Introduction
The capacity of the adult cortex to undergo reorganization was first demonstrated in the pioneering studies of Merzenich, Kaas and colleagues [42,43]. In these studies the median nerve, which innervates the medial volar surface of the hand in primates, was transected and electrophysiological recordings were carried out in the somatosensory cortex. Acute mapping revealed that islands of novel receptive field inputs conveyed by the intact radial and ulnar nerves were immediately expressed in the “median nerve cortex” [42]. These “latent inputs” [24] continued to expand over the ensuing weeks, with peripheral stimulation of innervated skin surface representations coming to reliably evoke neuronal activity throughout the former median nerve territory [43]. In a subsequent experiment employing a median nerve constriction paradigm, it was demonstrated that the cortex also went through a period of reorganization over the first month after nerve compression [59]. Continued remapping demonstrated the reinstatement of the original peripheral inputs as the median nerve regenerated down the intact neural sheath. These studies have provided useful platforms to study the mechanisms of adult neural plasticity [7, 8, 20, 21, 22, 23, 26, 49, 60].
More recently, we have begun exploring the molecular mechanisms that permit reorganizational plasticity. Specifically, we have investigated receptor subunit specific changes that occur within the cortex and brainstem during recovery from peripheral nerve compression. [45, 46, 47, 51]. The current study extends these investigations of AMPA and GABA receptor subunits to the cuneate nucleus one and five months after nerve compression.
Methods
We report data from the cuneate nucleus of 5 adult squirrel monkeys (Saimiri sciureus) that received median nerve compressions 1 (n = 2) and 5 (n = 3) months prior to immunohistochemical staining. Methods for nerve injury, immunohistochemical staining, and data quantification have been previously described in detail [45,46,47,51]. All procedures were approved by the Indiana University Institutional Animal Care and Use Committee. After 30 or 150 days of recovery, animals were anesthetized with isoflurane gas and transcardially perfused with cold 0.9% saline solution followed by 400 ml of 4% paraformaldehyde in .1 M phosphate buffer (pH 7.4). The brainstem was dissected out and coronal sections were cut (40 μm) using a freezing microtome. Sections containing the cuneate nucleus at the level of the pars rotunda, which contains the glabrous inputs [15,16, 65] were kept for immuno-histochemical staining. Alternating tissue sections were stained in antisera containing GluR1 (1:1000 Chemicon), GluR2/3 (1:1000 Thermo Scientific), GABAAα1 (1:1000 Thermo Scientific), GABAB1a (1:1000 Alpha Diagnostics International), or GABAB1b (1:1000 Alpha Diagnostics International) receptor subunits. These brainstem slices were stained contemporaneously with cortical slices prepared from the same animals.
The entire hand representation is present in each nucleus, and digit/palm representations were visible to the trained observer. Within the region corresponding to the injured median nerve, inputs from the intact nerve representations (ulnar, radial) were in close proximity, therefore soma staining intensity quantification was carried out in the more distal regions of the median nerve representation digit 2 in deprived and homologous intact regions of cuneate nucleus pars rotunda (see Figure 1A). A 100 μm by 100 μm bounding box was placed within the region of interest at low magnification (4×) and then cell contours were traced at higher magnification (40×) using the Stereo Investigator software (MBF Bioscience; Williston, VT, USA). Staining intensity measurements were generated using the luminance function (densitometry) in control and deprived cuneate nuclei (~30 per section × 3 sections).
Figure 1.
