Abstract
Background
Early-life human rhinovirus (RV) infection has been linked to asthma development in high risk infants and children. Nevertheless, the role of RV infection in the initiation of asthma remains unclear.
Objective
We hypothesized that, in contrast to infection of mature BALB/c mice, neonatal infection with RV promotes an IL-25-driven type 2 response which causes persistent mucous metaplasia and airway hyperresponsiveness.
Methods
Six day-old and eight week-old BALB/c mice were inoculated with sham HeLa cell lysate or RV. Airway responses from 1 to 28 days after infection were assessed by qPCR, ELISA, histology, immunofluorescence microscopy, flow cytometry and methacholine responsiveness. Selected mice were treated with a neutralizing antibody to IL-25.
Results
Compared to mature mice, RV infection in neonatal mice increased lung IL-13 and IL-25 production whereas IFN-γ, IL-12p40 and TNF-α expression were suppressed. In addition, the population of IL-13-secreting type 2 innate lymphoid cells (ILC2s) was expanded with RV infection in neonatal but not in mature mice. ILC2 cells were the major cell type secreting IL-13 in neonates. Finally, anti-IL-25 neutralizing antibody attenuated ILC2 expansion, mucous hypersecretion and airways responsiveness.
Conclusions
These findings suggest that early-life viral infection could contribute to asthma development by provoking age-dependent, IL-25-driven type 2 immune responses.
Keywords: Asthma, IL-25, mouse, neonatal, rhinovirus, type 2 innate lymphoid cells
Introduction
The precise mechanisms of asthma development are not fully understood. Initiation of asthma is highly associated with enhanced type 2 and reduced type 1 immunological responses which are in turn influenced by allergen exposure, respiratory infection and genetic background. Early-life exposures to immune-modulating factors in infancy may be particularly important in determining the susceptibility to lifelong asthma development.
Studies have found that the immature immune system is qualitatively different from that of adult, refractory to type 1 and permissive to type 2 responses. In contrast to mature T cells, human cord blood T cells demonstrate a permissive chromatin architecture at the IL-13 proximal promoter, favoring transcription 1. Murine neonatal CD4+ T cells harbor IL-4/IL-13 regulatory elements which are epigenetically modified to favor type 2 responses 2. With antigen challenge, secondary exposure to antigen causes IL-4-dependent depletion of T helper type 1 (Th1) cells in neonatal but not adult mice 3 Secretion of the type 1 cytokine IL-12 is suppressed in neonatal dendritic cells, thereby inhibiting Th1 cell differentiation 4–6. Finally, the innate type 1 response to TLR stimulation is significantly diminished in neonatal monocytes 7–9. Thus, in early life, type 2-biased neonatal adaptive and innate immune responses could provide a favorable environment for asthma development, particularly when maintained by appropriate stimuli.
Wheezing-associated acute respiratory viral infections in infancy, particularly those caused by respiratory syncytial virus (RSV), have long been considered risk factors for asthma 10, 11. However, recent studies suggest a possible role for the common cold virus, rhinovirus (RV). Epidemiologic studies now show a strong association between early-life RV infection and the development of asthma in infants and children with a family history of asthma 12. In Finnish infants hospitalized for respiratory infection-associated wheezing, RV was associated with asthma development in contrast to RSV, which was negatively associated 13. Retrospective analysis of a birth cohort of 90,000 children showed an increased risk of early childhood asthma following bronchiolitis during RV-predominant non-winter months vs. RSV-predominant winter months 14. However, while it is possible that early-life RV infection promotes asthma by maintaining an immature type 2 immune response, wheezing associated with RV may simply be a marker of pre-existing airways disease. To examine this question, we tested the effects of RV infection in neonatal and mature BALB/c mice and found that, in contrast to adults, neonatal infection induced type 2 cytokine expression, airway hyperresponsiveness and mucous metaplasia 15. Nevertheless, the mechanisms by which RV infection may lead to chronic airways disease, including the roles of development, remain unclear.
Interleukin 25 (IL-25/IL-17E), a cytokine belonging to IL-17 family, is a potent inducer of type 2 immunity 16. Systemic injection of IL-25 induces eosinophilia, mucus hyperplasia and type 2 cytokines such as IL-4, IL-5, and IL-13 16. Overexpression of IL-25 in epithelial cells induces goblet cell hyperplasia and a type 2 immune response 17, and intranasal IL-25 administration provokes airways hyperresponsiveness 18. Moreover, blocking of IL-25 reduces airways responsiveness in a mouse model of allergic asthma 19. Recent studies have found that, upon IL-25 stimulation, lineage-negative lymphoid cells expressing the IL-25 receptor IL-17RB secrete the type 2 cytokines IL-5 and IL-13, promoting a type 2 immune response 20–24. These cells, originally termed natural helper cells, nuocytes, innate helper cells or type 2 multipotent progenitor cells, are now referred to as type 2 innate lymphoid cells (ILC2s) 25. On this basis, we hypothesized that early-life infection with RV promotes an IL-25-driven ILC2-mediated type 2 response, leading to persistent mucous metaplasia and airway hyperresponsiveness.
Methods
Additional experimental procedures on the measurement of airways responsiveness, histology, immunohistochemistry, mRNA and protein quantification, and flow cytometric analysis and sorting are described in Online Repository Materials (see Methods in this article’s Online Repository at www.jacionline.org).
Generation of RV
RV1B (ATCC, Manassas, VA) were grown in HeLa cells, concentrated and partially purified 26. Similarly concentrated and purified HeLa cell lysates were used for sham infection. Viral titer was measured by fifty percent tissue culture infectivity doses (TCID50) using the Spearman-Karber method 27 or by plaque assay 28.
