Abstract
BACKGROUND
Transcranial magnetic stimulation (TMS) to the left dorsal-lateral prefrontal cortex (DLPFC) is used clinically for the treatment of depression. However the antidepressant mechanism remains unknown and its therapeutic efficacy remains limited. Recent data suggests that some left DLPFC targets are more effective than others, however the reasons for this heterogeneity and how to capitalize on this information remain unclear.
METHODS
Intrinsic (resting state) fMRI data from 98 normal subjects were used to compute functional connectivity with various left DLPFC TMS targets employed in the literature. Differences in functional connectivity related to differences in previously reported clinical efficacy were identified. This information was translated into a connectivity-based targeting strategy to identify optimized left DLPFC TMS coordinates. Results in normal subjects were tested for reproducibility in an independent cohort of 13 patients with depression.
RESULTS
Differences in functional connectivity were related to previously reported differences in clinical efficacy across a distributed set of cortical and limbic regions. DLPFC TMS sites with better clinical efficacy were more negatively correlated (anticorrelated) with the subgenual cingulate. Optimum connectivity-based stimulation coordinates were identified in BA46. Results were reproducible in patients with depression.
CONCLUSIONS
Reported antidepressant efficacy of different left DLPFC TMS sites is related to the anticorrelation of each site with the subgenual cingulate, potentially lending insight into the antidepressant mechanism of TMS and suggesting a role for intrinsically anticorrelated networks in depression. These results can be translated into a connectivity-based targeting strategy for focal brain stimulation that might be used to optimize clinical response.
Keywords: Transcranial magnetic stimulation, TMS, intrinsic connectivity, resting state functional connectivity, MRI, subgenual, dorsolateral prefrontal cortex, depression
Introduction
Transcranial magnetic stimulation (TMS) is a noninvasive technique that utilizes short, rapidly changing magnetic field pulses to induce electrical currents in underlying cortical tissue (for reviews see (1–3)). By applying repeated pulses (rTMS) at low frequencies (eg 1 Hz) one can suppress underlying cortical activity and high-frequency stimulation (eg 20 Hz) can result in excitatory changes (1–3). Further, the effects of TMS can propagate beyond the site of stimulation, impacting a distributed network of brain regions (4–10).
One of the first clinical uses of TMS and its only FDA-approved therapeutic indication is high-frequency stimulation to the left dorsal lateral prefrontal cortex (DLPFC) for the treatment of medication resistant depression (11–14). Depression involves a distributed network of cortical and limbic regions including the DLPFC (especially the left), hippocampus, and subgenual cingulate among others (15, 16). Of these, the subgenual region has shown some of the most reproducible abnormalities. The subgenual decreases its activity in response to multiple treatment modalities (Table 1) and is a successful target of deep brain stimulation (DBS) (16–18). Unfortunately TMS is largely limited to the cortical surface and deeper limbic regions including the subgenual cannot be directly or selectively stimulated with traditional stimulation coils. TMS studies have therefore focused on the left DLPFC as one accessible node of this depression network. It has been hypothesized that left DLPFC TMS might have distributed effects on deeper limbic regions such as the subgenual (12, 13, 19), however combined TMS imaging studies designed to investigate this hypothesis have produced conflicting results (20–34). It therefore remains unclear how TMS to the DLPFC exerts its antidepressant effect.
Table 1.
Coordinates used to generate a priori regions of interest (ROIs). A) Coordinates of treatment related decreases in the subgenual cingulate tied to antidepressant effect, the treatment modality used, and the average coordinates used to generate our a priori ROI. B) Coordinates of various left dorsal-lateral prefrontal cortex transcranial magnetic stimulation targets suggested in the literature. For all prior studies we show the published coordinates in either Talairach (Tx, Ty, Tz) or MNI (MNIx, MNIy, MNIz) space along with the transformed MNI coordinates used in the present study.
| A) SUBGENUAL REGION | |||||||
|---|---|---|---|---|---|---|---|
| Study | Tx | Ty | Tz | MNIx | MNIy | MNIz | Treatment |
| Wu et al. 1999 | 7 | 17 | −4 | 7 | 18 | −4 | Sleep Deprivation |
| Mayberg et al. 2000 | 4 | 2 | −4 | 4 | 2 | −5 | SSRI |
| Drevets et al. 2002 | 3 | 31 | −10 | 3 | 32 | −10 | SSRI |
| Mayberg et al. 2005 | −2 | 8 | −10 | −2 | 9 | −11 | DBS |
| Mayberg et al. 2005 | 10 | 20 | −4 | 10 | 21 | −4 | DBS |
| Kito et al. 2008 | 17 | 16 | −14 | 17 | 17 | −16 | TMS |
| Kito et al. 2011 | 8 | 21 | −9 | 8 | 22 | −9 | TMS |
| Nahas et al. 2007 | 0 | 8 | −16 | 0 | 9 | −19 | VNS |
| AVERAGE | 5.9 | 16.3 | −9.8 | ||||
| B) DLPFC REGIONS | |||||||
| Study / Site | Tx | Ty | Tz | MNIx | MNIy | MNIz | |
| Herwig 2001 5cm Stim. Site | −42 | 17 | 52 | ||||
| Herbsman 2009 5cm Stim. Site | −42 | 20 | 49 | ||||
| Herbsman 2009 5cm Sham Site | −39 | 17 | 47 | ||||
| AVERAGE 5cm Coordinates | −41 | 18 | 49 | −41 | 16 | 54 | |
| Herbsman 2009 Responders | −46 | 25 | 44 | −46 | 23 | 49 | |
| Herbsman 2009 Nonresponders | −41 | 19 | 50 | −41 | 17 | 55 | |
| Herwig 2003 EEG (F3) Site | −37 | 27 | 44 | −37 | 26 | 49 | |
| Rajkowska 1995 BA46 Definition | −44 | 40 | 25 | −44 | 40 | 29 | |
| Rajkowska 1995 BA9 Definition | −36 | 40 | 38 | −36 | 39 | 43 | |
| Paus 2001 TMS Target | −40 | 32 | 30 | −40 | 31 | 34 | |
| Cho 2009 TMS Target | −40 | 32 | 30 | −40 | 31 | 34 | |
| Fitgerald 2009 TMS Target | −46 | 45 | 35 | −46 | 45 | 38 | |
| Rusjan 2010 TMS Target | −50 | 31 | 32 | −50 | 30 | 36 |
Paralleling our limited understanding of the antidepressant mechanism of TMS, its therapeutic efficacy, while statistically significant, also remains limited (11–14). One problem known to contribute to limited average clinical efficacy is difficulty identifying the appropriate stimulation target in the left DLPFC (12, 35–38). The FDA approved Neuronetics®’ Neurostar protocol along with the majority of TMS depression studies identifies the left DLPFC stimulation site by moving 5 cm anterior to the motor cortex along the curvature of the scalp (11–14, 39). However this technique frequently misses the DLPFC (37, 38). Alternative approaches to DLPFC target identification have been examined including standardized EEG electrode positions (40), a variety of anatomical MR coordinates focused around Brodmann areas 9 and 46 (35, 36, 41), and individualized hypometabolic foci (42–44) (Table 1B). These alternative targeting strategies have not led to substantial clinical improvements beyond the 5 cm approach, however data from these studies suggests that some DLPFC stimulation sites are more effective than others (12, 35, 36, 42). Unfortunately, it remains unclear why some sites are more effective, making it difficult to capitalize on this information to optimize target selection or clinical effect.