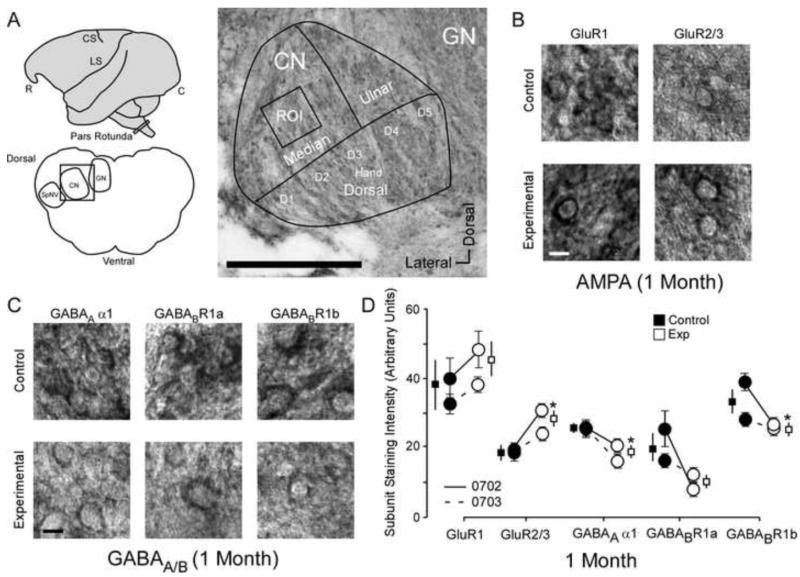
The changes to AMPA and GABAA/B receptor subunits 1 month after nerve injury. A: Left Top: Cartoon of the squirrel monkey brain identifying pars rotunda of the brainstem, CS; central sulcus, LS; lateral sulcus, R; rostral, C; caudal; Left bottom: Coronal section through brainstem identifying region of interest (black box) in cuneate nucleus CN, GN; Gracile Nucleus, SpNV: spinal nucleus. Right: Photo-micrograph of a control section indicating the sampling region for immunohistochemical quantification of staining intensity (Median Input): scale bar .25 mm; ROI; region of interest, CN; cuneate nucleus, GN; Gracile Nucleus. B: Photomicrographs showing qualitative examples of GluR1 and GluR2/3 soma staining between control and experimental cuneate nucleus 1 month after nerve injury: scale bar 5 μm. C: Photomicrographs showing qualitative examples of GABAA α1, GABABR1a,and GABABR1b soma staining between control and experimental cuneate nucleus 1 month after nerve injury: scale bar 5 μm. D: Quantitative plot comparing GluR1, GluR2/3, GABAA α1, GABABR1a, and GABABR1b staining intensity group averages and raw averages for all animals one month after nerve injury. * p < .05.
Results
AMPA and GABAA/B receptors 1 month
The average soma subunit staining intensities in the control cuneate nucleus were compared within animals to experimental cuneate nucleus in monkeys 1 month after median nerve compression (N = 2). Figure 1 presents the qualitative and quantitative differences in GluR1, GluR2/3, GABAA α1, GABABR1a, and GABABR1b staining intensity between experimental and control cuneate nuclei 1 month after nerve injury. Figure 1A illustrates the region of interest where subunit staining intensity data was collected. The pars rotunda of the brainstem (Cartoon top left) was dissected out and then sliced coronally (Illustration, bottom left) throughout the hand representation. Photomicrograph (Figure 1A, right) illustrates the hand representation of the cuneate nucleus and how the overlaid tracing grid framed a bounding box around the region of interest (~ digit 2) that was used for all data collection.
Representative examples of GluR1 and GluR2/3 subunit staining (Figure 1B) demonstrate the non-significant increase in GluR1 and significant increase in GluR2/3 subunit staining intensity 1 month after median nerve compression [Mean ± SEM; GluR1∷ Ctrl 39.6 ± 8.5 vs Exp 46.5 ± 5.0; t (3) = 2.68 p = .074; t (3) = 2.68 p = .074; GluR2/3: Ctrl 19.2 ± 1.9 vs Exp 27.4 ± 2.1; t (3) = 3.39 p = .042]. Representative examples of GABAA α1, GABABR1a, and GABABR1b subunit staining (Figure 1C) demonstrates the significant decrease in GABAA α1, the decrease in GABABR1a, and the significant decrease in GABABR1b subunit staining intensity 1 month after median nerve compression [Mean ± SEM; GABAA α1: Ctrl 25.3 ± 1.9 vs Exp 20.3 ± 1.0; t (3) = 3.22 p = .04; GABABR1a: Ctrl 19.6 ± 1.4 vs Exp 10.3 ± 2.5; t (3) = 3.13 p = .051; GABABR1b: Ctrl 33.6 ± 0.3 vs Exp 25.8 ± 3.5;t (3) = 3.95 p = .028]. Figure 1D shows the quantified data (average subunit intensity and SEMs) for each monkey (circles) and the group means and SEMs (squares).