RV infection
Experiments were approved by the University of Michigan Institutional Animal Care and Use Committee. BALB/c mice (Jackson Laboratories, Bar Harbor, ME) were inoculated through the intranasal route under Forane anesthesia with RV1B (1×108 PFU/ml) or sham HeLa cell lysates. To 5–24 day-old mice, 20 μl of RV1B (2×106 PFU) or an equal volume of sham was given. To 21 day-old mice, 30 μl of RV1B (3×106 PFU) or sham was given. To eight week-old mice, 50 μl of RV1B (5×106 PFU) or sham was administered.
Anti-IL-25 neutralizing antibody treatment
Six-day old neonatal mice were treated with either 100 μg of neutralizing antibody to IL-25 (clone 35B, Biolegend, San Diego, CA) or isotype control (rat, IgG1κ) intraperitoneally at days 0, 7 and 14 of infection. Mice were sacrificed 3–4 weeks after infection for analysis.
Flow cytometric analysis
Lung cells were stained with FITC-conjugated antibodies for lineage markers (CD3ε, TCRβ, B220/CD45R, Ter-119, Gr-1/Ly-6G/Ly-6C, CD11b, CD11c, F4/80 and FcεRIα, from Biolegend), anti-CD25-PerCP-Cy5.5 (Biolegend), anti-CD127-PE-Cy5 (eBioscience, San Diego, CA), anti-c-kit/CD117-APC (eBioscience), anti-sca-1-PE-Cy7 (eBioscience), anti-T1/ST2-PE (R&D Systems, Minneapolis, MN) and anti-IL-17RB (R&D Systems) conjugated with AF750. Cells were fixed, subjected to flow cytometry and analyzed on a FACSAria II (BD Biosciences, San Jose, CA). For analysis of intracellular IL-13, fresh aliquots of lung mince were stimulated for 5 h with cell stimulation cocktail (40.5 μM phorbol 12-myristate 13-acetate, 670 μM ionomycin, 5.3 mM brefeldin A, 1 mM monensin, eBioscience), fixed, permeabilized and incubated with anti-mouse IL-13 clone eBio13A (eBioscience).
Fluorescence-activated cell sorting of ILC2s
Lineage-negative CD25 and CD127 double-positive ILC2s or lineage-negative CD25 and CD127 double-negative cells were sorted at 9000 cells/200 μl into 96 well plates and stimulated with media, IL-25 (20 ng/ml), IL-2 (50 ng/ml) + IL-25 (20 ng/ml) or PMA + ionomycin for 3 days. To visualize ILC2s, cells were stained with Diff-Quick (Dade Behring, Newark, DE).
Data analysis
Data are represented as mean±standard error. Statistical significance was assessed by unpaired t-test, one-way or two-way analysis of variance (ANOVA), as appropriate. Group differences were pinpointed by Newman-Keuls multiple comparison test.
Results
Compared to adult mice, RV infection of neonatal mice provokes an enhanced type 2 immune response and attenuated type 1 response
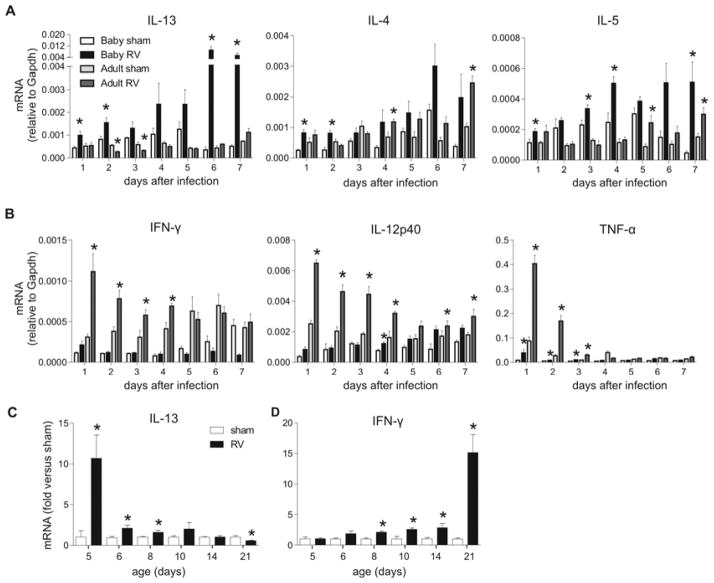
We infected 6 day-old and 8 week-old mice with RV1B and analyzed cytokine gene expression. Unlike mature mice, RV infection of neonatal mice increased expression of the type 2 cytokines IL-13, IL-4 and IL-5 immediately post-infection, with slightly different kinetics (Fig 1, A). In contrast, induction of type 1 cytokines IFN-γ and IL-12p40 gene was blunted in neonatal mice, whereas expression was increased in mature mice (Fig 1, B). Compared to sham infection, TNF-α gene expression was significantly increased in both neonates and adults after RV infection, but induction was significantly less in neonatal mice. Consistent with the attenuated type 1 response, viral replication and load tended to be greater 3–7 days after inoculation in neonatal mice compared to adults (see Fig E1 in the Online Repository). We performed additional studies examining the age-dependency of RV-induced cytokine responses. RV-induced IL-13 expression was increased, and IFN-γ expression decreased up to 8 days of age (Fig 1, C). These results show that early-life RV infection elicits exaggerated type 2 responses and mitigated type 1 responses.
FIG 1.
Cytokine expression after RV infection. A and B, Six-day-old and eight-week-old mice were inoculated with sham or RV (n=4–8 sham, n=5–14 RV) and lung mRNA measured 1–7 days later. *P<0.05 compared to sham (unpaired t-test). C and D, Mice of different ages (n=3–10/group) were inoculated with sham or RV and mRNA expression measured one day later. *P<0.05 versus sham (unpaired t-test).
