 expression and phosphorylation, the lack of miR-23b binding site in the 3’UTR of these CDKs suggests that the miR-23b regulation of these proteinsis not through direct targeting. We have identified CCNH in the CAK complex as a direct target for miR-23b (Fig.3); the CAK complex possesses the dual capabilities of modulating basal transcription and phosphorylating the T-loop of CDKs that controls cell cycle progression27.In addition toCDK2/4 phosphorylation, CAK activity is known to modulate the initiation event of transcription through phosphorylating the Ser5 residues of the CTD of Pol II (Online Fig. XI). A recent study has demonstrated that 6h-laminar shear increases Ser2 but not Ser5 phosphorylation of the CTD and its recruitment to eNOS gene28. In agreement with this finding, our present study shows that short-term PS (1 and 4 h) increases the CTD phosphorylation at Ser2 but not Ser5, where as prolonged PS (12 and 24 h) causes a reduction in the phosphorylation of both Ser2 and Ser5. The temporal changes of CTD phosphorylation are in agreement with the downregulation of CAK components in ECs under PS. Along the same line, overexpression of KLF2 mimicking PS condition led to the decreases of Ser5 phosphorylation of the CTD and CCNH expression (Online Fig. XII). Most importantly, AM23b restores Ser5 phosphorylation of the CTD and its recruitment to the TSS of CDK2 and CDK4, validating that PS-regulation of CAK activity is mediated by miR-23b. In this respect, the PS-induced miR-23b exerts the anti-proliferative effects on ECs through both inactivation and transcriptional suppression of cell cycle regulatory proteins.
Studies with culture ECs in flow chamber have shown that laminar flow with high shear stress causes ECs to be in a quiescent state whereas disturbed flow patterns increase EC turnover29–31. In agreement with these observations, our results have shown that BrdU-incorporation in ECs is significantly less under PS than OS. Our in vitro findings indicate that PS and OS cause differential effects on miR-23b expression, which acts through CAK pathway to cause opposite changes in EC proliferation in response to these two flow patterns. These results reflect the in vivo conditions that ECs in the arterial tree exposed to PS are primarily quiescent, and that the increase of EC turnover is usually associated with athero-prone sites where the blood flow is disturbed3. The disturbed flow created by partial carotid ligation has been reported to cause carotid intima-media thickening32 and advanced lesion development33. Using this partial ligation model, we have shown the differential effects of pulsatile flow vs. disturbed flow on the expression levels of miR-23b and CCNH, as well as EC proliferation, in rat carotid arteries. Thus, in comparison to sham with high shear stress, the ECs in PL exposed to low and oscillatory shear stress have lower miR-23b expression and higher CCNH expression, CTD phosphorylation, and EC proliferation. Furthermore, our study demonstrates the therapeutic role of miR-23b by showing that the pathological effects (e.g. CAK activation and EC proliferative phenotype) after partial carotid ligation can be suppressed by local delivery of miR-23b. These findings validate the significance of the “miR-23b → CAK/Pol II → cell proliferation” pathway in regulating vascular functionsin vivo.
In summary, we used in vitrocell perfusion systems and anin vivo partial-carotid-ligation animal model to demonstrate the functional role of miR-23b in effecting anti-proliferative actions by modulation of cell cycle regulatory proteins and inhibition of basal transcription. Our findings also implicate the flow-sensitive miR-23b as a potential therapeutic target for maintaining ECs homeostasis.
Supplementary Material
Significance.
MiR-23b is a mechano-sensitive miRNA that mediates the quiescent phenotype of endothelial cells in response to pulsatile shear stress. The current study demonstrates that the induction of miR-23b by pulsatile shear stress is mediated by the athero-protective transcription factor KLF2 and that miR-23b represses CDK-activating kinase and downstream functional targets to suppress cell cycle progression and basal transcription. These findings elucidated the molecular mechanisms by which pulsatile shear stress exerts anti-proliferative effect on endothelial cells. The in vitro results were validated by the in vivo findings that local hemodynamics in rat carotid artery modulate the levels of KLF2, miR-23b, and endothelial proliferation via the same mechanism. This study demonstrates a novel role of miR-23b in regulating the athero-protective phenotypes of endothelial cells.
Acknowledgments
The authors would like to thank Dr. Clark Z. Wu (Department of Radiology, University of California, San Diego) for the assistance of ultrasound imaging.
Sources of Funding
This work was supported in part by National Institutes of Health Research Grants HL106579 (to S.C., S.S., and John Shyy), HL108735 (to S.C., S. S., and John Shyy), HL121365 (to S.C. and Y.W.) and Taiwan National Science Council TMS-094-1A-004 (fellowship to K.W.).
Abbreviations
- EC
endothelial cell
- PS
pulsatile shear flow
- OS
oscillatory shear flow
- KLF2
Krüppel-like Factor 2
- Pri-miR-23b
primary transcript of miR-23b
- AM23b
synthetic Anti-miR of miR-23b
- PM23b
synthetic Pre-miR of miR-23b
- BrdU
bromodeoxyuridine
- Rb
retinoblastoma protein
- CCN
cyclin
- CDK
cyclin-dependent kinase
- 3’UTR
3’ untranslated region
- miRISC
miRNA-induced silencing complex
- CAK
CDK-activating kinase
- CTD
carboxyl-terminal domain
Footnotes
Disclosures
None.