AMPA and GABAA/B receptors 5 months
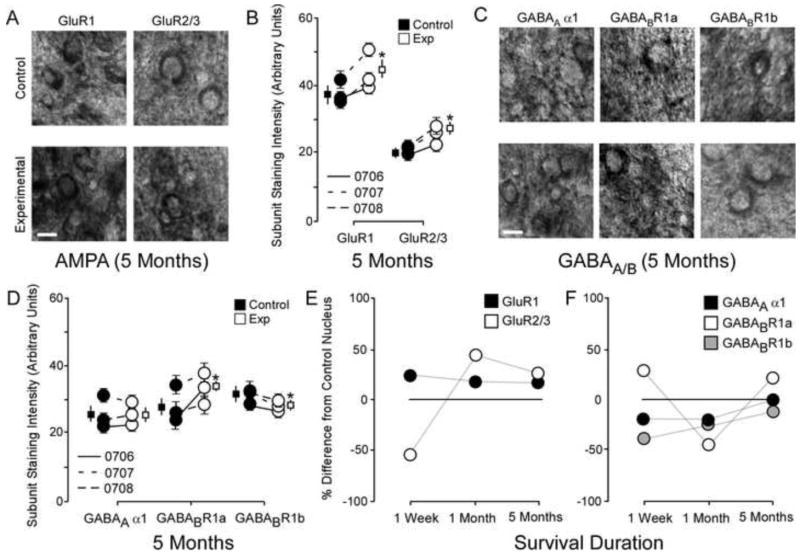
The average soma subunit staining intensities in the control cuneate nucleus were compared within animals (N = 3) to experimental cuneate nucleus in monkeys 5 months after median nerve compression. Representative examples of GluR1 and GluR2/3 subunit staining (Figure 2A) demonstrate the significant increase in GluR1 and GluR2/3 subunit staining intensity 5 months after median nerve compression [Figure 2B: Mean ± SEM; GluR1: Ctrl 38.0 ± 2.4 vs Exp 44.4 ± 3.4; t (5) = 3.7 p = .013; GluR2/3: Ctrl 20.7 ± 0.3 vs Exp 25.5 ± 0.8; t (5) = 3.9 p = .011]. Representative examples of GABAA α1, GABABR1a, and GABABR1b subunit staining (Figure 2C) demonstrate the lack of difference in GABAA α1 expression, the significant increase in GABABR1a expression, and the significant decrease in GABABR1b subunit staining intensity 5 months after median nerve compression [Figure 2D: Mean ± SEM; GABAA α1: Ctrl 25.1 ± 1.7 vs Exp 25.6 ± 1.2; t (5) = .01 p = .99; GABABR1a: Ctrl 28.4 ± 1.5 vs Exp 33.2 ± 1.7; t (5) = 3.3 p = .021; GABABR1b: Ctrl 31.5 ± 0.6 vs Exp 29.2 ± 0.6; t (5) = 2.8 p = .036]. Figure 2B/D shows the quantified data (average subunit intensity) for each monkey (circles) and the group means and SEMs (squares).
Figure 2.
The changes to AMPA and GABAA/B receptor subunits 5 months after nerve injury. A: Photomicrographs showing qualitative examples of GluR1 and GluR2/3 soma staining between control and experimental cuneate nucleus 5 months after nerve injury: scale bar 5 μm. B: Quantitative plot comparing GluR1 and GluR2/3 staining intensity data for all animals 5 months after nerve injury. * p < .05. C: Photomicrographs showing qualitative examples of GABAA α1, GABABR1a, and GABABR1b soma staining between control and experimental cuneate nucleus 5 months after nerve injury: scale bar 5 μm. D: Quantitative plot comparing GABAA α1, GABABR1a, and GABABR1b staining intensity data for group averages and raw averages 5 months after nerve injury. * p < .05. E: Qualitative line-plots comparing percent differences from control values for GluR1 and GluR2/3 staining intensity data one week, one month, and five months after nerve compression. F: Qualitative line-plots comparing percent differences from control values for GABAA α1, GABABR1a, and GABABR1b staining intensity data one week, one month, and five months after nerve compression.
AMPA and GABAA/B receptor changes throughout recovery
A ratio of the group average for control to experimental subunit staining intensities was used to illustrate changes in receptor subunit over time. These data are presented as percentage differences from control values 1 week, 1 month, and 5 months after nerve injury for both AMPA (Figure 2E) and GABA subunits (Figure 2F). Data for one week were derived from previously published data (46, 51).
Discussion
This study investigated the changes to AMPA and GABAA/B receptor subunit expression within the cuneate nucleus of adult primates at two specific points during the recovery from a regenerating peripheral nerve injury. The pattern of AMPA and GABAA/B subunit expression within cuneate nucleus shows signs of reorganizational plasticity after one month of recovery, and continues to display significant changes in the pattern of subunit expression at a time when peripheral nerve regeneration has presumably reinnervated its original cutaneous inputs [59]. At both one and five months of recovery, the brainstem displays a pattern of subunit expression that is very similar to that seen in the corticofugal layers of cortex from the same animals [47]. This suggests that the cortex and brainstem are governed by homogeneous types of receptor plasticity following peripheral nerve injury, which further agrees with the functional roles that exist between them in vivo [63,64,65].