Infection of neonatal mice with RV provokes long-term mucus metaplasia and airways hyperresponsiveness
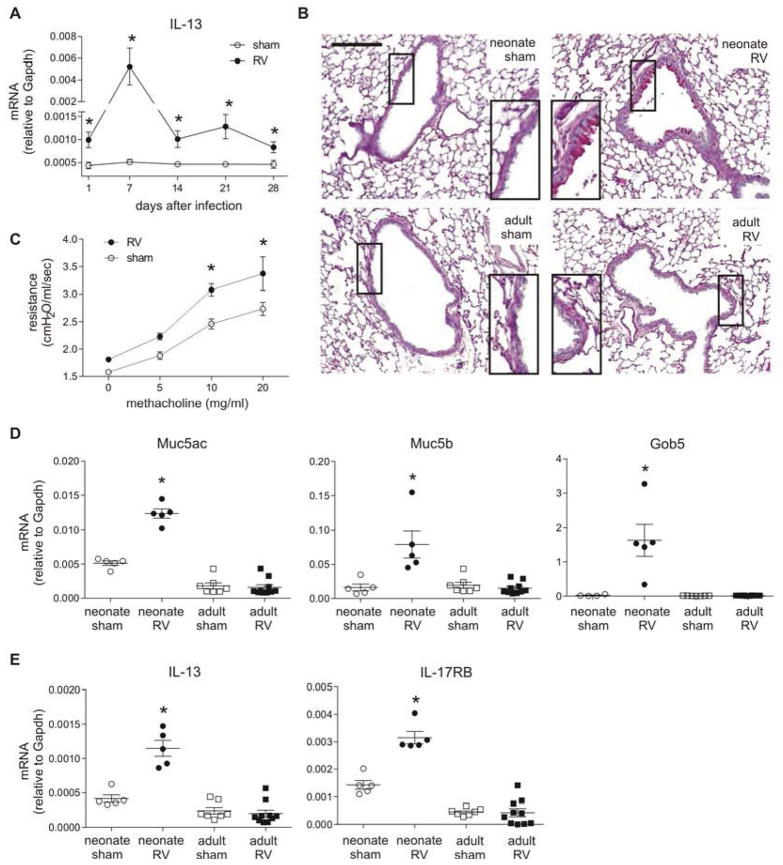
Neonatal RV infection led to persistent asthma-like pathophysiological changes including IL-13 expression, mucous metaplasia and airways hyperresponsiveness (Fig 2, A–C). The mucus-related genes Muc5ac, Muc5b and Gob5 were increased with neonatal but not adult RV infection (Fig 2, D). Induction of Muc5ac and Gob5 was maintained after 8 weeks of infection (Fig E2). We also found that IL-17RB gene expression was increased (Fig 2, E), suggesting a possible role for IL-25 in promoting the type 2 immune response.
FIG 2.
Mucous metaplasia and airway hyperresponsiveness after neonatal RV infection. A, Lung IL-13 from six day-old mice. *P<0.05 versus sham (unpaired t-test). B, PAS-stained lung sections prepared 3 weeks after inoculation of six day-old and eight week-old mice (magnification, 100X; bar. 200 μm). C, Airway responsiveness four weeks after inoculation of neonatal mice (n=4/group). * P<0.05 versus sham (two-way ANOVA). D and E, Lung mRNA expression three weeks after inoculation. *P<0.05 versus sham (unpaired t-test).
Epithelial IL-25 is increased with neonatal RV infection
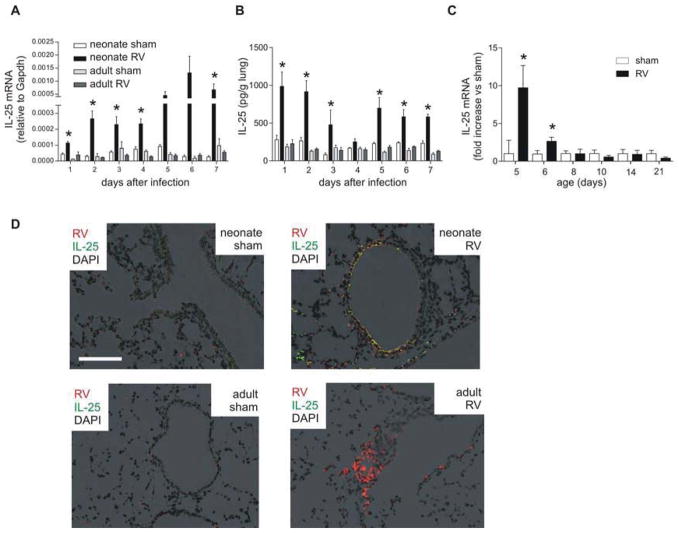
We next asked whether IL-25 expression is increased following neonatal RV infection and differentially regulated with age. Similar to the induction of type 2 cytokines, IL-25 mRNA expression was increased in RV-infected neonatal mice (6-day-old) but not mature mice (8–10 weeks old) (Fig 3, A). IL-25 protein production was induced with RV infection in neonates but not adults (Fig 3, B). Expression of IL-33 did not change with infection (Fig E3). Additional studies examining the age-dependency of RV-induced IL-25 expression showed significant induction only in mice younger than 6 days-old (Fig 3, C). UV-irradiated, replication-deficient virus did not generate a response (data not shown). We also asked whether viral dosage affects the neonatal type 2 cytokine response. Low-dose RV significantly increased IL-25 but not IFN-γmRNA 24 h after infection (Fig E3). These results show that RV-induced type 2 cytokine production in neonates is not dose-dependent. Lung immunofluorescent staining showed that RV-infected epithelial cells were the major source of IL-25 (Fig 3, D). Lungs of RV-infected mature mice showed minimal IL-25.
FIG 3.