In the current study we hypothesized that previously reported differences in clinical efficacy of different left DLPFC stimulation sites are related to differences in the connectivity of these sites to deeper limbic regions, especially the subgenual cingulate. We tested this hypothesis using intrinsic (resting state) functional connectivity MRI, a powerful imaging technique that utilizes correlations in spontaneous fluctuations in the blood oxygenation level-dependent (BOLD) signal to assess functional relationships between regions (45–47). We first examined a large cohort of normal subjects to detect subtle differences in connectivity between adjacent regions, then confirmed these findings in a smaller cohort of patients with major depressive disorder.
Methods
Full methodological details can be found in the Supplement. Two datasets collected at different sites were used in the present analysis. The first consisted of 98 healthy right-handed subjects (48 male, ages 22±3.2 years (mean±SD)). The second dataset consisted of 13 right-handed subjects with major depressive disorder (3 male, mean age 40.2 years, mean HAM-D 23.8) and eleven healthy controls (5 male, mean age 29 years, mean HAM-D 0.4). These cohorts differed in age, gender ratio, and MRI scanner parameters and therefore cannot be directly compared to look for cohort differences, however they can be used to test for reproducibility across cohorts. All subjects completed one or more resting state fcMRI scans. fcMRI data were processed in accordance with the strategy of Fox et al 2005 (48) as implemented in Van Dijk 2010 (47) including global signal regression. An a-priori region of interest (ROI) was defined in the subgenual cingulate cortex (Fig. S1 in the Supplement, Fig. 3A) based on coordinates from prior studies showing reductions in subgenual activity tied to antidepressant response (17, 23, 24, 49–52) (Table 1). Additionally, a-priori ROIs were defined in the left DLPFC based on coordinates previously used or proposed as TMS targets for depression (Fig. 1, Fig. 2, Table 1) (25, 35–37, 41, 42, 53, 54).
Figure 3.
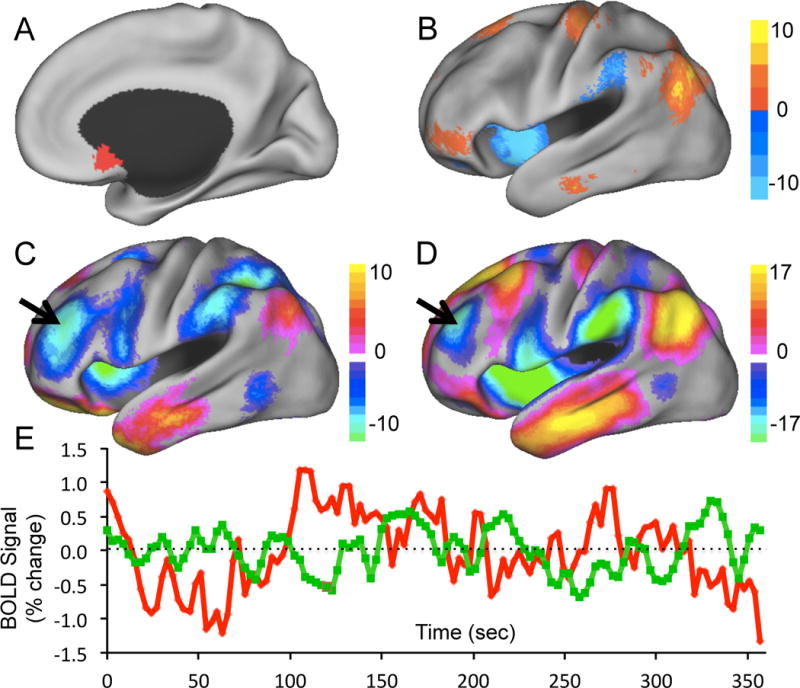
Identification of optimized left DLPFC TMS targets for depression based on functional connectivity. Regional time courses were extracted from our seed region in the subgenual cingulate (A) and our efficacy-based seed map (B) and used to generate resting state functional connectivity maps (C and D respectively). Peak anticorrelations were identified in the left DLPFC that could serve as optimized targets for focal brain stimulation. fMRI time courses from the subgenual region of interest (red) and the anticorrelated left dorsal lateral prefrontal cortex (green) are shown for a representative subject (r = −0.23).
Figure 1.