The time course of mechanisms driving brainstem plasticity
This study concludes an investigation into the similarities and differences between AMPA and GABA receptor subunit expression in the brainstem and cortex throughout recovery from nerve injury. In all of these studies, experimental data were compared to control data gathered from regions associated with homologous digit representations. Therefore it is important to note that bilateral cortical plasticity has been reported after unilateral nerve injuries [eg 3, 10]. Because of this we cannot rule out that plasticity could be occurring in the intact hand region contemporaneously. Figure 2E/F plots the reported changes in receptor expression between experimental and control cuneate nucleus one week, one month, and five months after nerve injury. It remains unknown whether these shifts in expression manifest lineally or abruptly; however, studies investigating developmental and adult plasticity offer important clues as to the time course of the plasticity mechanisms governing these changes.
1 week
Previously we have reported that brainstem and cortex undergo a period of developmental recapitulation around 1 week after nerve injury [45, 46, 51]. Developmental plasticity happens considerably faster (hours/days) than more prolonged forms of adult plasticity (weeks/months). Rapid shifts in subunit expression occur during the critical period of brainstem nuclei development [eg 34, 35] and a rapid decrease in cortical GABAAR expression occurs within hours of nerve injury in adults [60]. Our studies suggest that shifts in specific subclasses of receptor phenotype (eg increased homomeric, decreased heteromeric) can be hidden by autoradiography techniques that quantify net changes in receptors [26]. Therefore we would hypothesize that wide-scale shifts in receptor expression can occur less linearly and more abruptly approximate to nerve injury.
1 month
One month after nerve injury, the cortex [47] displays patterns of subunit expression that have been observed during somatosensory reorganization [26]. Currently we report a pattern of subunit expression in cuneate nucleus of these same animals that is very similar to that reported in infragranular cortex (Figure 1D). This suggests that both the infragranular cortex and the cuneate brainstem nuclei share conserved forms of plasticity. Considering that the cortex forms a feedback loop with cuneothalamic relay neurons via infragranular corticofugal output neurons, the question remains as to whether brainstem plasticity drives upstream reorganization or whether cortical plasticity governs subcortical reorganization.
Within this loop, the somatosensory cortex imposes center surround control of ascending somatotopic input. This has substantial influence on receptive field expression at lower levels of the ascending neuroaxis [1,2, 3,5, 34, 35, 38, 39, 40, 54, 56 57, 58]. While corticofugal feedback does play an important role in both immediate unmasking and reorganization within subcortical structures [6, 13, 17, 27, 31, 58], the time-course and extent of changes occurring in the brainstem impose important limitations on cortical reorganization.
The entire sensory neuroaxis immediately expresses unmasked latent inputs following loss of dominant sensory input [7, 8, 14, 24, 63, 64]; however, only receptive field changes occurring at the level of the brainstem are faithfully re-expressed in the thalamus and cortex during reorganization [29, 30, 61]. More importantly, receptive field changes at the level of the brainstem are essential for the expression of reorganization at the level of the cortex [32]. Thus, the pattern and extent of cortical reorganization is highly correlated with representational changes evident at subcortical levels [17].
Electrophysiological studies have revealed that the time course of reorganization for cortical [42, 43], thalamic, and brainstem [8] somatosensory regions occur over many weeks to months. During reorganization we would suggest that subunit shifts occur slowly as NMDAR plasticity potentiates local circuits across the sensory neuroaxis [49]. Significant changes in receptor subunit expression, like the ones reported in our studies, are presumably observed as system’s level changes to receptive field expression become predominant.
5 months
Five months after nerve compression, the pattern of AMPA and GABA subunit expression in the cuneate nucleus is very similar to the pattern of granular and infragranular cortical expression of these same animals (Figure 2B/D). This provides further evidence that both the infragranular cortex and the cuneate brainstem nuclei share conserved forms of plasticity. Recovery from peripheral nerve injury occurs slowly (~ 2mm/day), and therefore it takes many months for the distal digit tips to be reinnervated [59]. Therefore, receptor shifts associated with reinnervation would likely occur slowly as cutaneous receptors were reinnervated on a receptor by receptor basis. It is unknown how reorganized inputs would compete with original inputs prior to the re-emergence of original somatotopy [59]; however, traces of receptive field plasticity and original somatotopy are maintained after the induction of adult plasticity [28, 41, 44, 52, 53, 62].
