Abstract
Background
Aberrant methylation of the EYA4 gene (mEYA4) highly discriminates ulcerative colitis (UC) cases with colorectal neoplasia from UC controls in both tissue and stool. It is not known if mEYA4 is also present in nonadjacent non-neoplastic mucosa (NNM) of UC patients with colorectal neoplasia.
Methods
Formalin-fixed tissues from 25 UC cases with colorectal cancer (CRC) and 25 UC controls with neither CRC nor dysplasia were matched on gender, age, disease duration, disease extent, and coexistence of primary sclerosing cholangitis. DNA was extracted from sections of CRC and NNM from cases and UC control mucosae. Bisulfite-treated DNA was amplified using real-time methylation-specific PCR. The Wilcoxon rank-sum test assessed differences in mEYA4 levels from CRC, NNM, and control samples. Logistic regression was used to estimate sensitivity and specificity.
Results
Sufficient DNA was available for 20 cases and 20 controls. The median mEYA4 level (with interquartile range) was 2 (0–5.7) in control mucosae but 93 (38.5–306) in CRC (P < 0.0001) and 27.4 (3–140) in NNM (P = 0.0009). At 95% specificity, mEYA4 was present in 80% of CRC and 50% of NNM tissue samples. The odds ratio of mEYA4 in NNM as an indicator of synchronous CRC was 19 (95% confidence interval, 2–170).
Conclusions
The authors demonstrate significantly higher mEYA4 levels in NNM and synchronous CRC from UC cases than in colorectal mucosae of UC controls without neoplasia. The finding of this CRC-associated field change has important implications to the use of mEYA4 as a potential UC surveillance marker in tissue or stool.
Keywords: inflammatory bowel disease/complications, neoplasms/genetics, field cancerization defect, biological markers, DNA methylation
Patients with inflammatory bowel disease, specifically chronic ulcerative colitis (UC) and Crohn’s colitis, are at increased risk of colorectal cancer (CRC) and dysplasia.1,2 Chronic inflammation seems to promote UC-associated CRC (UC-CRC),3,4 and cancer risk increases with extent and duration of disease, concomitant primary sclerosing cholangitis, and presence of pseudopolyposis.5 UC-CRC arises from both endoscopically visible polypoid and flat dysplastic precursors.6 Low-grade flat dysplasia7 and high-grade polypoid dysplasia8 can predict synchronous UC-CRC. UC-CRC is also more likely than sporadic CRC to be synchronous at presentation.9
Across multiple diseases, investigators have sought to understand the etiological molecular mechanisms for multicentric neoplasms to develop within regions of common carcinogen exposure, including chronic inflammation. Originated by Slaughter et al,10 the field cancerization defect (or field effect) hypothesis has important implications in the UC-CRC disease model. An early report of abnormal p53 staining in cancerous, dysplastic, and histologically normal mucosa from colectomy specimens suggested clonal expansion of an early founder mutation that confers a growth advantage.11 Local and segmental field cancerization has been confirmed by mapping p53 and KRAS mutations in non-dysplastic crypts adjacent to UC-CRCs.12 Field cancerization is also supported by analysis of telomere length in non-neoplastic distant colon segments of UC patients with cancer or high-grade dysplasia.13
Aberrant methylation of gene promotors represents another molecular change that may be part of this field phenomenon. At least 1 of TGFB2, SLIT2, HS3ST2, or TMEFF2 was found to be methylated in 12 of 30 non-neoplastic mucosae adjacent to inflammatory bowel disease–associated neoplasms and also in inflamed but normal mucosae of 15 of 20 patients at high risk of inflammatory bowel disease–associated neoplasia.14 Methylation of some markers, such as RUNX3 and MINT1, are found in both CRC and nonadjacent non-neoplastic mucosa (NNM) of patients with UC.15 We have previously shown that aberrant methylation of the Eyes Absent 4 gene (mEYA4) highly discriminates UC cases with colorectal neoplasia from UC controls without neoplasia in both tissue16 and stool.17 It is not yet known if mEYA4 is confined to neoplastic tissue or if it is also found in NNM of UC patients with CRC.