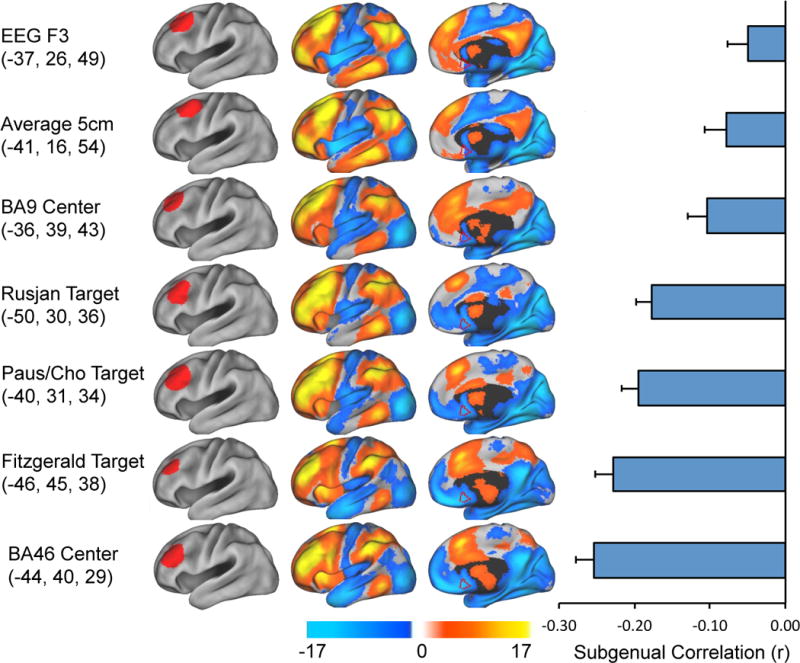
Different left DLPFC TMS targets show variability in resting state functional connectivity, especially with the subgenual cingulate. The left hand column shows the coordinates and regions of interest for various left DLPFC TMS targets employed in the literature. The middle columns show resting state functional connectivity maps for each DLPFC region of interest. The border of our a-priori defined subgenual region of interest is show for reference in red. The right hand column is the correlation coefficient between the timecourse from each DLPFC region of interest and that of the subgenual cingulate.
Figure 2.
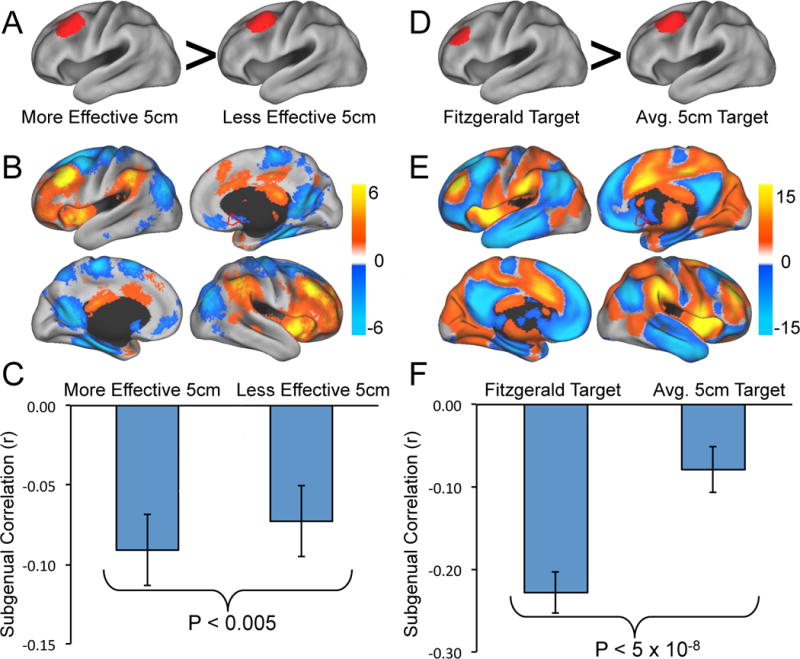
Differences in resting state functional connectivity between more effective versus less effective DLPFC stimulation sites. Coordinates are taken from Herbsman et al. 2009 (A–C) and Fitzgerald et al. 2009 (D–F). The top row (A, D) shows the DLPFC regions of interest compared in each study. The middle row (B, E) shows significant differences in resting state functional connectivity between the two sites (more effective – less effective). The border of our a-priori defined subgenual region of interest is show for reference in red. The bottom row (C, F) shows bar graphs of the correlation of each DLPFC site with the subgenual cingulate. In both cases the more effective DLPFC site is significantly more anticorrelated with the subgenual cingulate than the less effective site.
Three different analyses were used to relate functional connectivity of various left DLPFC TMS sites to previously reported clinical efficacy: 1) Paired comparison of functional connectivity between two TMS sites previously shown to differ in clinical efficacy (35, 36). 2) Correlation between functional connectivity and clinical efficacy as predicted by a previously reported equation (36): HDRS drop = −.84 + (X * −0.022) + (Y * 0.012). 3) Correlation between functional connectivity and clinical efficacy as previously reported in individual patients (42). Motivated by the results of the above analyses, coordinates were identified in the left DLPFC that could potentially serve as optimized TMS targets by computing seed-based functional connectivity with our a priori ROI in the subgenual and our effective-ineffective map. Principal findings in normal subjects were confirmed in patients with depression.
Results
We first determined whether the different left DLPFC stimulation sites suggested in the literature showed heterogeneity in their underlying functional connectivity, both on a voxelwise basis and specifically with our a-priori defined region of interest in the subgenual cingulate (Fig 1). Clear differences in functional connectivity were observed across multiple regions in the subgenual, medial prefrontal cortex, insula, and anterior cingulate. Interestingly, all DLPFC sites tested showed a significant negative correlation (anticorrelation) with the subgenual ranging from p < 0.01 for the F3 site to p < 10−26 for BA46. All sites except F3 remained significantly anticorrelated (p < 10−3) after Bonferroni correction for multiple comparisons. Stimulation sites relying on external skull-based landmarks including the 5 cm method and the EEG electrode method showed the weakest anticorrelation with the subgenual. Sites with strong physiological data showing distributed effects of TMS in the medial prefrontal cortex (25, 53) revealed a stronger anticorrelation. While both our BA9 and BA46 ROIs were anticorrelated, the stronger effect was for BA46. Finally, anatomical sites with either proven (35) or suggested (41) enhancement in clinical antidepressant response showed some of the strongest levels of anticorrelation.