Lung IL-25 after RV infection. A, Six-day-old and eight-week-old mice were inoculated with sham or RV (n=4–7/group) and mRNA measured 1–7 days after infection. *P<0.05 versus sham (unpaired t-test). B, IL-25 protein. *P≤0.05 versus sham (one-way ANOVA). C, Mice were inoculated at different ages (n = 3–10/group) and mRNA measured one day after treatment. *P<0.05 versus sham (unpaired t-test). D, Two days after infection, lungs were stained for IL-25 (green), RV (red) and nuclei (DAPI, black). (Bar, 200 μm; magnification, 200X).
RV infection of neonatal but not mature mice expands the population of IL-17RB-expressing ILC2s
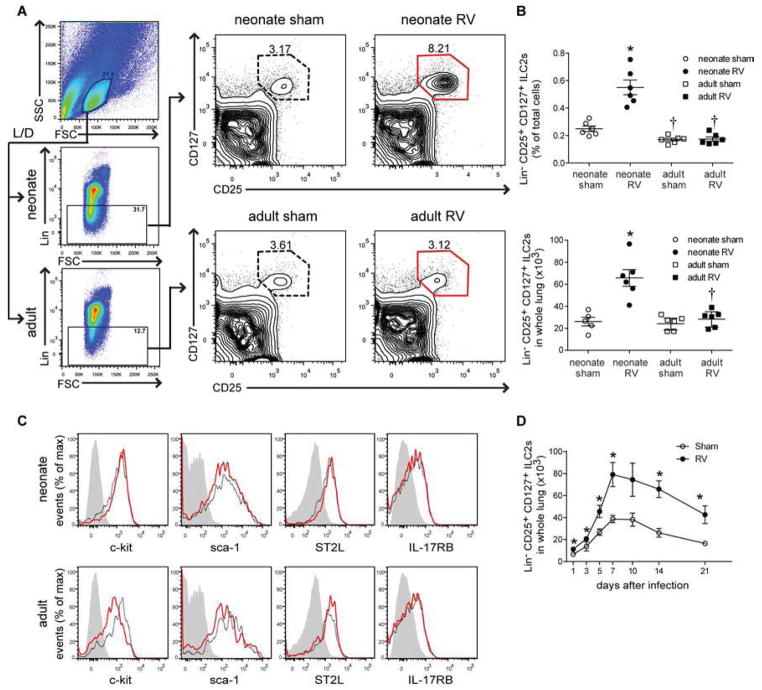
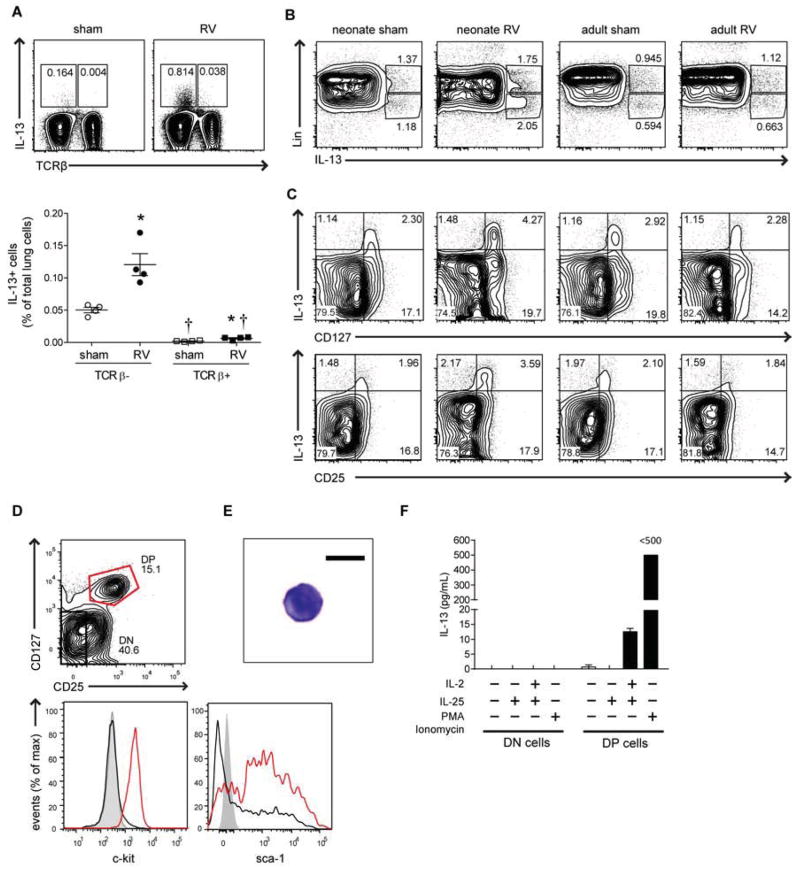
We collected lungs of neonates and adults two weeks after infection and analyzed ILC2s using flow cytometry. We gated on small cells, live cells and lineage-negative cells using mixture of hematopoietic lineage markers (CD3ε, TCRβ, B220, Ter-119, Gr-1, CD11b, CD11c, F4/80, FcεRIα) (Fig 4, A). Compared to adults, neonatal mice had nearly 2-fold more lineage-negative cells (Fig E5). After gating on the lineage-negative population, a discrete population of CD25 and CD127 double-positive ILC2s was found (Fig 4, A and B). The basal level of Lin-negative, CD25, CD127 double-positive cells in adult mice was comparable to the previous findings in C57BL/6 mice 29. Following RV infection, ILC2s were increased in neonates but not in adults, both in percentage and number. Further analysis showed that Lin-negative, CD25, CD127 double-positive cells express c-kit (CD117), sca-1, IL-17RB, and ST2L (Fig 4, C), suggesting that they are more closely related to lung natural helper cells than nuocytes, innate helper cells or type 2 multipotent progenitor cells, which are heterogeneous in c-kit or CD127 expression 21–24. ILC2s were increased as early as one day after infection and maintained three weeks after infection (Fig 4, D). Taken together, these results suggest that, following neonatal RV infection, the population of lung ILC2s is expanded in neonates, possibly through the induction of IL-25.