Accordingly, we aimed to measure mEYA4 levels in tissue sections of both primary tumors and NNM from UC patients with CRC and in mucosae from UC patients without CRC or dysplasia. We further sought to estimate the value of mEYA4 determination in NNM of patients with UC for predicting synchronous CRC.
MATERIALS AND METHODS
Patients and Tissue Samples
Patients with UC for >10 years who developed CRC were identified (UC-CRC cases). For each case, pathology slides from the surgical resection were recalled to confirm the diagnosis of UC, and complete patient chart reviews were then performed. Patients who did not have UC confirmed by review of the pathology or whose duration of disease was <10 years as documented in the clinical chart were excluded. A total of 25 UC-CRC cases, representing a subset of the UC-CRC cases described previously,15 were examined in the current study. Complete patient chart reviews were performed on all potential UC controls. These were defined as patients with UC who did not develop CRC, who had >10 years of disease, who underwent either colectomy or colonoscopy with biopsy at the Mayo Clinic, and who did not have previous dysplasia. The final selection of UC controls was based on the frequency matching to UC-CRC cases for age, gender, extent, and duration of UC. A total of 25 UC controls, a subset of the UC control group described previously,15 were analyzed in the present study. Among case subjects, NNM was defined as nonadjacent non-dysplastic mucosa histologically involved by UC. For nonadjacent NNM UC-CRC cases and controls, DNA was extracted from all non-neoplastic paraffin tissue sections that showed evidence of chronic inflammation (1–6 blocks per patient). For DNA extraction from tumors, only the section with confirmed CRC was used.15
Assay Technique
DNA was extracted with the Gentra Puregene Tissue Kit (Qiagen, Germantown, MD) and quantified using Quant-iT Pico-Green (Invitrogen, Carlsbad, CA). From each sample, 100 ng DNA was bisulfite treated with EZ DNA Methylation Kit (Zymo Research, Irvine, CA). One microliter bisulfite-treated DNA was used as a template for methylation quantification with fluorescence-based real-time PCR (MSP). Primers for mEYA4 were designed to target the bisulfite-modified methylated sequences of the gene promotor (IDT, Coralville, IA). A region without cytosine–phosphate–guanine sites in the β-actin gene was also quantified with real-time PCR using primers recognizing the bisulfite-converted sequence as a reference of the adequacy of bisulfite treatment and to normalize the MSP product. The MSP primers (in 5-prime to 3-prime direction) were as folllows: β-actin, forward, TTT TTT TTG GTG TTT GTT TTT TTG ATT; β-actin, reverse, CAC CAA CCT CAT AAC CTT ATC ACA C; mEYA4, forward, CGC CAC CGA CTA CTA CGA ACT CGT; and mEYA4, reverse, ATA AAA ACG GAG TGG GTT TTT CGC G. PCRs for tissue DNA samples were performed with SYBR Green master mix (Roche, Mannheim, Germany) on Roche 480 LightCyclers. Bisulfite-treated CpGenome Universal Methylated DNA (Millipore, Billerica, MA) was used as a positive control and serially diluted to create standard curves for all plates. Each plate consisted of bisulfite-treated DNA samples, positive and negative controls, and water blanks. Samples that did not amplify β-actin were not included in the analysis.
Statistical Analysis
Clinical characteristics of cases and controls were compared using the Student’s t test for continuous variables and chi-square or Fisher’s exact test for categorical variables. The primary quantitative variable was the absolute copy number of mEYA4, determined by MSP against standard dilutions. The Wilcoxon rank-sum test was used to compare the number of mEYA4 copies between CRC tumors versus control tissues and NNM tissues versus control tissues. Logistic regression was performed to assess the strength of association between mEYA4 (copies in tumors or NNM) and CRC or control status. Receiver operating characteristics curves were constructed, and the area under the curve value was calculated for each curve. From the receiver operating characteristics curves, sensitivity was estimated at 90% and 95% specificity cutoffs. The 95% confidence intervals for the sensitivity estimates were calculated using the efficient score method. Odds ratios were calculated at each cutoff and tested using a chi-square statistic. Using the univariate effect size reported in a previous study,15 we estimated that 50 patients would provide >85% power to detect an odds ratio of >10 at the 0.05 level. Analyses were performed using JMP v8.0 (SAS Institute, Cary, NC).
