Abstract
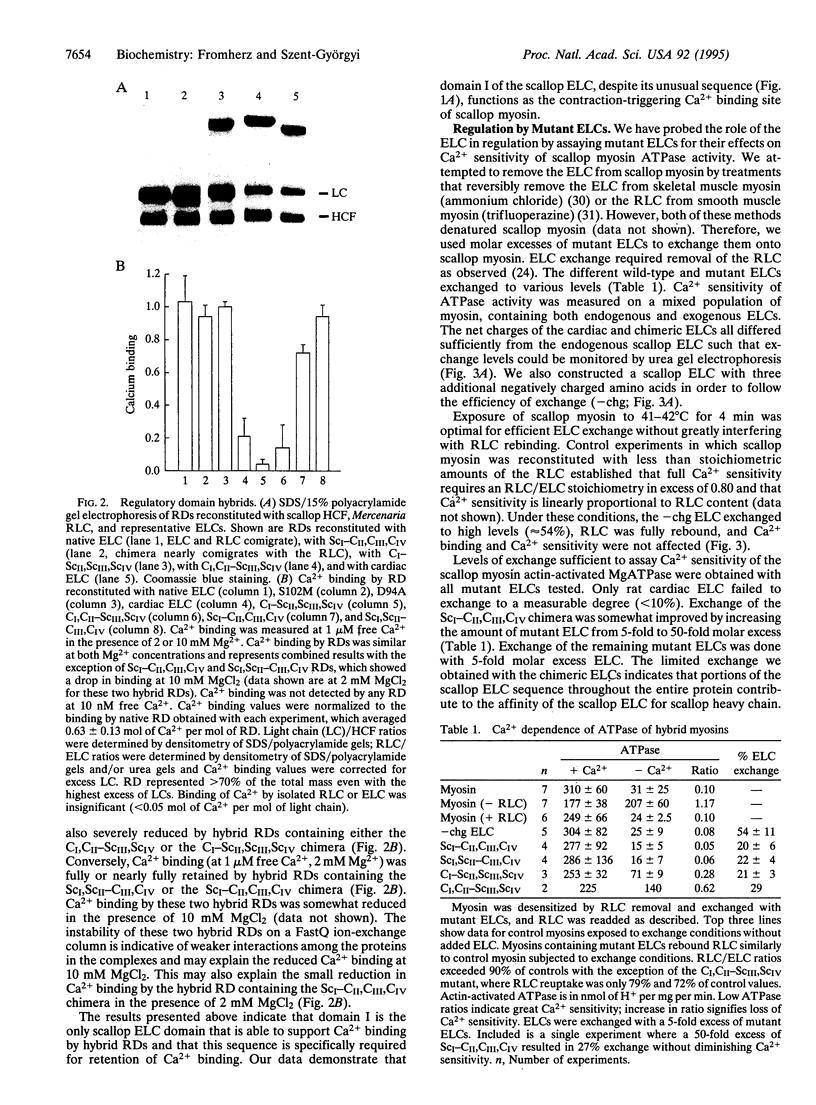
The specific Ca2+ binding site that triggers contraction of molluscan muscle requires the presence of an essential light chain (ELC) from a Ca2+ binding myosin. Of the four EF hand-like domains in molluscan ELCs, only domain III has an amino acid sequence predicted to be capable of binding Ca2+. In this report, we have used mutant ELCs to locate the Ca2+ binding site in scallop myosin and to probe the role of the ELC in regulation. Point mutations in domain III of scallop ELC have no effect on Ca2+ binding. Interestingly, scallop and rat cardiac ELC chimeras support Ca2+ binding only if domain I is scallop. These results are nevertheless in agreement with structural studies on a proteolytic fragment of scallop myosin, the regulatory domain. Furthermore, Ca2+ sensitivity of the scallop myosin ATPase requires scallop ELC domain I: ELCs containing cardiac domain I convert scallop myosin to an unregulated molecule whose activity is no longer repressed in the absence of Ca2+. Despite its unusual EF hand domain sequence, our data indicate that the unique and required contribution of molluscan ELCs to Ca2+ binding and regulation of molluscan myosins resides exclusively in domain I.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashiba G., Szent-Györgyi A. G. Essential light chain exchange in scallop myosin. Biochemistry. 1985 Nov 5;24(23):6618–6623. doi: 10.1021/bi00344a048. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Collins J. H. Homology of myosin DTNB light chain with alkali light chains, troponin C and parvalbumin. Nature. 1976 Feb 26;259(5545):699–700. doi: 10.1038/259699a0. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Jakes R., Kendrick-Jones J., Leszyk J., Barouch W., Theibert J. L., Spiegel J., Szent-Györgyi A. G. Amino acid sequence of myosin essential light chain from the scallop Aquipecten irradians. Biochemistry. 1986 Nov 18;25(23):7651–7656. doi: 10.1021/bi00371a056. [DOI] [PubMed] [Google Scholar]
- Goodwin E. B., Szent-Gyorgyi A. G., Leinwand L. A. Cloning and characterization of the scallop essential and regulatory myosin light chain cDNAs. J Biol Chem. 1987 Aug 15;262(23):11052–11056. [PubMed] [Google Scholar]
- Jancso A., Szent-Györgyi A. G. Regulation of scallop myosin by the regulatory light chain depends on a single glycine residue. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8762–8766. doi: 10.1073/pnas.91.19.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalabokis V. N., O'Neall-Hennessey E., Szent-Györgyi A. G. Regulatory domains of myosins: influence of heavy chain on Ca(2+)-binding. J Muscle Res Cell Motil. 1994 Oct;15(5):547–553. doi: 10.1007/BF00121160. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory light chains in myosins. J Mol Biol. 1976 Jul 15;104(4):747–775. doi: 10.1016/0022-2836(76)90180-7. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Nockolds C. E. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973 May 10;248(9):3313–3326. [PubMed] [Google Scholar]
- Kwon H., Goodwin E. B., Nyitray L., Berliner E., O'Neall-Hennessey E., Melandri F. D., Szent-Györgyi A. G. Isolation of the regulatory domain of scallop myosin: role of the essential light chain in calcium binding. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4771–4775. doi: 10.1073/pnas.87.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Melandri F. D., Szent-Györgyi A. G. Role of gizzard myosin light chains in calcium binding. J Muscle Res Cell Motil. 1992 Jun;13(3):315–320. doi: 10.1007/BF01766459. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowey S., Waller G. S., Trybus K. M. Skeletal muscle myosin light chains are essential for physiological speeds of shortening. Nature. 1993 Sep 30;365(6445):454–456. doi: 10.1038/365454a0. [DOI] [PubMed] [Google Scholar]