FIG 4.
Lung lineage-, CD25+, CD127+ ILC2s. A, Six day-old and eight week-old mice were inoculated with sham or RV and live ILC2s identified fourteen days later. B, Percentage (upper panel) and total (lower panel) ILC2s for each group. *P<0.05 versus sham, †P<0.05 versus mature mice (unpaired t-test). C, C-kit/CD117, Sca-1, T1/ST2 and IL-17RB expression in ILC2s from sham- (black, dotted) and RV-treated mice (red, solid). (Isotype control is grey, filled). D, ILC2 time course after neonatal infection (n = 3–6/group). *P<0.05 versus sham (unpaired t-test).
ILC2s are a major IL-13-producing cell in RV-infected neonatal mice
We asked whether Lin-negative CD25, CD127 double-positive ILC2 cells produce IL-13 after RV infection. First, we analyzed the role of T cells utilizing flow cytometric analysis. Lung samples were collected two weeks after infection and stimulated with phorbol 12-myristate 13-acetate, ionomycin, brefeldin A and monensin. The main population of IL-13-producing cells was TCRβ-negative (Fig 5, A). After RV stimulation, the number of IL-13-secreting TCRβ-negative cells was approximately 18-fold higher than the number of IL-13-secreting TCRβ-positive cells. To determine if ILC2s produced IL-13, lineage-positive and -negative cell populations were stimulated as above. The major IL-13-secreting cells were lineage negative (Fig 5, B). Among lineage-negative cells, CD25, CD127 double-positive cells contained a high IL-13-producing population (Fig 5, C) suggesting that ILC2s are a major source of IL-13 in the lungs of RV-infected neonatal mice. To further examine the capacity of these cells to produce IL-13, we sorted Lin-negative CD25, CD127 double-positive cells and CD25, CD127 double-negative cells (see Fig E6 for gating strategy). Double positive cells expressed c-kit and sca-1, whereas double-negative cells were negative for both markers (Fig 5, D). Similar to other ILC2s, double-positive cells were small cells with circular nuclei and scanty cytoplasm (Fig 5, E) 20, 30. Stimulation with IL-25 and IL-2 or PMA and ionomycin induced large amounts of IL-13 (Fig 5, F). These results show that an expanded population of ILC2s is the major source of IL-13 in RV-infected neonatal mice and likely contribute to the observed asthma-like phenotype.
FIG 5.
IL-13 producing cells. A, Percentages of IL-13+, TCRβ- and TCRβ+ cells two weeks after neonatal sham or RV. *P<0.05 versus sham, †P<0.05 versus TCRβ- cells (unpaired t-test). B and C, Percentages of lineage+ and lineage- IL-13+ cells (B). Percentages of lineage- IL-13+, CD127+ and CD25+ cells (C). D–F, Eight days after infection, Lin- CD25+ CD127+ double-positive (DP) and CD25- CD127- double-negative (DN) ILC2s were characterized for c-kit and Sca-1 (D). Image of ILC2 (E). IL-13 production by stimulated DP and DN cells (F).
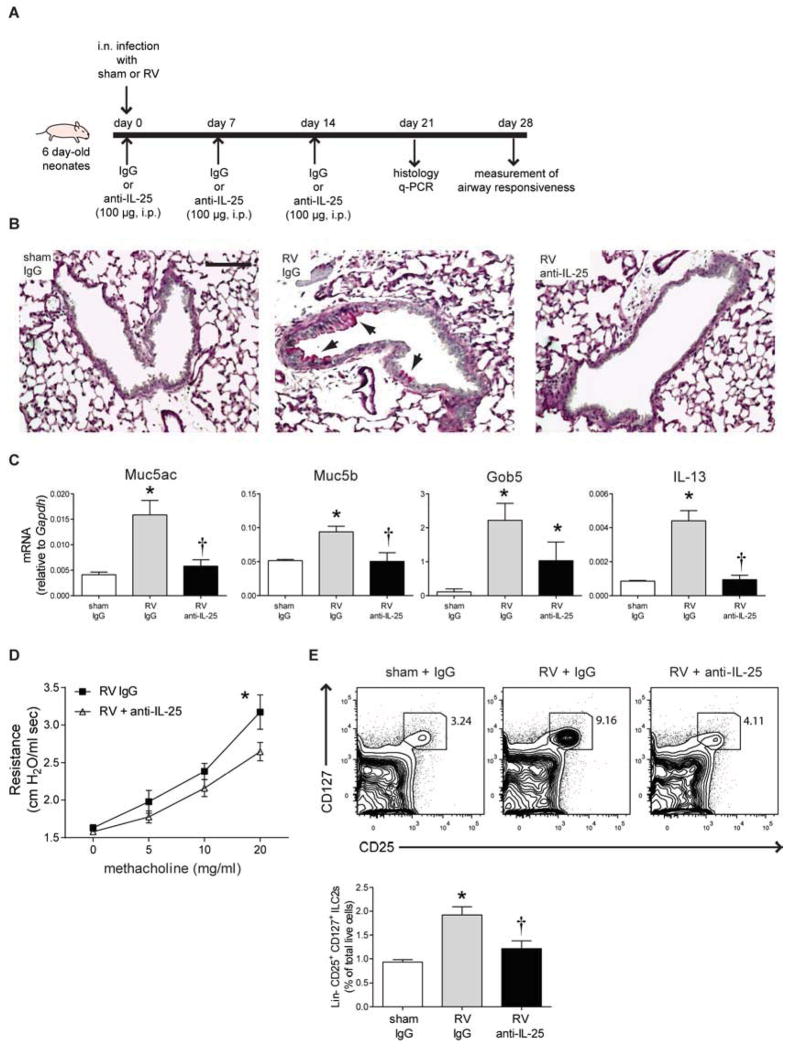
IL-25 mediates the development of mucus metaplasia and airways hyperresponsiveness in RV-infected neonatal mice
To test whether IL-25 is required for the development of an asthma-like phenotype, we treated RV-infected neonatal mice with a neutralizing antibody against IL-25 (Fig 6, A). Treatment with anti-IL-25 reduced mucus metaplasia (Fig 6, B). The mucus-related genes Muc5ac, Muc5b, Gob5 decreased with anti-IL-25 treatment (Fig 6, C). Anti-IL-25 blocked the development of airways hyperresponsiveness (Fig 6, D). Finally, anti-IL-25 decreased the expansion of ILC2s in RV-infected neonates (Fig 6, E), suggesting that ILC2 expansion is dependent on IL-25. These results show that IL-25 plays a key role in the development of mucous metaplasia and airways hyperresponsiveness in RV-infected neonatal mice, at least in part by increasing the number of ILC2s.