Direct comparison of effective and ineffective TMS sites
Next we directly compared the functional connectivity between pairs of coordinates from prior studies documenting that one coordinate was clinically superior to another for producing an antidepressant effect. In the first study (Fig 2A), Herbsman et al. recorded the stimulation coordinates from 54 subjects treated with the 5 cm method (36). They averaged the stimulation sites for responders (−46, 23, 49) and showed this was anterior and lateral to the average stimulation site for non-responders (−41, 17, 55). Despite the fact that these coordinates are very close to one another, significant differences in functional connectivity were apparent (Fig 2B). The more effective stimulation site was significantly more anticorrelated with the subgenual cingulate compared to the less effective site (Fig 2C, P<0.005). In the second study (Fig 2D) Fitzgerald et al. targeted a specific anatomical coordinate (−46, 45, 38) based on evidence from the depression neuroimaging literature and showed (in secondary analyses) that this was superior to the standard 5 cm target (−41, 16, 54, from our analysis) (35). The voxelwise distribution of significant differences in functional connectivity between these two targets (Fig 2E) is similar to that in Figure 2B, although more robust given the larger separation in the DLPF coordinates. Also similar to the comparison using the Herbsman et al’s coordinates, the more effective stimulation site was significantly more anticorrelated with the subgenual cingulate compared to the less effective site (Fig 2F, P<0.0001).
We combined results across these two pair-wise comparisons to generate a single map of voxels showing significant differences in functional connectivity between more effective versus less effective DLPFC stimulation sites (Fig S2 in the Supplement). Peaks in this map were identified (23 positive, 29 negative) and include the subgenual cingulate in addition to several other regions implicated in depression including the medial prefrontal cortex, orbitofrontal cortex, subgenual cingulate, insula, thalamus, hypothalamus, and hippocampus (Table S1 in the Supplement).
Correlation between fcMRI and equation-based clinical efficacy
In addition to the above pair-wise comparisons, we examined the relationship between functional connectivity and the clinical efficacy of different DLPFC stimulation sites on a continuous basis. First, we computed the average clinical efficacy expected across a group of subjects based on the coordinates of each stimulation site using an equation empirically derived by Herbsman et al (2009) (36). We then plotted the predicted group-level clinical efficacy of all DLPFC stimulation sites considered in the current study (see Table 1) versus the resting state correlation of each site with the subgenual cingulate (Fig S3 A in the Supplement). Similar to the paired comparisons, DLPFC sites with higher predicted clinical efficacy showed stronger anticorrelation with the subgenual (r = −0.842, P<0.001 two-tailed). In fact, anticorrelation with the subgenual cingulate accounted for over 70% of the variance in clinical efficacy as predicted by Herbsman’s empirically-derived equation.
Correlation between fcMRI and clinical efficacy from individual patients
Moving beyond estimated group-level clinical efficacy using an equation, we next determined whether the above relationship held true for data from individual patients. To test this, we utilized a published table of left DLPFC stimulation coordinates and changes in the Montgomery & Asberg Depression Rating Scale for 27 individual patients receiving therapeutic TMS for depression (42). For each patient, we plotted their antidepressant response versus the resting state correlation between their specific stimulation site and the subgenual cingulate (Fig S3B in the Supplement). Note that resting state correlation values in this analysis are average values across our 98 normal subjects, not values from these specific patients as no resting state fMRI data was collected in this prior study. Despite this limitation, left DLPFC sites with higher clinical efficacy in individual patients again showed stronger anticorrelation with the subgenual (r = −0.355, p < 0.05 one-tailed). Interestingly, when applied to this independent cohort there was not a significant relationship between clinical efficacy measured in individual patients and group-level clinical efficacy as predicted by the Herbsman equation (r = 0.122, p > 0.25 one-tailed, Fig S3C in the Supplement). This suggests that anticorrelation with the subgenual captures important variance not captured by the Herbsman equation alone.
Identification of optimized TMS targets
The above results are potentially of interest for understanding the antidepressant mechanism of TMS (see discussion), but perhaps more importantly this information can be directly translated into a method to identify connectivity-based coordinates in the left DLPFC that could serve as an optimized TMS target. For example, the above results suggest that anticorrelation with the subgenual is related to antidepressant response. We can therefore use the subgenual ROI as a seed region and identify the peak anticorrelation in the left DLPFC (−44 38 34, Fig 3A). Similarly, the above results provide a map of voxels more functionally connected to effective compared to less effective stimulation sites (see Fig S2 in the Supplement). One can use this map as a weighted seed region (minus the left DLPFC to avoid biasing results and inverted to maintain consistency with the subgenual results) to identify an optimized left DLPFC target (−38 44 26, Fig 3B). Note that despite some difference in the coordinates of the peak anticorrelation, these two maps are very similar both across all grey matter voxels (spatial r = 0.630) and specifically within the left DLPFC (spatial r = 0.806). Interestingly there were several other nodes, besides the DLPFC, that were anticorrelated with the subgenual including parietal cortex / intraparietal sulcus, anterior insula, anterior SMA, and thalamus which could potentially serve as novel targets of focal brain stimulation for the treatment of depression (Table S1 in the Supplement) (55, 56).