In humans with long standing nerve injuries (amputations) the original somatotopy is perceived following electrical activation of the nerve stump [44, 52].Similarly, after median nerve transection with ligation in monkeys, responsiveness to electrical stimulation of the proximal median nerve stump is still present after radial nerve inputs have established a “normal” profile in the “median nerve cortex”[53].
To that end we hypothesize that the consistent elevation in GluR1 and GluR2/3 receptor subunits within the brainstem could be representative of increased numbers of Glur1/2 and GluR2/3 containing AMPARs delivered to synapses that form both acutely [11, 12] and chronically [9, 30, 55, 61] following nerve injury. In this way the engram [see, 19]. of the reorganized circuit could be masked by corticofugal feedback [18, 32, 33 50, 58], while being maintained within the network.
Highlights.
Nerve compression injuries induce reorganization prior to reinnervation.
Changes to AMPAR and GABAR in cuneate nucleus during reinnervation are reported.
These results are compared to cortical data from the same adult animals
Similar changes occur in adult brainstem and cortex throughout reinnervation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aguilar J, Rivadulla C, Soto C, Canedo A. New corticocuneate cellular mechanisms underlying the modulation of cutaneous ascending transmission in anesthetized cats. J Neurophysiol. 2003;89:3328–3339. doi: 10.1152/jn.01085.2002. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar J, Soto C, Rivadulla C, Canedo A. The lemniscal-cuneate recurrent excitation is suppressed by strychnine and enhanced by GABAA antagonists in the anaesthetized cat. Eur J Neurosci. 2002;16:1697–1704. doi: 10.1046/j.1460-9568.2002.02230.x. [DOI] [PubMed] [Google Scholar]
- 3.Calford MB, Tweedale R. Interhemispheric transfer of plasticity in the cerebral cortex. Science. 1990;249:805–807. doi: 10.1126/science.2389146. [DOI] [PubMed] [Google Scholar]
- 4.Canedo A, Mariño J, Aguilar J. Lemniscal recurrent and transcortical influences on cuneate neurons. Neuroscience. 2000;97:317–334. doi: 10.1016/s0306-4522(00)00063-4. [DOI] [PubMed] [Google Scholar]
- 5.Cheema S, Rustioni A, Whitsel BL. Sensorimotor cortical projections to the primate cuneate nucleus. J Comp Neurol. 1985;240:196–211. doi: 10.1002/cne.902400209. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury SA, Reek KA. D. D. Rasmusson Changes in corticothalamic modulation of receptive fields during peripheral injury-induced reorganization. Proc Natl Acad Sci U S A. 2004;101:7135–7140. doi: 10.1073/pnas.0307840101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churchill JD, Muja N, Myers WA, Besheer J, Garraghty PE. Somatotopic consolidation: a third phase of reorganization after peripheral nerve injury in adult squirrel monkeys. Exp Brain Res. 1998;118:189–196. doi: 10.1007/s002210050271. [DOI] [PubMed] [Google Scholar]
- 8.Churchill JD, Arnold LL, Garraghty PE. Somatotopic reorganization in the brainstem and thalamus following peripheral nerve injury in adult primates. Brain Res. 2001;910:142–152. doi: 10.1016/s0006-8993(01)02703-2. [DOI] [PubMed] [Google Scholar]
- 9.Churchill JD, Tharp JA, Wellman CL, Sengelaub DR, Garraghty PE. Morphological correlates of injury-induced reorganization in primate somatosensory cortex. BMC Neurosci. 2004;5:43. doi: 10.1186/1471-2202-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarey JC, Tweedale R, Calford MB. Interhemispheric modulation of somatosensory receptive fields: evidence for plasticity in primary somatosensory cortex. Cereb Cortex. 1996;6:196–206. doi: 10.1093/cercor/6.2.196. [DOI] [PubMed] [Google Scholar]
- 11.Darian-Smith C. Primary afferent terminal sprouting after a cervical dorsal rootlet section in the macaque monkey. J Comp Neurol. 2004;470:134–150. doi: 10.1002/cne.11030. [DOI] [PubMed] [Google Scholar]
- 12.Darian-Smith C, Ciferri M. Cuneate nucleus reorganization following cervical dorsal rhizotomy in the macaque monkey: its role in the recovery of manual dexterity. J Comp Neurol. 2006;498:552–565. doi: 10.1002/cne.21088. [DOI] [PubMed] [Google Scholar]