Ethical Considerations
The Mayo Clinic Institutional Review Board approved this research protocol.
RESULTS
Sufficient DNA for EYA4 and β-actin amplifications was available for 20 cases and 20 controls (Table 1). Respectively, in cases and controls, 71% and 75% were male; mean (SD) age was 53 (14.1) and 51 (13.1). Mean (SD) UC duration was 20 years (9.8 years) and 21 years (8.5 years), disease was extensive in 17 (85%) and 15 (75%), and primary sclerosing cholangitis was present in 4 (20%) and 2 (10%). None of these characteristics were significantly different between cases and controls. Control subjects were significantly more likely to have active inflammation at the time of colectomy (P = 0.007).
TABLE 1.
Patient Characteristics
| Cases (n = 20) | Controls (n = 20)a | |
|---|---|---|
| Men | 14 | 15 |
| Mean age (SD), yr | 53 (14.1) | 51 (13.1) |
| Mean UC duration (SD), yr | 20 (9.8) | 21 (8.5) |
| Extensive disease (%)b | 17 (85) | 15 (75) |
| Primary sclerosing cholangitis (%) | 4 (20) | 2 (10) |
| Inflammation score15 | 0.19 | 0.78c |
β-Actin did not amplify in samples from 5 cases and 5 controls.
Proximal to the splenic flexure.
P = 0.0007, all other comparisons nonsignificant.
For cases, data were abstracted regarding the location of both NNM and tumor samples. The majority (75%) of NNM samples were obtained in an anatomical segment distinct from the tumor, e.g., for tumors in the cecum, samples were obtained from a colorectal segment other than the cecum. Median (interquartile range) tissue levels of mEYA4 were not significantly affected by distance from the tumor: 5 (1–192) methylated copies in the same segment versus 62 (7–141) copies in distinct segments, P = 0.3.
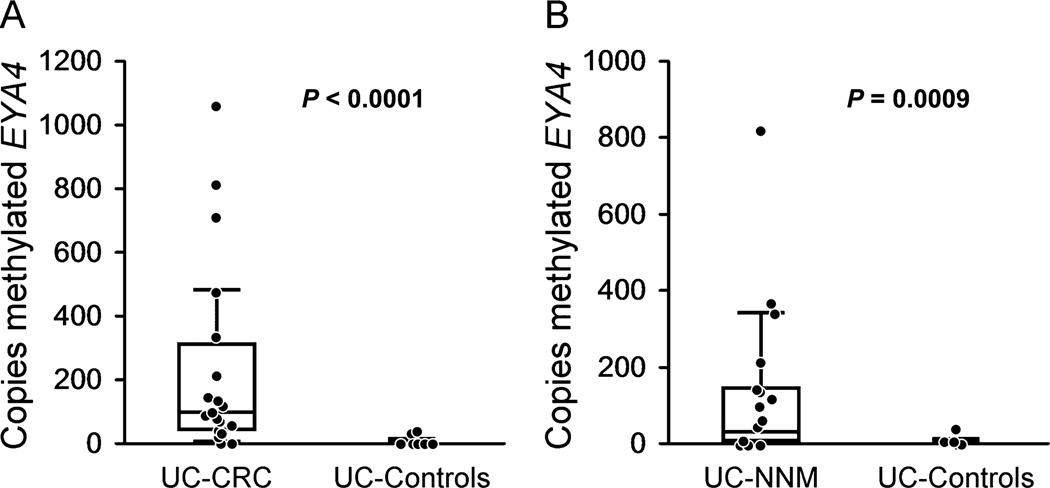
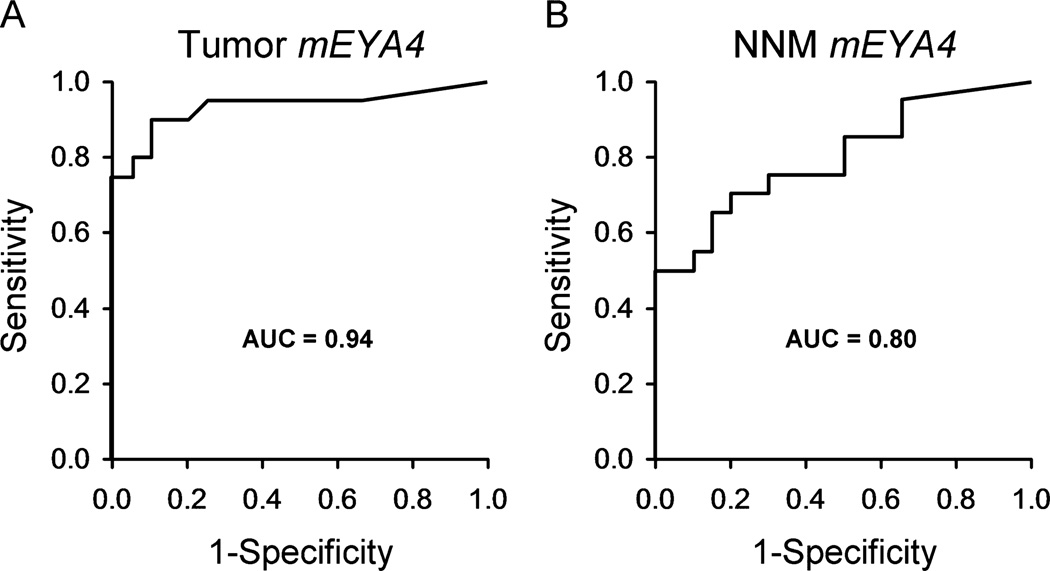