Replication of results in Depression
Since resting state functional connectivity can differ between normal subjects and patients with depression (57), we confirmed our results in an independent cohort of 13 patients with depression using both our subgenual seed region and our efficacy-based seed map. Similar to normal subjects, we found a significant anticorrelation between the subgenual and multiple left DLPFC TMS targets, including the optimized targets identified above (P<0.05, Fig 4A). In paired comparisons, more effective sites showed a trend towards stronger anticorrelation with the subgenual and the optimized left DLPFC site was significantly more anticorrelated with the subgenual than the standard 5 cm target (P<0.05, Fig 4B). As in normal subjects, there was a robust relationship between clinical efficacy as predicted by the Herbsman equation and anticorrelation with the subgenual (r = −0.812, P<0.005, Fig 4C). Results were even more robust using our distributed efficacy-based seed map rather than the smaller and noisier subgenual ROI (Fig 4 D–F). Similar to the subgenual, many DLPFC targets including our optimized sites showed a significant negative correlation with the seed map (Fig 4E). In paired comparisons, more effective sites were significantly more anticorrelated than less effective sites, including the Herbsman regions (P<0.05), the Fitzgerald regions (P<10−4), and our new optimized site compared to the standard 5 cm target (P<10−6). Finally, there was a highly significant relationship between predicted clinical efficacy and correlation with our efficacy-based seed map (r = −0.875, P<0.001).
Figure 4.
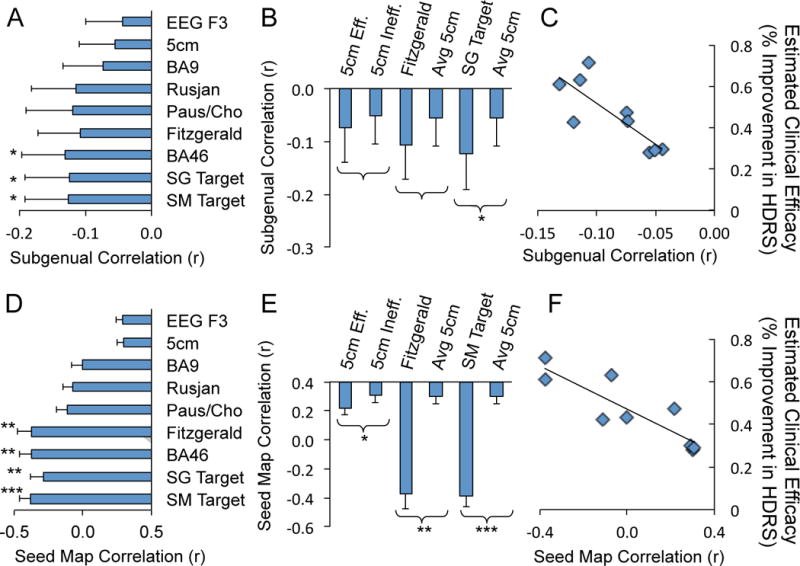
Replication of principal findings in patients with major depressive disorder. Time course correlations are shown between regions of interest in the dorsal lateral prefrontal cortex (DLPFC) and the subgenual seed region (A–C) or the efficacy-based seed map (D–F). Similar to normal subjects, there is an anticorrelation between TMS targets in the DLPFC and the subgenual (A). Paired comparisons of effective versus less effective DLPFC targets show the same trend as normal subjects and a significant difference between the optimized DLPF target identified using the subgenual seed region (SG Target) and the average 5 cm target (B). Also similar to normal subjects, there is a strong relationship between estimated clinical efficacy (using the Herbsman equation) and anticorrelation with the subgenual (C; r2 = 0.66, P<0.005). Using the efficacy-based seed map rather than the small subgenual seed region produces similar but more robust results including examination of regional time course correlations (D), paired comparisons (E), and the correlation between functional connectivity and estimated clinical efficacy (F; r2 = 0.76, P<0.001). Labels for DLPFC ROIs are as in Figures 1 and 2 with the addition of optimized DLPFC targets identified in normal subjects using the subgenual seed region (SG Target) and the efficacy-based seed map (SM Target). *P<0.05, **P<0.001, ***P<10−4.
Analyses were also replicated on the 11 control subjects from the same dataset as the 13 patients with depression (Fig S4 in the Supplement). There were no significant differences between these control subjects and patients with depression.
Discussion
In the current paper we used 1a novel connectivity-based approach to gain insight into why some left DLPFC TMS targets have proven more clinically effective than others. We identified robust differences in functional connectivity related to previously reported differences in clinical efficacy, particularly anticorrelation with the subgenual cingulate. We then demonstrated how one could translate this information into a connectivity-based targeting technique to identify coordinates in the left DLPFC that could potentially be used to optimize clinical response.
These results are likely relevant to understanding network models of depression, the antidepressant effect of TMS, and the functional relevance of intrinsic anticorrelations in resting state fMRI. Most importantly, the current results suggest that the clinical efficacy of focal brain stimulation might be optimized by targeting based on connectivity, a concept that remains to be tested in clinical trials but could find broad applicability across a number of diseases and stimulation techniques.
Relevance to network models of depression
Depression is becoming increasingly recognized as a network disorder associated with alterations in a distributed set of regions including DLPFC (especially left), medial prefrontal, orbitofrontal, subgenual cingulate, insula, thalamus, hypothalamus, and hippocampus (15, 16). Of these regions, the left DLPFC and the subgenual cingulate have received the most attention due to the consistency of their depression-related abnormalities, their modulation with treatment across a range of therapies, and their use as targets of focal brain stimulation (58). Although depression functional imaging studies have produced heterogeneous results (16, 59–61), on average the abnormalities in these two regions have been opposite one another (58). The subgenual has been observed to be hyperactive in depression and a decrease in this hyperactivity is associated with antidepressant response (16, 17, 58) (see Table 1). Conversely, the left DLPFC tends to be hypoactive in depression and an increase in activity is associated with antidepressant response (58, 59). Consistent with this dichotomy, lesions of the ventral medial prefrontal cortex can improve depression while lesions of the dorsal lateral prefrontal cortex can exacerbate it (62).