- 13.Ergenzinger ER, Glasier MM, Hahm JO, Pons TP. Cortically induced thalamic plasticity in the primate somatosensory system. Nat Neurosci. 1998;1:226–229. doi: 10.1038/673. [DOI] [PubMed] [Google Scholar]
- 14.Faggin BM, Nguyen KT, Nicolelis MA. Immediate and simultaneous sensory reorganization at cortical and subcortical levels of the somatosensory system. Proc Natl Acad Sci U S A. 1997;94:9428–9433. doi: 10.1073/pnas.94.17.9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florence SL, Wall JT, Kaas JH. Somatotopic organization of inputs from the hand to the spinal gray and cuneate nucleus of monkeys with observations on the cuneate nucleus of humans. J Comp Neurol. 1989;286:48–70. doi: 10.1002/cne.902860104. [DOI] [PubMed] [Google Scholar]
- 16.Florence SL, Wall JT, Kaas JH. Central projections from the skin of the hand in squirrel monkeys. J Comp Neurol. 1991;311:563–578. doi: 10.1002/cne.903110410. [DOI] [PubMed] [Google Scholar]
- 17.Florence SL, Kaas JH. Large-scale reorganization at multiple levels of the somatosensory pathway follows therapeutic amputation of the hand in monkeys. J Neurosci. 1995;15:8083–8095. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florence SL, Hackett TA, Strata F. Thalamic and cortical contributions to neural plasticity after limb amputation. J Neurophysiol. 2000;83:3154–3159. doi: 10.1152/jn.2000.83.5.3154. [DOI] [PubMed] [Google Scholar]
- 19.Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field Plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- 20.Garraghty PE, Sur M. Morphology of single intracellularly stained axons terminating in area 3b of macaque monkeys. J Comp Neurol. 1990;294:583–593. doi: 10.1002/cne.902940406. [DOI] [PubMed] [Google Scholar]
- 21.Garraghty PE, Kaas JH. Large-scale functional reorganization in adult monkey cortex after peripheral nerve injury. Proc Natl Acad Sci U S A. 1991;88:6976–680. doi: 10.1073/pnas.88.16.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garraghty PE, Kaas JH. Functional reorganization in adult monkey thalamus after peripheral nerve injury. NeuroReports. 1991;2:747–750. doi: 10.1097/00001756-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Garraghty PE, LaChica EA, Kaas JH. Injury-induced reorganization of somatosensory cortex is accompanied by reductions in GABA staining. Somatosens Mot Res. 1991;8:347–354. doi: 10.3109/08990229109144757. [DOI] [PubMed] [Google Scholar]
- 24.Garraghty PE, Hanes DP, Florence SL, Kaas JH. Pattern of peripheral deafferentation predicts reorganizational limits in adult primate somatosensory cortex. Somatosens Mot Res. 1994;11:109–117. doi: 10.3109/08990229409028864. [DOI] [PubMed] [Google Scholar]
- 25.Garraghty PE, Muja N. NMDA receptors and plasticity in adult primate somatosensory cortex. J Comp Neurol. 1996;367:319–326. doi: 10.1002/(SICI)1096-9861(19960401)367:2<319::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Garraghty PE, Arnold LL, Wellman CL, Mowery TM. Receptor autoradiographic correlates of deafferentation-induced reorganization in adult primate somatosensory cortex. J Comp Neurol. 2006;497:636–645. doi: 10.1002/cne.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graziano A, Jones EG. Early withdrawal of axons from higher centers in response to peripheral somatosensory denervation. J Neurosci. 2009;29:3738–3748. doi: 10.1523/JNEUROSCI.5388-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halligan PW, Marshall JC, Wade DT, Davey J, Morrison D. Thumb in cheek? Sensory reorganization and perceptual plasticity after limb amputation. Neuroreport. 1993;4:233–236. doi: 10.1097/00001756-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Jain N, Florence SL, Qi HX, Kaas JH. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc Natl Acad Sci USA. 2000;97:5546–5550. doi: 10.1073/pnas.090572597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones EG, Pons TP. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science. 1998;282:1121–1125. doi: 10.1126/science.282.5391.1121. [DOI] [PubMed] [Google Scholar]