Tissue mEYA4 levels were substantially and significantly higher in primary tumors and NNM from UC cases with CRC than in UC control mucosae. The median number of mEYA4 copies (with interquartile range) was 2 (0–5.7) in UC control mucosae, 93 (39–306) in case with CRC tumors (P < 0.0001 versus control), and 27 (3–140) in case with NNM (P = 0.0009 versus control) (Fig. 1). Receiver operating characteristics curves plotted from the logistic regression models are shown in Figure 2. Among DNA samples taken from case with CRC tumors, mEYA4 copy number was strongly associated with case versus control status with an area under the curve of 0.94. When UC-NNM case levels were modeled, mEYA4 was also strongly associated with case versus control status with an area under the curve of 0.80. No difference was observed when models were corrected by dividing the mEYA4 copy number by the copy number of β-actin, indicating that the DNA input into the MSP reaction was uniform and that quantification of the MSP products was accurate.
FIGURE 1.
Copy number of mEYA4 in tissues from patients with UC. A, Tumor tissue in UC cases with CRC versus colorectal mucosae in UC controls without CRC or dysplasia. B, Nonadjacent NNMs in cases with CRC versus mucosae in UC controls.
FIGURE 2.
Detection of CRC based on tissue mEYA4 levels in (A) primary tumors and (B) NNM: receiver operating characteristics curves plotted.
At 95% specificity, mEYA4 was elevated in primary tumor tissue from 16 of 20 patients with CRC (sensitivity for CRC 80% [56%–93%]). In 10 of 20 patients with CRC, levels of mEYA4 were elevated in NNM samples (sensitivity for CRC 50% [28%–72%]). The tissue level of mEYA4 in NNM was a strong predictor of synchronous CRC, odds ratio 19 (2–170).