The current finding that the subgenual and DLPFC are intrinsically anticorrelated during the resting state mirrors this dichotomy and suggests that there is a link between the depression-related abnormalities in these two regions. There are several implications of this result. First, observed depression-related abnormalities in one region could theoretically be due solely to pathology in the opposing region. Primary hyperactivity in the subgenual might result in secondary hypoactivity of the DLPFC without anything being abnormal in the DLPFC and vice versa. Second, this anticorrelation could mediate compensatory responses. The DLPFC could increase its activity in response to subgenual hyperactivity in an attempt to suppress or normalize activity in this region, a mechanism that could explain the occasional finding of DLPFC hyperactivity in depression (15, 59, 60). Finally, focal inhibition/excitation of one region could be expected to respectively enhance/suppress activity of the other region. Indeed, DBS of the subgenual (which suppresses activity locally) results in an increase in activity in the DLPFC (17).
While the above discussion focused on the subgenual and the DLPFC, it is important to remember that the current results include several other regions previously implicated in the pathology of depression (15, 61). Our results suggest two anticorrelated groups of regions. The first consists of the subgenual, medial prefrontal, superior frontal, hippocampus, posterior cingulate / precuneus, middle temporal gyrus, and cerebellar tonsils while the second consists of the DLPFC, anterior insula, dorsal anterior cingulate / pre-SMA, thalamus, DLPFC, and parietal cortex.
Understanding the antidepressant mechanism of TMS
There has been much research into the antidepressant mechanism of DLPFC TMS in the hopes that this knowledge would facilitate optimization of the effect and improve clinical utility. Many hypotheses have been proposed (12, 63), however one idea that has been pursued aggressively is the propagation of TMS effects through anatomical connections to deeper limbic regions (12). A number of groups have attempted to localize the remote effects of DLPFC TMS by pairing it with neuroimaging techniques both in normal subjects and patients with depression. A full review of these heterogeneous results is beyond the scope of this article, however given the current findings we examined the results of these studies with respect to changes in the subgenual cingulate or adjacent medial prefrontal cortex (Table S2 in the Supplement). Although many studies found TMS-induced decreases in subgenual activity (20–24) or adjacent medial prefrontal activity (25–27), other studies found no significant changes in these regions (28, 29, 31–33), and one study observed increased medial prefrontal activity (29). The present findings using a novel connectivity-based approach are consistent with eight of the above thirteen studies and suggest that part of the antidepressant mechanism of DLPFC TMS may be remote suppression of activity in the subgenual cingulate and other limbic regions.
Relevance to the debate surrounding anticorrelations
There has been substantial debate surrounding the appropriate interpretation of anticorrelations observed with resting state fcMRI in the setting of a preprocessing step termed global signal regression (47, 64–67). This processing can improve the specificity of resting state correlations and the correspondence with anatomy (65), however there are mathematical concerns that anticorrelations could emerge as “processing artifact.” While the technical issues surrounding processing strategy and anticorrelations are beyond the scope of this article (see Fox et al. 2009 for discussion), the current results add information to be considered in the ongoing debate. First, the fact that the resting state anticorrelation between the subgenual and DLPFC is recapitulated in patterns of pathological abnormalities seen in depression provides additional evidence that anticorrelations may reflect functionally meaningful relationships. Second, the focal brain stimulation interventions used in depression might serve as a causal test of the functional importance of anticorrelations. If stimulation/inhibition of one node suppresses/augments the activity of the anticorrelated node in a spatially specific manner and in proportion to the strength of the anticorrelation this would support the biological importance of anticorrelations.
An interesting issue is determining how anticorrelations observed with resting state fcMRI are mediated. In the case of the subgenual and DLPFC, the anticorrelation is unlikely to be the result of direct inhibitory connections. Monkey track tracing studies suggest that there are not direct anatomical connections between BA46 and BA25 (68, 69). However there are direct anatomical connections between the subgenual (BA25) and the anterior insula and mediodorsal nucleus of the thalamus, both of which are anticorrelated with the subgenual in the current analysis. Previous studies have implicated the fronto-insular cortex as a potential node mediating anticorrelations (70), and other studies have suggested the thalamus, especially the mediodorsal nucleus, as the site of integration of otherwise separate cortical-subcortical loops (71).
Targeting focal brain stimulation based on connectivity
The idea that targets for focal brain stimulation should be selected at least partly based on their connectivity to other regions is not new, however implementing this strategy in practice has been difficult and empiric evidence supporting the utility of this approach has been limited (for review see (10)). It has been suggested that stimulation should be targeted to the portion of the DLPFC with connectivity to deeper limbic regions (12, 19). Unfortunately, the connectivity between the DLPFC and various limbic regions is complicated even in monkeys (68, 69), and the DLPFC is one of the areas that has expanded the most throughout evolution (54, 72). It has remained unclear which part of the human DLPFC should be stimulated and which limbic regions are important even if the human connectivity between the DLPFC and limbic regions was well established.
In the current manuscript we use intrinsic fcMRI with the subgenual and our efficacy-based seed map to identify left DLPFC TMS coordinates designed to optimize antidepressant response. These coordinates might serve as the basis for a clinical trial, however this connectivity-based targeting approach can be taken further. First, our results suggest the existence of other connectivity-based TMS targets for depression besides the DLPFC (see Fig 3, Table S1 in the Supplement). Of these, the cerebellum and parietal cortex have previously been suggested as potential TMS targets in depression based on mood effects in normal subjects (56). A recent trial of low-frequency parietal stimulation failed to show a significant response beyond sham (55), however the present results suggest that high-frequency stimulation to the peak parietal node anticorrelated with the subgenual may be more effective. Second, the current study reports average group-level coordinates. Although average coordinates have previously been used in clinical trials of TMS for depression (35), an advantage of the current targeting approach is it might be applied at the single subject level. Given cross-subject heterogeneity in the location of the DLPFC (54), the full potential of connectivity-based targeting may be realized with identification of individualized TMS targets tailored to individual patients. Finally, the current targeting approach is potentially applicable across other diseases and brain stimulation techniques. Cortical correlates of deep brain stimulation sites based on fcMRI could serve as important TMS targets in Parkinson’s disease, dystonia, obsessive compulsive disorder, or any other disease for which DBS provides clinical benefit (73). The converse of this approach also holds promise. Specifically, intrinsic fcMRI could be used to identify optimized DBS sites in individual patients based on connectivity with distributed cortical networks know to be impacted by disease.