FIG 6.
Effect of IL-25 neutralization on RV-infected neonatal mice. A, Protocol for anti-IL-25 treatment. B, Three weeks after inoculation, lungs were harvested and stained with PAS. Bar, 100 μm. C, Lung mRNA expression (n = 4–10/group). *P<0.05 versus sham, †P<0.05 versus RV+IgG (unpaired t-test). D, Airway resistance four weeks after RV infection and antibody treatment (n = 4–5 in each group). *P<0.05 versus RV+IgG (two-way ANOVA). E, Lineage-negative CD25+ CD127+ ILC2s four weeks after infection (top). Group percentages of live ILC2s (n = 3–8/group, bottom). *P<0.05 versus sham, †P<0.05 versus RV+IgG (unpaired t-test).
Discussion
In this study, we showed that infection of mice with RV induces an age-dependent type 2 immune response in the airways. Neonatal RV infection, but not adult infection, increased expression of IL-13 and IL-25. In contrast, induction of the type 1 cytokines IFN-γ, IL-12 p40 and TNF-α was diminished in neonates compared to adults. The increase in IL-25 production in neonatal mice was associated with long-term expansion of IL-25-responsive ILC2s in the lungs. Further, ILC2s were a significant source of IL-13 after RV infection. Finally, RV-induced mucous cell metaplasia and airways hyperresponsiveness were attenuated by anti-IL-25. Together, these studies indicate that RV induces an age-dependent asthma-like phenotype which is driven by IL-25 and ILC2s. These studies provide a mechanism by which viral infection in early-life could lead to persistent type 2 immune responses and asthma development.
The immature immune system is qualitatively different from that of adult, refractory to type 1 and permissive to type 2 responses 1–9. In our experiments, RV-induced IL-25 was regulated in an age-dependent manner and required for the development of mucous metaplasia and airways hyperresponsiveness. IL-25 appeared to be produced by RV-infected epithelial cells, though uninfected cells, including submucosal cells, may also have been involved. To our knowledge, this is the first report showing a developmental difference in the IL-25 response. Considering the epigenetic modification favoring type 2 cytokine induction in T cells 2, it is possible that the regulatory region of IL-25 is also epigenetically favored transcription in neonates compared to adults. Alternatively, blunted induction of type 1 cytokine IFN-γ in RV-infected neonates could be permissive for IL-25 induction. In NK cell-deficient mice, RSV infection leads to an exaggerated IL-25 response which is blocked by recombinant IFN-γ treatment, consistent with the notion that IFN-γ blocks IL-25 expression 31. Finally, it is possible that neonates experienced a greater total IL-25 response based on a higher viral load 3–7 days after inoculation. However, treatment of neonatal mice with low-dose RV also induced lung IL- 25 expression, and NK cell-deficient mice with exaggerated IL-25 production and attenuated IFN-γ responses have similar viral loads as wild-type mice 31, suggesting the primacy of IFN regulation.
The cytokine IL-33 has been associated with development of lung ILC2s and type 2 cytokine responses in mice 32. However, IL-33 was not increased with RV infection. Thymic stromal lymphopoietin (TSLP) has also been shown to expand skin ILC2s in mice 33. RV16 infection increases TSLP expression in human airway epithelial cells 34. It is therefore conceivable that TSLP plays a role in RV-induced ILC2 expansion.
We have previously shown that this IL-13 induction is required for the development of RV-induced mucous metaplasia and airways hyperresponsiveness in neonatal mice 15. Persistent induction of IL-13-driven changes in airway inflammation and function following viral infection were first reported in Sendai-infected C57BL/6J mature mice 35. Subsequently, persistent IL-13 production has been noted following neonatal infection by RSV, pneumonia virus of mice (PVM) and influenza exposure 36–38. In the case of mature Sendai-infected mice, IL-13 was secreted by a combination of M2-polarized macrophages and invariant NKT cells 35. In the present study, the major cells persistently secreting IL-13 were lineage-negative, CD25, CD127 double-positive ILC2s. These cells expressed c-kit, Sca-1, ST2, and IL-17RB, closely resembling lung natural helper cells and dissimilar from nuocytes, innate helper cells or type 2 multipotent progenitor cells 21–24.. Induction of ILC2s with viral infection has previously been shown following in mature mice with influenza virus 29,39. Finally, an acute increase in ILC2s was recently shown in neonatal TLR7 null mice with a severe PVM infection 40. However, IL-13 production by ILC2s was not assessed, and virus-induced airway inflammation and airway hyperreactivity was dependent on memory CD4+ T cells. Our experiments extend previous reports in the following respects. First, we found for the first time that ILC2s are expanded following infection with RV, perhaps the most common infection of humans. Second, we established that ILC2s make IL-13 in response to viral infection in neonatal animals. Third, we found a developmental difference in the ILC2 response between neonates and adults (see above). This difference was based on RV-induced production of ILC2-activating cytokines such as IL-25, rather than the infection itself. Fourth, following neonatal RV infection, IL-13 secretion and ILC2 expansion were long-lived, at least 21 days after infection. It is therefore conceivable that ILC2s could produce type 2 cytokines for extended periods, perhaps in response to subsequent infection or allergen exposure. Finally, we showed for the first time that, in addition to IL-33, IL-25 is required for ILC2 expansion following viral infection.