DISCUSSION
This study demonstrates that a CRC-associated field change of aberrantly methylated EYA4 occurs in patients with UC. The finding of mEYA4 in random NNM samples was predictive of synchronous CRC.
Based on our earlier observations,16 mEYA4 is typically present in colorectal neoplasia tissue from patients with UC but absent in mucosa from UC controls without neoplasia. More recently, our preliminary data suggested feasibility of mEYA4 assay in stool for the detection of both CRC and precancers in patients with UC or Crohn’s disease.17 The findings in the present study have additional important implications for the use of mEYA4 as a potential stool or tissue marker in UC surveillance. Aberrant methylation of EYA4 may augment the predictive value of conventional white light colonoscopy with random biopsies. However, because we have detected mEYA4 in NNM tissue from UC cases with CRC, an individual with persistent EYA4 methylation may be at increased risk of UC-CRC, despite normal pathology findings on surveillance biopsies. If the strong predictive association between mEYA4 field changes and CRC risk holds in corroborative observations, then assay of mEYA4 has potential to serve as a useful adjunct to routine histology.
The mechanisms underlying field cancerization effects in UC require elucidation. A key question is whether or not the diffuse methylation changes reflect clonal expansion, as is suggested by studies that mapped DNA mutations. Investigators examining mutations in 5 exons of the p53 gene have demonstrated clonal expansion across multiple colonic crypts.11,12 We have previously sequenced the UC-CRC tissues of the case patients for mutations in p53, KRAS, BRAF, APC, and PIK3CA; however, only 14 of 25 tumors contained a mutation on any of the genes sequenced.17 Therefore, we chose not to repeat mutation sequencing in the UC-NNM tissues in the present study. A recent study that followed the expansion of mutant non-neoplastic colonic epithelial stem cells did not show a consistent methylation pattern.18 These findings suggest that methylation markers may not reflect clonality or that the methylation markers studied may be subject to epigenetic drift. Alternatively, the studied markers may undergo dynamic methylation changes that control tissue-specific gene regulation. Clonal expansion of UC-CRC may also be limited to the same or adjacent anatomical segment as the primary tumor11,12; in contrast, distant synchronous UC-associated12 and sporadic19 neoplasms do not necessarily share clonal ancestry by mutation or DNA mismatch repair analysis.
Methylated EYA4 may act as a record of mitotic age20 and better reflect cumulative inflammatory or other environmental exposures. Chronic inflammation seems to alter microbial composition, possibly promoting tumorigenesis. In an interleukin 10–deficient murine colitis model, expansion of genotoxic colibactin-producing Escherichia coli strains was related to the presence of inflammation rather than introduction of a carcinogen, azoxymethane.21 Importantly, colibactin-producing E. coli have been shown to induce aneuploidy and double-stranded DNA breaks in mammalian cells.22 Although the direct connections between inflammation, microbial factors, and DNA methylation are not yet fully understood, gene silencing due to aberrant methylation was observed following a defined double-stranded DNA break in the tumor suppressor gene E-cadherin.23 The tyrosine phosphatase domain of EYA proteins dephosphorylates histone protein H2AX, implicating EYA in DNA repair. Although this has been directly studied in embryonic development,24 this observation also implicates EYA proteins in carcinogenesis.25 Because we have previously shown reversible methylation-dependent downregulation of EYA4 expression,16 it may be that methylation of the EYA4 promotor silences a tumor suppressor function.