Reference
- 1.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 2.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang KC, Garmire LX, Young A, Nguyen P, Trinh A, Subramaniam S, Wang N, Shyy JY, Li YS, Chien S. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc Natl Acad Sci U S A. 2010;107:3234–3239. doi: 10.1073/pnas.0914825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, Li JY, Chien S. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107:13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boon RA, Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie. 2009;29:39–40. 41–43. [PubMed] [Google Scholar]
- 9.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 10.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 11.Young A, Wu W, Sun W, Benjamin Larman H, Wang N, Li YS, Shyy JY, Chien S, Garcia-Cardena G. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler Thromb Vasc Biol. 2009;29:1902–1908. doi: 10.1161/ATVBAHA.109.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, Li YS, Chien S and Wang N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2010;107:3240–3244. doi: 10.1073/pnas.0914882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011;300:H1762–H1769. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son DJ, Kumar S, Takabe W, Woo Kim C, Ni CW, Alberts-Grill N, Jang IH, Kim S, Kim W, Won Kang S, Baker AH, Woong Seo J, Ferrara KW, Jo H. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun. 2013;4:3000. doi: 10.1038/ncomms4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin S, Sun C, Ma M, Huang Y, Xi JJ. Genome-wide functional screening of miR-23b as a pleiotropic modulator suppressing cancer metastasis. Nat Commun. 2011;2:554. doi: 10.1038/ncomms1555. [DOI] [PubMed] [Google Scholar]
- 16.Majid S, Dar AA, Saini S, Arora S, Shahryari V, Zaman MS, Chang I, Yamamura S, Tanaka Y, Deng G, Dahiya R. miR-23b represses proto-oncogene Src kinase and functions as methylation-silenced tumor suppressor with diagnostic and prognostic significance in prostate cancer. Cancer research. 2012;72:6435–6446. doi: 10.1158/0008-5472.CAN-12-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Q, Gallagher R, Ufret-Vincenty R, Li X, Olson EN, Wang S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters. Proc Natl Acad Sci U S A. 2011;108:8287–8292. doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ham O, Song BW, Lee SY, Choi E, Cha MJ, Lee CY, Park JH, Kim IK, Chang W, Lim S, Lee CH, Kim S, Jang Y, Hwang KC. The role of microRNA-23b in the differentiation of MSC into chondrocyte by targeting protein kinase A signaling. Biomaterials. 2012;33:4500–4507. doi: 10.1016/j.biomaterials.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Yeung CLA, Tsang TY, Yau PL, Kwok TT. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene. 2011;30:2401–2410. doi: 10.1038/onc.2010.613. [DOI] [PubMed] [Google Scholar]
- 20.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Lolli G, Johnson LN. CAK-Cyclin-dependent Activating Kinase: a key kinase in cell cycle control and a target for drugs? Cell Cycle. 2005;4:572–577. [PubMed] [Google Scholar]
- 22.Hendrickson DG, Hogan DJ, Herschlag D, Ferrell JE, Brown PO. Systematic identification of mRNAs recruited to argonaute 2 by specific microRNAs and corresponding changes in transcript abundance. PLoS One. 2008;3:e2126. doi: 10.1371/journal.pone.0002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe Y, Fujimoto H, Watanabe T, Maekawa T, Masutani C, Hanaoka F, Ohkuma Y. Modulation of TFIIH-associated kinase activity by complex formation and its relationship with CTD phosphorylation of RNA polymerase II. Genes Cells. 2000;5:407–423. doi: 10.1046/j.1365-2443.2000.00336.x. [DOI] [PubMed] [Google Scholar]
- 24.Miyashiro JK, Poppa V, Berk BC. Flow-induced vascular remodeling in the rat carotid artery diminishes with age. Circ Res. 1997;81:311–319. doi: 10.1161/01.res.81.3.311. [DOI] [PubMed] [Google Scholar]
- 25.Ishteiwy RA, Ward TM, Dykxhoorn DM, Burnstein KL. The microRNA-23b/-27b cluster suppresses the metastatic phenotype of castration-resistant prostate cancer cells. PLoS One. 2012;7:e52106. doi: 10.1371/journal.pone.0052106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr, Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JY. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paternot S, Bockstaele L, Bisteau X, Kooken H, Coulonval K, Roger PP. Rb inactivation in cell cycle and cancer: the puzzle of highly regulated activating phosphorylation of CDK4 versus constitutively active CDK-activating kinase. Cell Cycle. 2010;9:689–699. doi: 10.4161/cc.9.4.10611. [DOI] [PubMed] [Google Scholar]
- 28.Moore JP, Weber M, Searles CD. Laminar shear stress modulates phosphorylation and localization of RNA polymerase II on the endothelial nitric oxide synthase gene. Arterioscler Thromb Vasc Biol. 2010;30:561–567. doi: 10.1161/ATVBAHA.109.199554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Jr, Gimbrone MA., Jr Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci U S A. 1986;83:2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin K, Hsu PP, Chen BP, Yuan S, Usami S, Shyy JY, Li YS, Chien S. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci U S A. 2000;97:9385–9389. doi: 10.1073/pnas.170282597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo D, Chien S, Shyy JY. Regulation of endothelial cell cycle by laminar versus oscillatory flow: distinct modes of interactions of AMP-activated protein kinase and Akt pathways. Circ Res. 2007;100:564–571. doi: 10.1161/01.RES.0000259561.23876.c5. [DOI] [PubMed] [Google Scholar]
- 32.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 33.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–H1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.