Limitations and Future Work
The current work was limited in several respects and these limitations suggest important avenues for future research. First, our results were generated on normal subjects then confirmed in a small cohort of patients with depression. While this makes it likely that our findings will further generalize to a larger cohort of patients with medication-refractory depression undergoing TMS, our results remain to be confirmed in this specific population. Second, measures of clinical efficacy in the current article were based on previously published data and not obtained denovo. Ideally one would measure clinical efficacy and resting state functional connectivity in the same cohort of patients. However, the fact that our connectivity results in normal subjects predicted clinical efficacy in an independent set of patients suggests that future work measuring both parameters in the same cohort should only increase the strength of the relationship. Finally, the current findings suggest that the antidepressant effect of TMS might be optimized through connectivity-based targeting, however this remains a hypothesis. The clinical utility of this method remains to be tested in a clinical trial.
Supplementary Material
Acknowledgments
We thank the Brain Genomics Superstruct Project for contributing data. MDF was supported by NIH Grant R25NS065743. Work on this study was also supported by grants from the National Institutes of Health and National Center for Research Resources: Harvard Clinical and Translational Science Center (UL1 RR025758), the Howard Hughes Medical Institute, and the Dana Foundation. APL serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Allied Mind, Neosync, and Novavision, and is listed as inventor in issued patents and patent applications on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 3.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Valero-Cabre A, Payne BR, Rushmore J, Lomber SG, Pascual-Leone A. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: a 14C-2DG tracing study in the cat. Experimental brain research Experimentelle Hirnforschung. 2005;163:1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- 5.Valero-Cabre A, Payne BR, Pascual-Leone A. Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Experimental brain research Experimentelle Hirnforschung. 2007;176:603–615. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- 6.Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, Mochizuki H, et al. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2:58–80. doi: 10.1016/j.brs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Ruff CC, Driver J, Bestmann S. Combining TMS and fMRI: from ‘virtual lesions’ to functional-network accounts of cognition. Cortex. 2009;45:1043–1049. doi: 10.1016/j.cortex.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreri F, Pasqualetti P, Maatta S, Ponzo D, Ferrarelli F, Tononi G, et al. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 9.Lisanby SH, Belmaker RH. Animal models of the mechanisms of action of repetitive transcranial magnetic stimulation (RTMS): comparisons with electroconvulsive shock (ECS) Depress Anxiety. 2000;12:178–187. doi: 10.1002/1520-6394(2000)12:3<178::AID-DA10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.03.035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol. 2009;219:2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 13.George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6:1853–1856. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- 14.Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 15.Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61:729–730. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS spectrums. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George MS, Wassermann EM, Kimbrell TA, Little JT, Williams WE, Danielson AL, et al. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. The American journal of psychiatry. 1997;154:1752–1756. doi: 10.1176/ajp.154.12.1752. [DOI] [PubMed] [Google Scholar]
- 20.George MS, Stallings LE, Speer AM, Nahas Z, Spicer KM, Vincent DJ, et al. Prefrontal repetitive transcranial magnetic stimulation (rTMS) changes relative perfusion locally and remotely. Human Psychopharmacology: Clinical and Experimental. 1999;14:161–170. [Google Scholar]
- 21.Kimbrell Ta, Dunn RT, George MS, Danielson AL, Willis MW, Repella JD, et al. Left prefrontal-repetitive transcranial magnetic stimulation (rTMS) and regional cerebral glucose metabolism in normal volunteers. Psychiatry research. 2002;115:101–113. doi: 10.1016/s0925-4927(02)00041-0. [DOI] [PubMed] [Google Scholar]
- 22.Narushima K, McCormick LM, Yamada T, Thatcher RW, Robinson RG. Subgenual cingulate theta activity predicts treatment response of repetitive transcranial magnetic stimulation in participants with vascular depression. The Journal of neuropsychiatry and clinical neurosciences. 2010;22:75–84. doi: 10.1176/appi.neuropsych.22.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kito S, Fujita K, Koga Y. Regional cerebral blood flow changes after low-frequency transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in treatment-resistant depression. Neuropsychobiology. 2008;58:29–36. doi: 10.1159/000154477. [DOI] [PubMed] [Google Scholar]
- 24.Kito S, Hasegawa T, Koga Y. Neuroanatomical correlates of therapeutic efficacy of low-frequency right prefrontal transcranial magnetic stimulation in treatment-resistant depression. Psychiatry Clin Neurosci. 2011;65:175–182. doi: 10.1111/j.1440-1819.2010.02183.x. [DOI] [PubMed] [Google Scholar]
- 25.Paus T, Castro-Alamancos Ma, Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. European Journal of Neuroscience. 2001;14:1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Nahas Z, Kozel FA, Anderson B, Bohning DE, George MS. Acute left prefrontal transcranial magnetic stimulation in depressed patients is associated with immediately increased activity in prefrontal cortical as well as subcortical regions. Biological psychiatry. 2004;55:882–890. doi: 10.1016/j.biopsych.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Teneback CC, Nahas Z, Speer AM, Molloy M, Stallings LE, Spicer KM, et al. Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. Journal of Neuropsychiatry and Clinical Neurosciences. 1999;11:426. doi: 10.1176/jnp.11.4.426. [DOI] [PubMed] [Google Scholar]
- 28.Nahas Z, Lomarev M, Roberts DR, Shastri a, Lorberbaum JP, Teneback C, et al. Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biological psychiatry. 2001;50:712–720. doi: 10.1016/s0006-3223(01)01199-4. [DOI] [PubMed] [Google Scholar]
- 29.Speer aM, Kimbrell Ta, Wassermann EM, D Repella J, Willis MW, Herscovitch P, et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biological psychiatry. 2000;48:1133–1141. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- 30.Mottaghy FM, Keller CE, Gangitano M, Ly J, Thall M, Parker JA, et al. Correlation of cerebral blood flow and treatment effects of repetitive transcranial magnetic stimulation in depressed patients. Psychiatry research. 2002;115:1–14. doi: 10.1016/s0925-4927(02)00032-x. [DOI] [PubMed] [Google Scholar]