Although methylation of many gene promotors may correlate with active inflammation,26 we have demonstrated that mEYA4 levels were not influenced by acute inflammatory activity at the time point of colectomy; these results are similar to those reported for UC-CRC–associated methylation markers.15
This study has both strengths and limitations. DNA did not amplify in 5 case and 5 control samples; as a result, the total number of samples analyzed was below the intended target. Despite the change in estimated statistical power, the results remained statistically significant. Samples were randomly selected from a large well-characterized cohort, and controls were tightly matched on known risk factors for colitis-associated cancers. However, patients were enrolled at a tertiary referral center and may not be representative of the general at-risk population. Additionally, the use of available archived tissue blocks did not permit detailed mapping of the field size. It is possible that the samples contained stromal DNA; however, this would be expected to dilute the difference in mEYA4 copy numbers between NNM and control samples, which was still quite significant. Use of paraffin-embedded tissue may result in excessive DNA fragmentation,27 and DNA recovery rates and yield of mEYA4 may be higher with frozen tissues. Although aberrantly methylated DNA is a very robust and chemically stable biomarker, it was beyond the scope of our aims to make direct comparisons to other classes of biomarkers for which there are promising preliminary data.28–33
Methylated EYA4 in NMM biopsies may serve as a useful predictive marker of synchronous CRC in patients with UC; these novel findings warrant further investigation. Our results also have potential clinical relevance to the use and interpretation of stool tests that incorporate mEYA4 and other methylation markers for surveillance CRC in UC.
Acknowledgments
Supported by the Charles Oswald Foundation and the Maxine and Jack Zarrow Family Foundation of Tulsa Oklahoma.
Footnotes
Portions of this manuscript were presented in abstract form at Digestive Diseases Week 2011, May 7, 2011 in Chicago, IL.
Mayo Clinic has entered into a licensing agreement with Exact Sciences (Madison, WI) whereby inventors (W.RT., J.B.K., and D.A.A.) could share in equity or future royalties in accordance with Mayo Clinic policy.
The authors have no other conflicts of interest to disclose.
REFERENCES
- 1.Herrinton LJ, Liu L, Levin TR, et al. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382–389. doi: 10.1053/j.gastro.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 2.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639–645. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. doi: 10.1053/j.gastro.2007.08.001. quiz 1340-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baars JE, Looman CW, Steyerberg EW, et al. The risk of inflammatory bowel disease-related colorectal carcinoma is limited: results from a nationwide nested case-control study. Am J Gastroenterol. 2011;106:319–328. doi: 10.1038/ajg.2010.428. [DOI] [PubMed] [Google Scholar]
- 6.Rutter MD, Saunders BP, Wilkinson KH, et al. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004;60:334–339. doi: 10.1016/s0016-5107(04)01710-9. [DOI] [PubMed] [Google Scholar]
- 7.Thomas T, Abrams KA, Robinson RJ, et al. Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment Pharmacol Ther. 2007;25:657–668. doi: 10.1111/j.1365-2036.2007.03241.x. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71–74. doi: 10.1016/s0140-6736(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 9.Greenstein AJ, Slater G, Heimann TM, et al. A comparison of multiple synchronous colorectal cancer in ulcerative colitis, familial polyposis coli, and de novo cancer. Ann Surg. 1986;203:123–128. doi: 10.1097/00000658-198602000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Brentnall TA, Crispin DA, Rabinovitch PS, et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369–378. doi: 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 12.Leedham SJ, Graham TA, Oukrif D, et al. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology. 2009;136:542–550. e546. doi: 10.1053/j.gastro.2008.10.086. [DOI] [PubMed] [Google Scholar]
- 13.Risques RA, Lai LA, Himmetoglu C, et al. Ulcerative colitis-associated colorectal cancer arises in a field of short telomeres, senescence, and inflammation. Cancer Res. 2011;71:1669–1679. doi: 10.1158/0008-5472.CAN-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azuara D, Rodriguez-Moranta F, de Oca J, et al. Novel methylation panel for the early detection of neoplasia in high-risk ulcerative colitis and Crohn’s colitis patients. Inflamm Bowel Dis. 2013;19:165–173. doi: 10.1002/ibd.22994. [DOI] [PubMed] [Google Scholar]