Our finding that RV infection elicits a significant ILC2 response in immature but not mature mice, one which is based on the age-dependent expression of IL-25 and other cytokines, suggests that it is the stage of development, rather than the specific virus, that drives establishment of the asthma-like phenotype. Accordingly, it is possible that other early-life viral infections also induce mucous metaplasia and airways responsiveness through the early expansion of the ILC2 cells. As noted above, neonatal infection by RSV, PVM and influenza have been shown to induce a persistent asthma-like phenotype in mice 36–38. IL-25 production has been noted following neonatal infection with RSV 37. Recently, neonatal PVM infection of TLR7−/− mice showed an IFN-low, IL-25-high cytokine response similar to that we observed, which was associated with recruitment of ILC2 cells 40. In humans, early-life infection with RV 13, 14, 41 and RSV 10, 11 have each been associated with asthma development.
The association between respiratory viral infection and asthma is likely to be complex, with asthma development requiring the repeated infections, appropriate genetic background and allergen exposure 42–46. For example, variants of the ORMDL3/GSDMB locus on chromosome 17q21 have recently been associated with both childhood-onset asthma 47 and RV wheezing illnesses in early life 48. In addition, studies have shown additive or synergistic effects of allergen exposure and neonatal RSV 36, PVM 37, 40, influenza 38 and RV infection 15. Further studies are needed to characterize the role of innate immune cells, including the ILC2, in combined responses. For example, it is conceivable that IL-13-producing ILC2s, perhaps along with FcεRI-expressing dendritic cells,49, 50 promote non-specific IgE production, leading to atopy development.
We would like to add a few caveats about our mouse model of RV infection. We 49 and others 50 have found that a much higher viral titer is required to infect mice compared with humans. This finding is to be expected, because differences in the homology of viral receptors and intracellular signaling mechanisms are likely to restrict viral infection and replication in mice. Nevertheless, we have clearly shown that human RV1B replicates in mouse lungs, as evidenced by: 1) the presence of negative-strand viral RNA in the lungs of inoculated mice, 2) transmissibility of RV infection from the lung homogenates of inoculated mice to cultured HeLa cells; and 3) the induction of a robust lung interferon response 49. RV replication appears to be augmented in neonatal mice. Nevertheless, as in humans, RV infection was not associated with neonatal mortality. In fact, viral titers were relatively low, suggesting that even mild respiratory viral infections may elicit an ILC2 response. These data are consistent with recent speculation by workers in the field that relatively innocuous RV infections in early childhood lead to sustained changes in the immune response which contribute to the development of asthma.
We conclude that early-life rhinoviral infection could contribute to asthma development by provoking age-dependent, IL-25-driven type 2 immune response. IL-25 induction was regulated in an age-dependent manner and required for ILC2 expansion and the development of asthma phenotype in neonates. Further characterization of this immune pathway may lead to new molecular and cellular targets for the prevention of asthma.
Supplementary Material
Key Messages.
Infection of neonatal but not adult mice with human rhinovirus (RV) induces mucous metaplasia and airways hyperresponsiveness which is associated with enhanced expression of IL-13 and IL-25, reduced expression of IFN-g, IL-12p40 and TNF-α, and expansion of type 2 innate lymphoid cells (ILC2s).
Neutralization of IL-25 attenuates RV-induced ILC2 expansion, mucous metaplasia and airways responsiveness.
Neonatal mice are susceptible to rhinovirus-induced airways disease mediated by IL-25 and ILC2s
Acknowledgments
This work was supported by NIH HL081420 (M.B.H.)
We thank Drs. Nicholas Lukacs, Carey Lumeng, Bethany Moore and Cheong-Hee Chang (all University of Michigan Medical School) for their constructive critiques of this research.
Abbreviations
- ILC2
type 2 innate lymphoid cells
- PVM
pneumonia virus of mouse
- RSV
respiratory syncytial virus
- RV
rhinovirus
- Th1
T helper type 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Webster RB, Rodriguez Y, Klimecki WT, Vercelli D. The human IL-13 locus in neonatal CD4+ T cells is refractory to the acquisition of a repressive chromatin architecture. J Biol Chem. 2007;282:700–9. doi: 10.1074/jbc.M609501200. [DOI] [PubMed] [Google Scholar]
- 2.Rose S, Lichtenheld M, Foote MR, Adkins B. Murine neonatal CD4+ cells are poised for rapid Th2 effector-like function. J Immunol. 2007;178:2667–78. doi: 10.4049/jimmunol.178.5.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Lee H-H, Bell JJ, Gregg RK, Ellis JS, Gessner A, et al. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–40. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 4.Lee H-H, Hoeman CM, Hardaway JC, Guloglu FB, Ellis JS, Jain R, et al. Delayed maturation of an IL-12–producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med. 2008;205:2269–80. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128:118–23. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goriely S, Van Lint C, Dadkhah R, Libin M, De Wit D, Demonté D, et al. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004;199:1011–6. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chelvarajan RL, Collins SM, Doubinskaia IE, Goes S, Van Willigen J, Flanagan D, et al. Defective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigens. J Leuk Biol. 2004;75:982–94. doi: 10.1189/jlb.0403179. [DOI] [PubMed] [Google Scholar]