- McNally E. M., Buttrick P. M., Leinwand L. A. Ventricular myosin light chain 1 is developmentally regulated and does not change in hypertension. Nucleic Acids Res. 1989 Apr 11;17(7):2753–2767. doi: 10.1093/nar/17.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Moncrief N. D., Kretsinger R. H. Evolution of EF-hand calcium-modulated proteins. II. Domains of several subfamilies have diverse evolutionary histories. J Mol Evol. 1992 May;34(5):416–448. doi: 10.1007/BF00162998. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. The "megaprimer" method of site-directed mutagenesis. Biotechniques. 1990 Apr;8(4):404–407. [PubMed] [Google Scholar]
- Scholey J. M., Taylor K. A., Kendrick-Jones J. The role of myosin light chains in regulating actin-myosin interaction. Biochimie. 1981 Apr;63(4):255–271. doi: 10.1016/s0300-9084(81)80115-0. [DOI] [PubMed] [Google Scholar]
- Simmons R. M., Szent-Györgyi A. G. A mechanical study of regulation in the striated adductor muscle of the scallop. J Physiol. 1985 Jan;358:47–64. doi: 10.1113/jphysiol.1985.sp015539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Strynadka N. C., James M. N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- Szebenyi D. M., Moffat K. The refined structure of vitamin D-dependent calcium-binding protein from bovine intestine. Molecular details, ion binding, and implications for the structure of other calcium-binding proteins. J Biol Chem. 1986 Jul 5;261(19):8761–8777. [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Szentkiralyi E. M., Kendrick-Jonas J. The light chains of scallop myosin as regulatory subunits. J Mol Biol. 1973 Feb 25;74(2):179–203. doi: 10.1016/0022-2836(73)90106-x. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Waller G. S., Chatman T. A. Coupling of ATPase activity and motility in smooth muscle myosin is mediated by the regulatory light chain. J Cell Biol. 1994 Mar;124(6):963–969. doi: 10.1083/jcb.124.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufty R. M., Kretsinger R. H. Troponin and parvalbumin calcium binding regions predicted in myosin light chain and T4 lysozyme. Science. 1975 Jan 17;187(4172):167–169. doi: 10.1126/science.1111094. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Szent-Gyorgyi A. G., Sheetz M. P. Movement of scallop myosin on Nitella actin filaments: regulation by calcium. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6775–6778. doi: 10.1073/pnas.81.21.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M., Pope B., Gooch J., Hawkins M., Weeds A. G. Identification of a region in segment 1 of gelsolin critical for actin binding. EMBO J. 1990 Dec;9(12):4103–4109. doi: 10.1002/j.1460-2075.1990.tb07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C., Warriner K. E., Bagshaw C. R. Fluorescence studies on the nucleotide- and Ca2+-binding domains of molluscan myosin. Biochem J. 1985 Oct 1;231(1):31–38. doi: 10.1042/bj2310031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Harrison D. H., Schlichting I., Sweet R. M., Kalabokis V. N., Szent-Györgyi A. G., Cohen C. Structure of the regulatory domain of scallop myosin at 2.8 A resolution. Nature. 1994 Mar 24;368(6469):306–312. doi: 10.1038/368306a0. [DOI] [PubMed] [Google Scholar]