- 31.Krupa DJ, Ghazanfar AA, Nicolelis MA. Immediate thalamic sensory plasticity depends on corticothalamic feedback. Proc Natl Acad Sci USA. 1999;96:8200–8205. doi: 10.1073/pnas.96.14.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lane RD, Pluto CP, Kenmuir CL, Chiaia NL, Mooney RD. Does reorganization in the cuneate nucleus following neonatal forelimb amputation influence development of anomalous circuits within the somatosensory cortex? J Neurophysiol. 2008;99:866–875. doi: 10.1152/jn.00867.2007. [DOI] [PubMed] [Google Scholar]
- 33.Lane RD, Bennett-Clarke CA, Chiaia NL, Killackey HP, Rhoades RW. Lesion-induced reorganization in the brainstem is not completely expressed in somatosensory cortex. Proc Natl Acad Sci USA. 1995;92:4264–4268. doi: 10.1073/pnas.92.10.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q, Wong-Riley MT. Postnatal development of N-methyl-D-aspartate receptor subunits 2A, 2B, 2C, 2D, and 3B immunoreactivity in brain stem respiratory nuclei of the rat. Neuroscience. 2010;171:637–654. doi: 10.1016/j.neuroscience.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Q, Wong-Riley MT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol. 1985;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- 36.Lue JH, Jiang-Shieh YF, Shieh JY, Ling EA, Wen CY. Multiple inputs of GABA-immunoreactive neurons in the cuneate nucleus of the rat. Neurosci Res. 1997;27:123–132. doi: 10.1016/s0168-0102(96)01139-x. [DOI] [PubMed] [Google Scholar]
- 37.Lue JH, Lai SM, Wang TJ, Shieh JY, Wen CY. Synaptic relationships between corticocuneate terminals and glycine-immunoreactive neurons in the rat cuneate nucleus. Brain Res. 1997;771:167–171. doi: 10.1016/s0006-8993(97)00907-4. [DOI] [PubMed] [Google Scholar]
- 38.Lue JH, Chen SH, Shieh JY, Wen CY. Afferent synaptic contacts on glycine-immunoreactive neurons in the rat cuneate nucleus. Synapse. 2001;41:139–149. doi: 10.1002/syn.1068. [DOI] [PubMed] [Google Scholar]
- 39.Malmierca E, Nuñez A. Corticofugal action on somatosensory response properties of rat nucleus gracilis cells. Brain Res. 1998;810:172–180. doi: 10.1016/s0006-8993(98)00920-2. [DOI] [PubMed] [Google Scholar]
- 40.Martin JH, Kably B, Hacking A. Activity-dependent development of cortical axon terminations in the spinal cord and brain stem. Exp Brain Res. 1999;125:184–199. doi: 10.1007/s002210050673. [DOI] [PubMed] [Google Scholar]
- 41.Merzenich MM, WM Jenkins. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- 42.Merzenich MM, Kaas JH, Wall JT, Nelson RJ, Sur M, Felleman DJ. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983a;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 43.Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983b;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- 44.Moore CE, Schady W. Investigation of the functional correlates of reorganization within the human somatosensory cortex. Brain. 2000;123:1883–1895. doi: 10.1093/brain/123.9.1883. [DOI] [PubMed] [Google Scholar]
- 45.Mowery TM, Garraghty PE. Nerve-injury induced changes to GluR1 and GluR2/3 sub-unit expression in area 3b of adult squirrel monkeys: Developmental recapitulation? Front Syst Neurosci. 2009;3:1. doi: 10.3389/neuro.06.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mowery TM, Sarin RM, Elliott KS, Garraghty PE. Nerve injury-induced changes in GABA(A) and GABA(B) sub-unit expression in area 3b and cuneate nucleus of adult squirrel monkeys: further evidence of developmental recapitulation. Brain Res. 2011;1415:63–75. doi: 10.1016/j.brainres.2011.07.066. [DOI] [PubMed] [Google Scholar]
- 47.Mowery TM, Walls SM, Garraghty PE. AMPA and GABAA/B receptor subunit expression in the cortex of adult squirrel monkeys during peripheral nerve regeneration. Brain Res. 2013;1520:80–94. doi: 10.1016/j.brainres.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray GM, Taub DR, Mackie PD, Zhang HQ, Ghosh S, Rowe MJ. The effects of neonatal median nerve injury on the responsiveness of tactile neurones within the cuneate nucleus of the cat. J Physiol. 1997;505:759–678. doi: 10.1111/j.1469-7793.1997.759ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers WA, Churchill JD, Muja N, Garraghty PE. Role of NMDA receptors in adult primate cortical somatosensory plasticity. J Comp Neurol. 2000;418:373–382. [PubMed] [Google Scholar]