- 8.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective Impairment of TLR-Mediated Innate Immunity in Human Newborns: Neonatal blood plasma reduces monocyte TNF-α induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–34. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 9.Sadeghi K, Berger A, Langgartner M, Prusa A-R, Hayde M, Herkner K, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 10.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 11.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–7. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 12.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotaniemi-Syrjänen A, Vainionpää R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, et al. Season of infant bronchiolitis and estimates of subsequent risk and burden of early childhood asthma. J Allergy Clin Immunol. 2009;123:964–6. doi: 10.1016/j.jaci.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider D, Hong JY, Popova AP, Bowman ER, Linn MJ, McLean AM, et al. Neonatal rhinovirus infection induces persistent mucous metaplasia and airways hyperresponsiveness. J Immunol. 2012;188:2894–904. doi: 10.4049/jimmunol.1101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 17.Angkasekwinai P, Park H, Wang Y-H, Wang Y-H, Chang SH, Corry DB, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–17. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rickel EA, Siegel LA, Yoon B-RP, Rottman JB, Kugler DG, Swart DA, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 19.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–31. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 20.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 21.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halim Timotheus YF, Krauß Ramona H, Sun Ann C, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes Th2 cytokine responses. Nature. 2010;464:1362–6. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price AE, Liang H-E, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13–expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells -- a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 26.Newcomb DC, Sajjan U, Nanua S, Jia Y, Goldsmith AM, Bentley JK, et al. Phosphatidylinositol 3-kinase is required for rhinovirus-induced airway epithelial cell interleukin-8 expression. J Biol Chem. 2005;280:36952–61. doi: 10.1074/jbc.M502449200. [DOI] [PubMed] [Google Scholar]
- 27.Johnston SL, Tyrrell DAJ. Rhinoviruses. In: Lennette EH, Schmidt NJ, editors. Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections. Washington D.C: American Public Health Association; 1997. pp. 553–63. [Google Scholar]
- 28.Martin S, Casasnovas JM, Staunton DE, Springer TA. Efficient neutralization and disruption of rhinovirus by chimeric ICAM-1/immunoglobulin molecules. J Virol. 1993;67:3561–8. doi: 10.1128/jvi.67.6.3561-3568.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brickshawana A, Shapiro VS, Kita H, Pease LR. Lineage-Sca1+c-Kit-CD25+ cells are IL-33–responsive Type 2 innate cells in the mouse bone marrow. J Immunol. 2011;187:5795–804. doi: 10.4049/jimmunol.1102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaiko GE, Phipps S, Angkasekwinai P, Dong C, Foster PS. NK cell deficiency predisposes to viral-induced Th2-type allergic inflammation via epithelial-derived IL-25. J Immunol. 2010;185:4681–90. doi: 10.4049/jimmunol.1001758. [DOI] [PubMed] [Google Scholar]
- 32.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33–responsive lineage-CD25+CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33–independent innate lymphoid cell responses to promote skin inflammation. Science Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato A, Favoreto S, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–7. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–40. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.You D, Becnel D, Wang K, Ripple M, Daly M, Cormier S. Exposure of neonates to respiratory syncytial virus is critical in determining subsequent airway response in adults. Respir Res. 2006;7:107. doi: 10.1186/1465-9921-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegle J, Hansbro N, Herbert C, Rosenberg H, Domachowske J, Asquith K, et al. Early-life viral infection and allergen exposure interact to induce an asthmatic phenotype in mice. Respir Res. 2010;11:14. doi: 10.1186/1465-9921-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Garawi A, Fattouh R, Botelho F, Walker TD, Goncharova S, Moore CL, et al. Influenza A facilitates sensitization to house dust mite in infant mice leading to an asthma phenotype in adulthood. Mucosal Immunol. 2011;4:682–94. doi: 10.1038/mi.2011.35. [DOI] [PubMed] [Google Scholar]
- 39.Chang Y-J, Kim HY, Albacker LA, Baumgarth N, McKenzie ANJ, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–8. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaiko GE, Loh Z, Spann K, Lynch JP, Lalwani A, Zheng Z, et al. Toll-like receptor 7 gene deficiency and early-life Pneumovirus infection interact to predispose toward the development of asthma-like pathology in mice. J Allergy Clin Immunol. 2013;131:1331–9. e10. doi: 10.1016/j.jaci.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 41.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee W-M, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–5. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kusel MMH, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janssen R, Bont L, Siezen CLE, Hodemaekers HM, Ermers MJ, Doornbos G, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis. 2007;196:826–34. doi: 10.1086/520886. [DOI] [PubMed] [Google Scholar]
- 44.Stensballe LG, Brunbjerg Simonsen J, Thomsen SF, Hellesøe Larsen A-M, Hovmand Lysdal S, Aaby P, et al. The causal direction in the association between respiratory syncytial virus hospitalization and asthma. J Allergy Clin Immunol. 2009;123:131–7. e1. doi: 10.1016/j.jaci.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 45.Miller EK, Williams JV, Gebretsadik T, Carroll KN, Dupont WD, Mohamed YA, et al. Host and viral factors associated with severity of human rhinovirus–associated infant respiratory tract illness. J Allergy Clin Immunol. 2011;127:883–91. doi: 10.1016/j.jaci.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bacharier LB, Cohen R, Schweiger T, Yin-DeClue H, Christie C, Zheng J, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2012;130:91–100. e3. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Çalişkan M, Bochkov YA, Kreiner-Møller E, Bønnelykke K, Stein MM, Du G, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nat Med. 2012;18:726–35. doi: 10.1038/nm.2768. [DOI] [PubMed] [Google Scholar]
- 50.Potaczek DP, Michel S, Sharma V, Zeilinger S, Vogelberg C, von Berg A, et al. Different FCER1A polymorphisms influence IgE levels in asthmatics and non-asthmatics. Pediatric Allergy and Immunology. 2013;24:441–9. doi: 10.1111/pai.12083. [DOI] [PubMed] [Google Scholar]
- 51.Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, et al. Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med. 2008;177:1111–21. doi: 10.1164/rccm.200708-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, Zhu J, et al. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.