- 31.Eisenegger C, Treyer V, Fehr E, Knoch D. Time-course of “off-line” prefrontal rTMS effects–a PET study. NeuroImage. 2008;42:379–384. doi: 10.1016/j.neuroimage.2008.04.172. [DOI] [PubMed] [Google Scholar]
- 32.Knoch D, Treyer V, Regard M, Muri RM, Buck A, Weber B. Lateralized and frequency-dependent effects of prefrontal rTMS on regional cerebral blood flow. NeuroImage. 2006;31:641–648. doi: 10.1016/j.neuroimage.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 33.Speer AM, Willis MW, Herscovitch P, Daube-Witherspoon M, Shelton JR, Benson BE, et al. Intensity-dependent regional cerebral blood flow during 1-Hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with H215O positron emission tomography: II. Effects of prefrontal cortex rTMS. Biological psychiatry. 2003;54:826–832. doi: 10.1016/s0006-3223(03)00324-x. [DOI] [PubMed] [Google Scholar]
- 34.Ferrarelli F, Haraldsson HM, Barnhart TE, Roberts AD, Oakes TR, Massimini M, et al. A [17F]-fluoromethane PET/TMS study of effective connectivity. Brain research bulletin. 2004;64:103–113. doi: 10.1016/j.brainresbull.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald PB, Hoy K, McQueen S, Maller JJ, Herring S, Segrave R, et al. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009;34:1255–1262. doi: 10.1038/npp.2008.233. [DOI] [PubMed] [Google Scholar]
- 36.Herbsman T, Avery D, Ramsey D, Holtzheimer P, Wadjik C, Hardaway F, et al. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry. 2009;66:509–515. doi: 10.1016/j.biopsych.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 37.Herwig U, Padberg F, Unger J, Spitzer M, Schonfeldt-Lecuona C. Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard” coil positioning by neuronavigation. Biol Psychiatry. 2001;50:58–61. doi: 10.1016/s0006-3223(01)01153-2. [DOI] [PubMed] [Google Scholar]
- 38.Ahdab R, Ayache SS, Brugières P, Goujon C, Lefaucheur J-P. Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiologie clinique = Clinical neurophysiology. 2010;40:27–36. doi: 10.1016/j.neucli.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 39.George MS, Wassermann EM, Williams WA, Steppel J, Pascual-Leone A, Basser P, et al. Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci. 1996;8:172–180. doi: 10.1176/jnp.8.2.172. [DOI] [PubMed] [Google Scholar]
- 40.Herwig U, Satrapi P, Schonfeldt-Lecuona C. Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16:95–99. doi: 10.1023/b:brat.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- 41.Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB, et al. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Human brain mapping. 2010;31:1643–1652. doi: 10.1002/hbm.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paillère Martinot M-L, Galinowski A, Ringuenet D, Gallarda T, Lefaucheur J-P, Bellivier F, et al. Influence of prefrontal target region on the efficacy of repetitive transcranial magnetic stimulation in patients with medication-resistant depression: a [(18)F]-fluorodeoxyglucose PET and MRI study. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2010;13:45–59. doi: 10.1017/S146114570900008X. [DOI] [PubMed] [Google Scholar]
- 43.Herwig U, Lampe Y, Juengling FD, Wunderlich A, Walter H, Spitzer M, et al. Add-on rTMS for treatment of depression: a pilot study using stereotaxic coil-navigation according to PET data. J Psychiatr Res. 2003;37:267–275. doi: 10.1016/s0022-3956(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Toro M, Salva J, Daumal J, Andres J, Romera M, Lafau O, et al. High (20-Hz) and low (1-Hz) frequency transcranial magnetic stimulation as adjuvant treatment in medication-resistant depression. Psychiatry research. 2006;146:53–57. doi: 10.1016/j.pscychresns.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Biswal B, Yetkin F, Haughton V, Hyde J. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 46.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 47.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of neurophysiology. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, et al. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry. 1999;156:1149–1158. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- 50.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 51.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 52.Nahas Z, Teneback C, Chae JH, Mu Q, Molnar C, Kozel FA, et al. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology. 2007;32:1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 53.Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PloS one. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- 55.Schutter DJ, Laman DM, van Honk J, Vergouwen AC, Koerselman GF. Partial clinical response to 2 weeks of 2 Hz repetitive transcranial magnetic stimulation to the right parietal cortex in depression. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2009;12:643–650. doi: 10.1017/S1461145708009553. [DOI] [PubMed] [Google Scholar]
- 56.Schutter DJ, van Honk J. A framework for targeting alternative brain regions with repetitive transcranial magnetic stimulation in the treatment of depression. Journal of psychiatry & neuroscience : JPN. 2005;30:91–97. [PMC free article] [PubMed] [Google Scholar]
- 57.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural brain research. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry research. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Steele JD, Currie J, Lawrie SM, Reid I. Prefrontal cortical functional abnormality in major depressive disorder: a stereotactic meta-analysis. Journal of affective disorders. 2007;101:1–11. doi: 10.1016/j.jad.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Human brain mapping. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12341–12348. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paus T, Barrett J. Transcranial magnetic stimulation (TMS) of the human frontal cortex: implications for repetitive TMS treatment of depression. Journal of psychiatry & neuroscience : JPN. 2004;29:268–279. [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong E-K, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Human brain mapping. 2010;00 doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. The European journal of neuroscience. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 69.Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. The Journal of comparative neurology. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- 70.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Groenewegen HJ, Galis-de Graaf Y, Smeets WJ. Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. J Chem Neuroanat. 1999;16:167–185. doi: 10.1016/s0891-0618(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 72.Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 73.Hamani C, Nobrega JN, Lozano aM. Deep brain stimulation in clinical practice and in animal models. Clinical pharmacology and therapeutics. 2010;88:559–562. doi: 10.1038/clpt.2010.133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