- 50.Rhoades RW, Wall JT, Chiaia NL, Bennett-Clarke CA, Killackey HP. Anatomical and functional changes in the organization of the cuneate nucleus of adult rats after fetal forelimb amputation. J Neurosci. 1993;13:1106–1119. doi: 10.1523/JNEUROSCI.13-03-01106.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarin RM, Mowery TM, Garraghty PE. AMPA receptor subunit expression in the cuneate nucleus of adult squirrel monkeys after peripheral nerve injury. Neurosci Lett. 2012;516:193–196. doi: 10.1016/j.neulet.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schady W, Braune S, Watson S, Torebjörk HE, Schmidt R. Responsiveness of the somatosensory system after nerve injury and amputation in the human hand. Ann Neurol. 1996;36:65–78. doi: 10.1002/ana.410360114. [DOI] [PubMed] [Google Scholar]
- 53.Schroeder CE, Seto S, Garraghty PE. Emergence of radial nerve dominance in median nerve cortex after median nerve transection in an adult squirrel monkey. J Neurophysiol. 1997;7:522–526. doi: 10.1152/jn.1997.77.1.522. [DOI] [PubMed] [Google Scholar]
- 54.Sclabassi RJ, Kroin JS, Hinman CL, Risch HA. The effect of cortical ablation on afferent activity in the cat somatosensory system. Electroencephalogr Clin Neurophysiol. 1986;64:31–40. doi: 10.1016/0013-4694(86)90041-6. [DOI] [PubMed] [Google Scholar]
- 55.Sengelaub DR, Muja N, Mills AC, Myers WA, Churchill JD, Garraghty PE. Denervation-induced sprouting of intact peripheral afferents into the cuneate nucleus of adult rats. Brain Research. 1997;769:256–262. doi: 10.1016/s0006-8993(97)00708-7. [DOI] [PubMed] [Google Scholar]
- 56.Soto C, Aguilar J, Martín-Cora F, Rivadulla C, Canedo A. Intracuneate mechanisms underlying primary afferent cutaneous processing in anaesthetized cats. Eur J Neurosci. 2004;19:3006–3016. doi: 10.1111/j.0953-816X.2004.03432.x. [DOI] [PubMed] [Google Scholar]
- 57.Urasaki E, Wada S, Yasukouchi H, Yokota A. Effect of transcutaneous electrical nerve stimulation (TENS) on central nervous system amplification of somatosensory input. J Neurol. 1998;245:143–148. doi: 10.1007/s004150050194. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Wall JT. Cortical influences on sizes and rapid plasticity of tactile receptive fields in the dorsal column nuclei. J Comp Neurol. 2005;489:241–248. doi: 10.1002/cne.20642. [DOI] [PubMed] [Google Scholar]
- 59.Wall JT, Felleman DJ, Kaas JH. Recovery of normal topography in the somatosensory cortex of monkeys after nerve crush and regeneration. Science. 1983;221:771–773. doi: 10.1126/science.6879175. [DOI] [PubMed] [Google Scholar]
- 60.Wellman CL, Arnold LL, Garman EE, Garraghty PE. Acute reductions in GABAA receptor binding in layer IV of adult primate somatosensory cortex after peripheral nerve injury. Brain Res. 2002;954:68–72. doi: 10.1016/s0006-8993(02)03343-7. [DOI] [PubMed] [Google Scholar]
- 61.Woods TM, Cusick CG, Pons TP, Taub E, Jones EG. Progressive transneuronal changes in the brainstem and thalamus after long-term dorsal rhizotomies in adult macaque monkeys. J Neurosci. 2000;20:3884–3899. doi: 10.1523/JNEUROSCI.20-10-03884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woolf CJ, Shortland P, Reynolds M, Ridings J, Doubell T, Coggeshall RE. Reorganization of central terminals of myelinated primary afferents in the rat dorsal horn following peripheral axotomy. J Comp Neurol. 1995;360:121–34. doi: 10.1002/cne.903600109. [DOI] [PubMed] [Google Scholar]
- 63.Xu J, Wall JT. Rapid changes in brainstem maps of adult primates after peripheral injury. Brain Res. 1997;774:211–215. doi: 10.1016/s0006-8993(97)81706-4. [DOI] [PubMed] [Google Scholar]
- 64.Xu J, Wall JT. Evidence for brainstem and supra-brainstem contributions to rapid cortical plasticity in adult monkeys. J Neurosci. 1999;19:7578–7590. doi: 10.1523/JNEUROSCI.19-17-07578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu J, Wall JT. Functional organization of tactile inputs from the hand in the cuneate nucleus and its relationship to organization in the somatosensory cortex. J Comp Neurol. 1999;411:369–389. [PubMed] [Google Scholar]