- 15.Garrity-Park MM, Loftus EV, Jr, Sandborn WJ, et al. Methylation status of genes in non-neoplastic mucosa from patients with ulcerative colitis-associated colorectal cancer. Am J Gastroenterol. 2010;105:1610–1619. doi: 10.1038/ajg.2010.22. [DOI] [PubMed] [Google Scholar]
- 16.Osborn NK, Zou H, Molina JR, et al. Aberrant methylation of the eyes absent 4 gene in ulcerative colitis-associated dysplasia. Clin Gastroenterol Hepatol. 2006;4:212–218. doi: 10.1016/j.cgh.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Kisiel JB, Yab TC, Nazer Hussain FT, et al. Stool DNA testing for the detection of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:546–554. doi: 10.1111/apt.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham TA, Humphries A, Sanders T, et al. Use of methylation patterns to determine expansion of stem cell clones in human colon tissue. Gastroenterology. 2011;140:1241–1250. e1249. doi: 10.1053/j.gastro.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Aslanian HR, Burgart LJ, Harrington JJ, et al. Altered DNA mismatch repair expression in synchronous and metachronous colorectal cancers. Clin Gastroenterol Hepatol. 2008;6:1385–1388. doi: 10.1016/j.cgh.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Chu MW, Siegmund KD, Eckstam CL, et al. Lack of increases in methylation at three CpG-rich genomic loci in non-mitotic adult tissues during aging. BMC Med Genet. 2007;8:50. doi: 10.1186/1471-2350-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arthur JC, Perez-Chanona E, Muhlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuevas-Ramos G, Petit CR, Marcq I, et al. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A. 2010;107:11537–11542. doi: 10.1073/pnas.1001261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Hagan HM, Mohammad HP, Baylin SB. Double strand breaks can initiate gene silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island. PLoS Genet. 2008;4:e1000155. doi: 10.1371/journal.pgen.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook PJ, Ju BG, Telese F, et al. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature. 2009;458:591–596. doi: 10.1038/nature07849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnan N, Jeong DG, Jung SK, et al. Dephosphorylation of the C-terminal tyrosyl residue of the DNA damage-related histone H2A.X is mediated by the protein phosphatase eyes absent. J Biol Chem. 2009;284:16066–16070. doi: 10.1074/jbc.C900032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito S, Kato J, Hiraoka S, et al. DNA methylation of colon mucosa in ulcerative colitis patients: correlation with inflammatory status. Inflamm Bowel Dis. 2011;17:1955–1965. doi: 10.1002/ibd.21573. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Wang L, Briggs C, et al. DNA degradation test predicts success in whole-genome amplification from diverse clinical samples. J Mol Diagn. 2007;9:441–451. doi: 10.2353/jmoldx.2007.070004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato I, Badsha KZ, Land S, et al. DNA/RNA markers for colorectal cancer risk in preserved stool specimens: a pilot study. Tumori. 2009;95:753–761. doi: 10.1177/030089160909500619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y, Choi KR, Chae MJ, et al. Stability of DNA, RNA, cytomorphology, and immunoantigenicity in Residual ThinPrep Specimens. [published online ahead of print April 9, 2013] APMIS. doi: 10.1111/apm.12082. [DOI] [PubMed] [Google Scholar]
- 30.Zou H, Harrington JJ, Klatt KK, et al. A sensitive method to quantify human long DNA in stool: relevance to colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2006;15:1115–1119. doi: 10.1158/1055-9965.EPI-05-0992. [DOI] [PubMed] [Google Scholar]
- 31.de Wit M, Fijneman RJ, Verheul HM, et al. Proteomics in colorectal cancer translational research: biomarker discovery for clinical applications. Clin Biochem. 2013;46:466–479. doi: 10.1016/j.clinbiochem.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 32.Bosch LJ, de Wit M, Oudgenoeg G, et al. Abstract 4523: stool proteomics reveals new candidate biomarkers for colorectal cancer screening. Cancer Res. 2012;72:4523. [Google Scholar]
- 33.Zou H, Harrington JJ, Sugumar A, et al. Detection of colorectal disease by stool defensin assay: an exploratory study. Clin Gastroenterol Hepatol. 2007;5:865–868. doi: 10.1016/j.cgh.2007.03.013. [DOI] [PubMed] [Google Scholar]